Abstract
The practice of directly applying unfermented and decomposed organic matter to plants is rare in the growth process of terrestrial plants. The organic matter content at the discharge outlet of shrimp ponds is usually high. Therefore, it is necessary to collect soil from the discharge outlet of shrimp ponds and simulate the migration and transformation pathways of organic acids and related metabolic microorganisms in soil of mangrove wetlands through laboratory soil columns and the HYDRUS-1D model. Results showed that the content of oxalic acid remained relatively stable in the soil column at different depths, citric acid settled downward along the vertical direction, the concentration of acetic acid in the depth range of 30–50 cm increased. The organic acids formed insoluble or slightly soluble precipitates in the form of organic acid calcium, the organic acids in 40–50 cm were completely neutralized on the 18th day. The abundance of acid-producing Acinetobacter Johnsonii increased during the later stages of anaerobic acidification and disappeared after the addition of Ca(OH)2. The results of HYDRUS-1D simulation showed that the adsorption, deposition and transport of organic acids in the mangrove wetland were poor, the results of vertical infiltration modelling were in agreement with the soil column experiments.
Keywords: Mangrove wetland, RT-HPLC method, Numerical modelling, Microbial sequencing
Subject terms: Ocean sciences, Marine chemistry, Environmental impact
Introduction
Mangrove wetlands are one of the most diverse and productive marine ecosystems which can capture pollutants and purify water quality1, with Hainan Province is the richest province in China in terms of mangrove species. Although Dongzhai Harbour Protected Area has taken a series of ecological restoration measures, such as returning ponds to forests, artificial restoration, environmental remediation, etc., the mangrove forests in the protected area have been damaged to varying degrees until 2016, with the mangrove forests in decline covering an area of about 100 hm2(accounting for 19% of the total area of mangrove forests in Dongzhai Harbour). More than 80% of the existing mangrove forests being inefficient degraded secondary forests, with the mangrove forest ecosystems health index continuously decreasing, and the mangrove ecosystem in Dongzhai Harbour is still deteriorating2.
In Dongzhai Harbour, Hainan, the discharge of high-elevation shrimp culture ponds is the main source of pollution in mangroves, and a large amount of residual bait, excreta, chemical disinfectants and antibiotics discharged from shrimp ponds culture has caused serious pollution to the natural environment. Five year monitoring results of soil and pollution sources in Dongzhai Habour showed that soils in difficult mangrove restoration areas were usually acidic. The pH of soils in the mangrove restoration area of Tashi Town was < 4, and the pH of soils in the mangrove restoration area of Shanwei Village was < 6, so it can be preliminarily determined that acidic discharged from shrimp aquaculture in the high-elevation ponds are the main factors affecting the mangrove forests’ restoration3. The results of literature research indicated that research on the toxicity of organic acids has focused on the impact of organic acids on crops4,5. Therefore, the study of the impact mechanism of organic acid substances in the mangrove ecosystem of the estuarine region is a pioneering research direction in the field of mangrove ecosystem research. The increasing demand for aquatic products in human society led to the gradual expansion of aquaculture around mangroves, which greatly increased the discharge of organic acids and organic matter. Moreover, the organic acids was also generated from the emitted organic matter. Numerous studies have shown that when organic acids reach a certain concentration, they have a certain toxic effect on plant tissue cells, embryonic axis germination, and root, stem, and leaf growth6,7. The accumulation of high concentration acidic substances in mangrove soil in a short period of time may cause the accumulation of acidic substances, which have toxic effects on mangrove plants. If acidic substances persist for a long time, it will lead to deterioration of soil conditions and difficulty in restoring mangroves8. More seriously, organic acids (citric acid, oxalic acid, acetic acid, etc.) can promote the weathering and dissolution of carbonate minerals in mangrove wetlands9, leading to the destruction of the carbon sequestration capacity of terrestrial ecosystems10. Accordingly, the increase in CO2production can seriously affecting the carbon sequestration function of mangrove ecosystems and lead to the greenhouse effect11,12. Therefore, it is crucial to explore the impact of organic acid enrichment and transformation in mangrove soil on the mangrove ecosystem.
The emissions from high-elevation shrimp ponds include acidic chlorine dioxide disinfectants and iodine solutions such as povidone iodine solution, organic acid substances such as oxalic acid, citric acid, acetic acid, and other detoxifying agents are also presented in emissions13. In addition, a large amount of residual bait, excreta fish and shrimp carcasses discharged from shrimp pond culture into the mangrove forests usually underwent a large amount of enriched sedimentation in the mangrove forest inlet, which was decomposed by anaerobic microorganisms to generate organic acids14. This practice of directly applying unfermented and decomposed organic matter to plants is rare in the growth process of terrestrial plants, while it is a common phenomenon in China’s mangrove wetland ecosystem. The unfermented and decomposed organic matter directly discharged into the mangrove forest will accumulate at the discharge outlet and produce acid under the action of anaerobic microorganisms, which will lower the soil pH; In addition, the decomposition of organic matter by microorganisms generates a large amount of heat, which leads to the massive death of mangrove plants at the discharge outlet. Other studies mainly focused on the impact of pollutant emissions during aquaculture on nitrogen, phosphorus, and soil organic carbon in soil, with little exploration of changes in organic acids15. When the concentration of organic acids reaches 30 mg/L, mangrove plants will be poisoned and soil conditions in local areas within the protected area will be deteriorated16. In addition, shrimp pond aquaculture uses a large amount of quicklime disinfectant during pond cleaning, which is discharged into mangroves in the form of Ca(OH)2. The biological and chemical transformation of the soil during the discharge of such alkaline disinfectants, the temporal and spatial changes in the production of organic acids during the transformation process, as well as potential methane emissions from organic acids, all of these required the establishment of research methods to confirm. Therefore, it is necessary to conduct soil column simulation experiments in the laboratory to explore the chemical and biological changes of soil organic acids in mangrove ecosystems under the influence of some simple factors.
This study aims to clarify the migration and transformation patterns of organic acids emitted and transformed from high-elevation shrimp ponds in mangrove wetland soil. Soil samples were collected from the discharge outlet of high-elevation shrimp ponds in Dongzhai Harbor mangrove wetland for column loading (Figs. 1 and 2). Based on the establishment of RT-HPLC method for simultaneous detection of multiple organic acids, the physical, chemical, and biological changes related to organic acids in the sediment at the discharge outlet of shrimp ponds in the Yanfeng area was studied. The effect of Ca(OH)2 on the anaerobic digestion process using alkaline disinfectant in high-elevation shrimp ponds was examined after the end of anaerobic acidification, and the microbial changes were detected by high throughput sequencing at the beginning and end of anaerobic acidification and after the addition of alkaline disinfectant. The research results in this paper can provide a reference for the restoration and management of rapidly degrading mangrove wetlands.
Fig. 1.
Sampling point at shrimp ponds outlet in Yanfeng Town.
Fig. 2.
Experimental setup of soil column.
Results
Establishment of organic acid standard curve
The six organic acid standards were effectively separated by RT-HPLC when 10 µL of organic acid control samples of different mass concentrations were aspirated into the sample. A linear regression was performed on the peak area Y with the mass concentration X of organic acid standards, and the correlation coefficient R2 of the regression equation ranged from 0.9975 to 0.9999, which indicated that a variety of organic acids could be detected at the same time by adopting the chromatographic conditions of RT-HPLC.
Results of soil column experiments
The water content, temperature and conductivity of the soil column device were monitored during the experiment using the ECH2O soil monitoring system, and a total of 76,758 records were recorded with Port1, Port2 and Port3 representing the monitoring of the upper, middle and lower monitoring positions. The monitoring results were shown in Fig. 3, which showed that the water content of the soil column decreases gradually from the top to the bottom, while the water content at the same position remains relatively stable. The results of the conductivity showed that the deeper the subsoil, the lower the total dissolved solids content, which coincided with the results of the water content. the results of the temperature monitoring showed that the maximum temperature difference at the same depth in the course of the experiment of the soil column is 3.7 °C, which is related to the weather condition. The maximum temperature difference at the same time was 2.5 °C, which was caused by the fact that the upper layer of the subsoil is more likely to receive heat from the environment. The deeper the layer, the lower the efficiency of heat transfer in the subsoil.
Fig. 3.
Plot of conductivity and water content versus temperature for soil column experiments.
By sampling the effluent from the side outlet of the soil column device and using high performance liquid chromatography to determine the content of organic acids therein, the types and concentrations of organic acids in the substrate at different depths were obtained and varied with time as shown in Fig. 4. The results of the soil column experiments showed that oxalic acid was the most widely distributed and stable organic acid under different spatial and temporal changes, with concentrations ranging from 6.3 to 7.5 mg/L. The concentration of citric acid in the shallow substrate was higher than that in the deep substrate at the beginning of the experiment. As the experiment proceeded, the concentration of citric acid in the 0–20 cm soil gradually decreased to 0 mg/L, while the concentration of citric acid in the 20–30 cm soil first increased from 0 to 0.32 mg/L, and then decreased to 0 mg/L. When it comes to the 30–40 cm soil, the concentration of citric acid increased from 0 mg/L to 0.39 mg/L, and the concentration of citric acid in the 40–50 cm soil increased from 0 mg/L to 0.50 mg/L, indicating that citric acid adsorption and sedimentation occurred in the substrate of the mangrove wetland. Acetic acid was not detected in the initial experiment, as the experiment progressed, the content of acetic acid in the deeper substrate of 30–50 cm gradually increased from 0 to 0.44 mg/L until it remained stable, indicating that the organic matter present in the substrate, such as fish and shrimp carcasses, bait from shrimp ponds, and excreta, etc., would undergo an anaerobic reaction to produce acetic acid in the anaerobic environment.
Fig. 4.
Plot of organic acids with time at different depths.
The changes of organic acids after adding Ca(OH)2 are shown in Fig. 5. The concentration of Ca(OH)2 used in the simulation was 1.84 g/L, which was the discharge concentration during the dredging of shrimp ponds. This concentration was much higher than the content of organic acids in the substrate, so the organic acids were immediately neutralised when Ca(OH)2 diffused to different depths, and the rate-limiting factor for the reduction of organic acids at this time mainly depended on the Ca(OH)2 diffusion rate in the substrate. The organic acids in the 0–10 cm soil were completely neutralized on the 4th day, while the organic acids in the 40–50 cm soil were completely neutralized on the 18th day. Therefore, the calculated permeation rate of Ca(OH)2 molecules was approximately 50/18 = 2.8 cm/d, indicating that the pores in the mangrove sediment were small and the permeability was poor, resulting in a slower permeation rate of Ca(OH)2 molecules. After the neutralisation of organic acids in the 40–50 cm sediment, excess hydrochloric acid was added to the effluent, and the organic acids were detected by RT-HPLC. The total amount of organic acids in the effluent was 6.7 mg/L, while the total amount of each organic acid was 7.2 mg/L after the end of the anaerobic acidification, which indicated that the organic acids mainly existed as insoluble or slightly soluble precipitates of calcium organic acids after the addition of Ca(OH)2.
Fig. 5.
Plot of organic acids with time after adding Ca(OH)2.
Model fitting results
The soils catalog item in the HYDRUS-1D water flow module contains 12 typical soil media such as sandy soil, silt, clay and other soil moisture characteristic curve related parameters. According to the column filled with soil media and the neural network prediction function in HYDRUS-1D17, adjusted to speculate the moisture Characteristic parameters in the subsoil, θr, θs, α, n, Ks, and l were 0.074 m3/m3, 0.355 m3/m3, 0.5 m−1, 1.09, 0.01 m/d, and 0.5, respectively, where the smaller Ksindicated the worse ability of water transport in the soil18.
In the parameter setting for solute transport, c was entered as the concentration of organic acids in the 10 cm subsoil at each sampling point19, where the partition coefficient of organic acids in the solid phase was 0.04; the integrated dispersion coefficient D was calculated according to the formula: D = 2.71 × 10−4/M0.71, with M being the molar mass of the solute, and the smaller the value of D indicated the poorer ability of the soil to transport the substance. The reaction rate constant was 1.8 × 10−4.
The upper boundary of solute transport was selected as the concentration flux boundary according to the actual situation, and the lower boundary was selected as the zero-concentration gradient boundary without considering the background value of organic acid in soil, and at the same time, the concentration of the liquid phase was selected as the initial condition of the model, and five observation points were uniformly distributed from the top to the bottom of the term.
The initial time step was set to be 0.1 d, and the minimum and maximum time steps were 0.01 d and 10 d, respectively; the permissible deviations of soil water content and pressure head were 0.0005 cm and 1 cm, respectively, and the pressure head was set to be 1 m. The simulation duration of this experiment was 1 a.
The set parameters were used to predict the concentrations of oxalic acid and acetic acid at the depths of 0–10 cm, 10–20 cm, 20–30 cm, 30–40 cm, and 40–50 cm, and the actual measured concentrations were compared with those of oxalic acid and acetic acid, as shown in Fig. 6.
Fig. 6.
Comparison of simulated and measured values of oxalic acid and acetic acid in the bottom mud.
The simulation accuracy of the model was evaluated using the coefficient of determination (R2) and standard error (RMSE) based on the validation of soil measured soil oxalic acid concentration against the simulation results17. The R2 and RMSE thresholds ranged from 0 to 1, where the larger the value of R2 and the smaller the value of RMSE, the higher the simulation accuracy. The simulation accuracies are shown in Tables 1 and 2, the R2 between the measured and simulated values of oxalic acid content in the substrate is between 0.8 and 0.9, and the RMSE is between 0.014 and 0.034. R2 between the measured and simulated values of acetic acid content in the substrate is between 0.7 and 0.9, and the RMSE is between 0.013–0.019.The simulated values of the simulation and the measured values are in a higher degree of agreement, and the results of the model simulation can be better compared to the measured values. The model simulation results can better reflect the dynamic change characteristics of soil organic acids in each treatment. Moreover, the monitoring results showed that the temperature and humidity changes under laboratory conditions had little effect on the temperature and moisture of the soil column.
Table 1.
Evaluation criteria for simulation results of oxalic acid content in 10–50 cm sediment.
| 10 cm | 20 | 30 | 40 cm | 50 cm | |
|---|---|---|---|---|---|
| R2 | 0.901 | 0.874 | 0.881 | 0.891 | 0.807 |
| RMSE | 0.014 | 0.019 | 0.024 | 0.016 | 0.034 |
Table 2.
Evaluation criteria for simulation results of acetic acid content in 30–50 cm sediment.
| 30 cm | 40 cm | 50 cm | |
|---|---|---|---|
| R2 | 0.898 | 0.846 | 0.884 |
| RMSE | 0.017 | 0.013 | 0.019 |
According to the neural network prediction function, Ks was 1.01 cm/d, which was lower than the permeation rate of Ca(OH)2. The diffusion coefficient of oxalic acid was 0.111 cm2/d, and the diffusion coefficient of acetic acid was 0.148 cm2/d, which indicated that the vertical mobility of water and organic acid in the subsoil during the experiment of the soil column was relatively poor, and the subsoil had a strong adsorption capacity of organic acid, so that the concentration of organic acid in the subsoil in each level remained relatively stable, the overall potential contamination distribution pattern of organic acids was shown to be consistent.
Microbial sequencing results
The heatmap of species distribution of the top 30 species with the highest relative abundance at different stages during the soil column experiment is shown in Fig. 7. As a widely distributed microorganism in soil, Woeseiacan not only synthesize some compounds that are beneficial to plant growth21but also help to maintain the structure and water balance of the soil22. Sulfurovumhas the capacity to oxidize sulfur, which played a key role in the sulphur cycle that can interact with iron and sulphides, leading to the precipitation and formation of minerals23. It can be inferred that the significant enrichment of Woeseia and Sulfurovumplayed an important role in maintaining ecosystem health. Their indispensable ability can provide essential substances for mangrove forests and contribute to the material cycling of ecosystem24,25, which is beneficial for maintaining ecosystem health.
Fig. 7.
Heatmap of distribution at species level.
The most obvious change in the whole process was Acinetobacter Johnsonii, which has the ability to produce acids and can degrade large organic molecules to produce organic acids, such as gluconic acid, acetic acid, amino carboxylic acid in various environments26,27. Its abundance increased after stabilization of the anaerobic phase, and finally disappeared after the addition of Ca(OH)2 solution, which coincided with the process of acetic acid change detected by HPLC.
Discussion
It’s determined that the practice of directly applying unfermented and decomposed organic matter to plants is rare in the growth process of terrestrial plants, but it is a common phenomenon in mangrove wetland ecosystems in China. The results of soil column experiments indicated that oxalic acid might mainly come from the residues of organic acid detoxifiers and the decomposition of fallen leaves of mangrove plants widely presented in protected areas. Citric acid mainly came from organic acid detoxifiers, and it underwent adsorption and sedimentation processes in the sediment of mangrove wetlands, exhibiting a top-down migration process in the sediment. Acetic acid was mainly generated from anaerobic acidification of organic matter in sediment.
The results of HYDRUS-1D simulation showed that the adsorption, deposition and transport of organic acids in Hainan Dongzhai Harbour Mangrove Wetland Reserve were poor, and the results of the vertical infiltration model were in agreement with the results of the samples taken from the Dongzhai Harbour Mangrove Wetland Reserve, so the model can be applied to the study of the vertical transport of organic acid pollutants in the soil of the Dongzhai Harbour Mangrove Wetland Reserve.
There were significant differences in the diversity and structure of the bacterial communities at different stages of the soil column experiment. Acetic acid was not detected and Acinetobacter Johnsonii abundance was the lowest at the Origin stage, while Acetic acid appeared at the beginning of the anaerobic stage and the abundance of Acinetobacter Johnsonii increased, and Acetic acid and Acinetobacter Johnsonii disappeared after alkalinization, thus, there were differences in the structure of the bacterial communities. Therefore, differences in bacterial community structure were significantly correlated with differences in organic acids at different levels of the substrate, and Acinetobacter Johnsonii was the main factor influencing microorganisms in the concentration of acetic acid in the substrate. Unfortunately, the succession process of microbial communities is completely consistent with that in mangrove wetlands cannot be determined from these data, as the environmental conditions of actual mangrove ecosystems are constantly changing. Despite its limitations, It’s clearly demonstrated that alkaline disinfectants entering the soil can quickly lead to the disappearance of acid producing bacteria.
The large amount of organic matter emitted from shrimp ponds entering mangrove ecosystems can lead to significant emissions of greenhouse gases such as carbon dioxide and methane, weakening the carbon storage capacity of mangrove ecosystems. One of the ways to address the impact of organic acids on mangrove ecosystems is to improve aerobic digestion of waste such as feed and excrement by adding personalized microbial inoculants, reducing organic emissions while increasing waste recycling28. In the future, it’s suggested that the discharge of organic matter from shrimp ponds into mangroves should be reduced, and the organic matter discharged from high-elevation shrimp ponds should be collected for further utilization.
By combining experimental data on organic acid conversion in the laboratory with the content of organic acids in mangrove ecosystems and using laboratory soil column experimental data to determine relevant parameters, the conversion process and amount of organic matter emissions from shrimp ponds in mangrove wetland ecosystems can be further predicted in the future, and the production of greenhouse gases methane and CO2 in different regions. The results of this study can provide data support for the discharge of wastewater after shrimp farming in Dongzhai Harbour high-elevation ponds, early warning of organic acid pollution, and the formulation and implementation of management policies.
The area of shrimp ponds around the protected area should be reasonable planned and converted to forests. The amount of feed should be controlled and the sediment of the shrimp pond can be regularly cleaned to reduce the accumulation of organic matter. Using suitable biological treatment agents and increasing the oxygen content in shrimp pond water can also help reduce the production of organic acids. Furthermore, artificial intelligence technology can be used to build a long-term prediction model to monitor the discharge situation at the shrimp pond discharge outlet in real time, predict possible problems, and help managers take timely measures to restore the mangrove ecosystem, which deserves further research in the future.
Materials and methods
Experimental materials
In this study, the sampling points were set up according to the growth of mangrove forests and the distribution of shrimp ponds, and the latitude and longitude of the specific sampling points were 110°584′E 19°957′N based on Google Earth (Fig. 1). Mud samples were manually drilled at low tide using a sampler with sampling depths of 0–10 cm, 10–20 cm, 20–30 cm, 30–40 cm and 40–50 cm, and the amount of mud taken from each section was about 3000–5000 g. The samples were put into sampling bags, numbered for refrigeration and transported back to the laboratory by air.
Simultaneous detection of multiple organic acids by RT-HPLC
Chromatographic conditions for the simultaneous determination of multiple organic acids by RT-HPLC
The chromatographic conditions were as follows: Xtimate XB-C18 column (4.6 mm×250 mm, 5 μm); mobile phase: 0.01 mol/L potassium dihydrogen phosphate solution (adjusted by phosphoric acid at pH = 2.5); G4212-60008 diode array detector at a detection wavelength of 215 nm; flow rate: 0.8 mL/min; column temperature: 40 °C; injection volume: 10 µL29. Under the above chromatographic conditions, the standard curves for the simultaneous determination of various organic acids were obtained.
Solution Preparation
The standards of oxalic acid, formic acid, malic acid, lactic acid, acetic acid and citric acid were weighed precisely, and then diluted with ultrapure water into 1, 5, 10, 15 and 20 mg/L standard solutions. The reagents used were Macklin purity of AR and GR grade.
Experimental method of soil column
Firstly, petroleum jelly was evenly applied to the inner wall of the drenching column to prevent the formation of preferential flow of raw water along the inner pipe wall. The soil for the experiment was collected from Dongzhai Harbour Mangrove Wetland Ecological Reserve and filled in layers with a height of 50 cm. The soil column experimental setup is shown in Fig. 2, with a column height of 63 cm and a diameter of 15 cm; 1 represents the outlets, with a spacing of 10 cm between the outlets; 2 represents the monitoring plugs, which were used to monitor the changes of the indexes of the upper, middle, and lower subsoils in the operation of the column setup, respectively; and 3 represents the ECH2O workstation for timely data storage. The experiment was conducted using the upper water inlet and the left side outlet, and the monitoring plug of the ECH2O soil monitoring system was inserted into the right-side hole to record the data, using the ECH2O software, which recorded one piece of data per minute, with each piece of data containing 9 readings. Seawater was used to simulate local tides by giving 10–20 cm of water pressure for 12 h of the day, and the remaining 12 h were spent sucking water out using the siphon principle to maintain water flow in the in-situ treatment model. Due to the slow rate of natural infiltration, samples were taken instantly at the side outlets and the organic acid content was determined using HPLC. The experiment was carried out for a total of 31 days after the organic acid content in the soil column remained stable, and then the experiment on the effect of alkaline disinfectant was carried out by adding Ca(OH)2 solution at a concentration of 1.84 g/L to the top every 12 h, keeping the same experimental operation as before. The experiment was ended when the organic acid content in the soil column device was 0. After the neutralisation of organic acids in the soil column device, excess hydrochloric acid was added to the effluent, and the reduced organic acid content was measured by RT-HPLC. The average temperature of the soil inside the soil column during the soil column experiment was 25 ± 2 °C, and the indoor temperature during the experiment ranged from 19.2 to 27.3 °C. The humidity in the air was maintained at 10 −32%. The experimental condition was only set to tidal conditions, while temperature and humidity were only recorded for dynamic changes without special settings. During the experiment, the sample was collected every 3 days to conduct relevant detection of organic acids.
Modelling
The Hydrus model was used to simulate the diffusion of organic acids in the mangrove area of Dongzhai Harbour, and the results obtained were combined with the measured results in order to obtain the migration and transformation law of organic acids in the mangrove wetland of Dongzhai Harbour.
The flow of water can be described using the Richards equation:
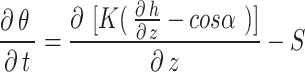 |
where θ is the soil volumetric moisture content; H is the soil pressure head; T is the simulation time; α represents the angle between the flow direction and the vertical direction. According to the above physical experimental model, the water flow is one-dimensional vertical seepage, i.e. α= 0; K is the unsaturated permeability coefficient; S is the source and sink term30.
Soil moisture transport models can be used to describe the process of water transport in the soil, and the HYDRUS-1D software water flow model includes various soil moisture transport models such as the single pore medium model and the double pore/double permeable medium model. In this paper, the soil hydraulic model proposed by VanGenuchten-Mualem is used for simulation prediction, and the phenomenon of water flow hysteresis is not considered in the simulation19. The equation is:
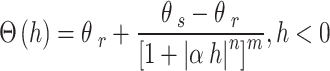 |
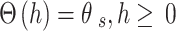 |
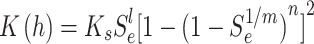 |
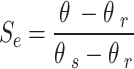 |
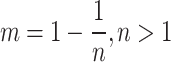 |
where θr is the residual moisture content of the soil; θs is the saturated soil moisture content; Se is the effective saturation; α is the bubbling pressure; n is the distribution index of soil pore size; Ks is the saturated hydraulic conductivity coefficient; l is the soil pore connectivity parameter, usually taken as 0.5.
There are three main processes of soil solute transport, namely convection, molecular diffusion and mechanical dispersion. The classical convection-dispersion equation was used in the simulation to describe the one-dimensional solute transport in saturated-unsaturated pore media31.
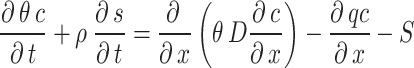 |
where c is the liquid phase concentration of the solution; ρ is the soil bulk density; s is the solid phase concentration of the solute; D is the comprehensive dispersion coefficient; q is the volumetric flow flux density; S is the source and sink term.
The effectiveness of the model simulation was evaluated by the following statistical parameters, mainly the coefficient of determination (R2) and root mean square error (RMSE).
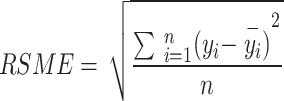 |
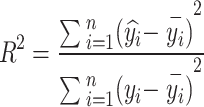 |
where n is the number of samples; yi is the true value of the target variable; 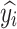 is the predicted value of the model; and
is the predicted value of the model; and 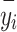 is the mean value of the target variable.
is the mean value of the target variable.
Determination of microorganisms in the substrate at different depths
At the early and late stages of anaerobic acidification and after the addition of alkaline disinfectant Ca(OH)2 solution in the soil column experiment, respectively, an appropriate amount of soil was taken at 0–10 cm, 20–30 cm and 30–40 cm in self-sealing bags, and the samples at the early stage of the experiment were recorded as Origin 1–3, the samples after the anaerobic acidification were recorded as Acidification 1–3, and the samples collected after adding Ca(OH)2 solution were recorded as Alkalisation1-3, and were stored in a refrigerator for 16 S rDNA whole-pass sequencing by Suzhou Jinwei Zhi Biotechnology Co. The samples collected after the addition of Ca(OH)2 solution were recorded as Alkalisation1-3, and stored in the refrigerator for 16 S rDNA full-throughput sequencing by Suzhou Jinwei Zhi Biotechnology Co.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant numbers 31971549], the National Key Project of International Science and Technology Cooperation Program of China [grant number 2016YFE0106800] and the Guangxi Innovation-driven Development Special Fund Project [grant numbers AA17202032].
Author contributions
Xinyu Liu: Formal Analysis, Methodology, Investigation, Writing-Original Draft.Yunan Yang: Conceptualization, Data Curation, Formal Analysis, Funding Acquisition, Project Administration, Resources, Supervision, Writing-Review & Editing. Yangang Lin: Investigation, Writing-Review & Editing.
Data availability
All data supporting the finding of this study are available within this article.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meng, L. et al. Biofilms in plastisphere from freshwater wetlands: biofilm formation, bacterial community assembly, and biogeochemical cycles. J. Hazard. Mater.476 (5), 134930. 10.1016/j.jhazmat.2024.134930 (2024). [DOI] [PubMed] [Google Scholar]
- 2.Liu, J. & Myat, T. Contaminants and heavy metals along the Mangrove area of Dongzhai Harbor, China: distribution and assessment. SN Appl. Sci.3 (10). 10.1007/s42452-021-04802-2 (2021).
- 3.Liu, Y. et al. Factors influencing the accumulation of Pd in Mangrove wetland sediments in Dongzhai Harbor, Hainan, China. J. Coast Conserv.23 (6), 1039–1045. 10.1007/s11852-019-00710-1 (2019). [Google Scholar]
- 4.Sun, N. et al. Effects of Organic Acid Root Exudates of Malus hupehensis Rehd. Derived from Soil and Root Leaching Liquor from Orchards with Apple Replant Disease. Plants 11(21): 2968. (2022). 10.3390/plants11212968 [DOI] [PMC free article] [PubMed]
- 5.Ren, W. et al. Effects of hydrosoluble calcium ions and organic acids on citrus oil emulsions stabilized with citrus pectin. Food Hydrocoll.100, 105413. 10.1016/j.foodhyd.2019.105413 (2020). [Google Scholar]
- 6.Wang, Y., Gao, M., Wang, Z., Huang, T. & Li, H. How organic acids affect plant nitrogen and phosphorus uptake under different fertilization treatments. J. Soil. Sci. Plant. Nut. 23, 6048–6058 (2023). [Google Scholar]
- 7.Faika, Y. et al. Revealing the effects of amino acid, organic acid, and phytohormones on the germination of tomato seeds under salinity stress. Open. Life Sci.19 (1), 20220892 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aschenbroich, A. et al. Spatio-temporal variations in the composition of organic matter in surface sediments of a Mangrove receiving shrimp farm effluents (New Caledonia). Sci. Total Environ.512 (15), 296–307. 10.1016/j.scitotenv.2014.12.082 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Tian, D. et al. Influences of phosphate addition on fungal weathering of carbonate in the red soil from karst region. Sci. Total Environ.755 (2), 142570. 10.1016/j.scitotenv.2020.142570 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Du, C. C. et al. The restoration of karst Rocky desertification has enhanced the carbon sequestration capacity of the ecosystem in Southern China. Global Planet. Change. 243, 104602. 10.1016/j.gloplacha.2024.104602 (2024). [Google Scholar]
- 11.Bai, X. Y. et al. Resolving controversies surrounding carbon sinks from carbonate weathering. Sci. China Earth Sci.67 (9), 2705–2717. 10.1007/S11430-024-1391-0 (2024). [Google Scholar]
- 12.Lin, N. P. et al. Black biodegradable mulching increases grain yield and net return while decreasing carbon footprint in rain-fed conditions of the loess plateau. Field Crop Res.318 (1), 109590. 10.1016/j.fcr.2024.109590 (2024). [Google Scholar]
- 13.Silva, C., Sternberg, L. D. L., Dávalos, P. B. & de Souza, F. E. S. The impact of organic and intensive farming on the tropical estuary. Ocean. Coast Manage.141, 55–64. 10.1016/j.ocecoaman.2017.03.010 (2017). [Google Scholar]
- 14.Zhang, T. et al. Effects of coastal wetland reclamation on soil organic carbon, total nitrogen, and total phosphorus in China: A meta-analysis. Land. Degrad. Dev.34, 3340–3349 (2023). [Google Scholar]
- 15.Li, T. et al. Effects of an ex situ shrimp-rice Aquaponic system on the water quality of aquaculture ponds in the Pearl river estuary, China. Aquaculture545, 737179. 10.1016/j.aquaculture.2021.737179 (2021). [Google Scholar]
- 16.Yang, Y., Liu, X. & Lin, Y. The organic acid environmental capacity of Mangrove ecosystem in Dongzhai Harbor, Hainan, China. Mar. Pollut Bull.205, 116622. 10.1016/j.marpolbul.2024.116622 (2024). [DOI] [PubMed] [Google Scholar]
- 17.Qi, G. et al. Moisture effect on carbon and nitrogen mineralization in topsoil of Changbai mountain, Northeast China. J. Sci.57, 340–348 (2011). [Google Scholar]
- 18.Imunek, J., Genuchten, M. T. V. & Ejna, M. H. Y. D. R. U. S. Model use, calibration, and validation. T Asabe. 55 (4), 1561–1574 (2012). [Google Scholar]
- 19.Jyotiprava Dash, C., Sarangi, A., Singh, D. K., Singh., A. K. & Partha, P. A. Prediction of root zone water and nitrogen balance in an irrigated rice field using a simulation model. Paddy Water Environ.13 (3), 281–290. 10.1007/s10333-014-0439-x (2015). [Google Scholar]
- 20.Cheviron, B. & Coquet, Y. Sensitivity analysis of Transient-MIM HYDRUS-1D: case study related to pesticide fate in soils. Geoscience World. 10.2136/vzj2009.0023 (2009). [Google Scholar]
- 21.Zhang, Y. M. et al. Metagenomic resolution of functional diversity in copper Surface-Associated marine biofilms. Front. Microbiol.10, 2863. 10.3389/fmicb.2019.02863 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharya, S., Bachani, P., Jain, D., Patidar, K. S. & Mishra, S. Extraction of potassium from K-feldspar through potassium solubilization in the halophilic Acinetobacter soli (MTCC 5918) isolated from the experimental salt farm. Int. J. Min. Process.152, 53–57. 10.1016/j.minpro.2016.05.003 (2016). [Google Scholar]
- 23.Sheng, X. F., Zhao, F., He, L. Y., Qiu, G. & Liang, C. Isolation and characterization of silicate mineral-solubilizing Bacillus globisporus Q12 from the surfaces of weathered feldspar. Can. J. Microbiol.54 (12), 1064–1068. 10.1139/W08-089 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Liu, Y. et al. Diversity and structure of vegetation rhizosphere bacterial community in various habitats of Liaohekou coastal wetlands. Sustainability14, 10396. 10.3390/su142416396 (2022). [Google Scholar]
- 25.Fernandez-Cadena, J. C. et al. Detection of Sentinel bacteria in Mangrove sediments contaminated with heavy metals. Mar. Pollut Bull.150, 110701. 10.1016/j.marpolbul.2019.110701 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Wang, X. Y. & Xie, J. Characterization of metabolite, genome and volatile organic compound changes provides insights into the spoilage and cold adaptive markers of Acinetobacter johnsonii XY27. LWT- Food Sci. Technol.162, 113453. 10.1016/j.lwt.2022.113453 (2022). [Google Scholar]
- 27.Petra, M. M. P. & Stefan, K. Genomic repertoire of the Woeseiaceae/JTB255, cosmopolitan and abundant core members of microbial communities in marine sediments. ISME J. 11(5), 1276–1281. 10.1038/ismej.2016.185 (2017). [DOI] [PMC free article] [PubMed]
- 28.Han, Y. et al. Improving aerobic digestion of food waste by adding a personalized microbial inoculum. Curr. Micro. 81 (9), 277. 10.1007/S00284-024-03796-5 (2024). [DOI] [PubMed] [Google Scholar]
- 29.Arnetoli, M., Montegrossi, G., Buccianti, A. & Cristina, G. Determination of organic acids in plants of Silene paradoxa L. by HPLC. J. Agric. Food Chem.56 (3), 789–795. 10.1021/jf072203d (2008). [DOI] [PubMed] [Google Scholar]
- 30.Ranjeet, K. J., Sahoo, B., Rabindra, K. & Panda Modeling the water and nitrogen transports in a soil–paddy–atmosphere system using HYDRUS-1D and lysimeter experiment. Paddy Water Environ.15 (4), 831–846. 10.1007/s10333-017-0596-9 (2017). [Google Scholar]
- 31.D. S. K., Sharma, P., Shukla, M. K., Ashigh, J. & Šimůnek, J. Numerical evaluation of nitrate distributions in the onion root zone under conventional furrow fertigation. J. Hydrol. Eng.21 (2), 1–12. 10.1061/(ASCE)HE.1943-5584.0001304 (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the finding of this study are available within this article.









