Abstract
Peroxiredoxin 6 (Prdx6), a unique non-seleno peroxidase, is a bifunctional protein with GSH peroxidase at pH 7.4 and calcium independent phospholipase A2 (aiPLA2) activities at pH 4.0. Changes in pH brings about alteration in the conformational and thermodynamic stability of Prdx6. For instance, under acidic condition (pH 4.0), Prdx6 forms higher oligomers with concommittant gain in aiPLA2 activity that is resistant to thermal denaturation. However, there has been no molecular level understanding of how low pH induces formation of oligomers. In the present study, site directed mutagenesis of two conserved amino acid residues, Asp42 and His79, was used to study the molecular basis for the influence of pH on the oligomeric state of Prdx6. We observed that mutation at Asp42 and His79 residues by Ala did not result in a significant change in its peroxidase activity at neutral pH 7.4, but its aiPLA2 activity at low pH 4.0 decreased significantly. At this pH condition, both mutants exhibit highly conserved alpha-helix content but fluctuating tryptophan micro-environment with partly exposed hydrophobic patches that render the formation of oligomers. DLS measurements and analytical SEC revealed that Wt Prdx6 forms oligomers at low pH but not the mutant proteins suggesting the importance of these residues in pH sensing and oligomerization. These results suggest that Asp42 and His79 interact each other to induce conformational change of Prdx6 that triggers the oligomerization of Prdx6 at low pH.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-91218-2.
Keywords: Peroxiredoxin 6; Calcium independent phospholipase A2; 1, 2,-bis palmitoyl-sn-glycero-3-phosphocholine; Circular dichroism; Thioredoxin; π glutathione S-transferase
Subject terms: Biochemistry, Chemical biology, Structural biology
Introduction
Peroxiredoxins (Prdx) are a family of antioxidant enzymes that play a crucial role in protecting cells from oxidative stress by detoxifying reactive oxygen species (ROS). Prdx proteins are ancestral thiol-dependent selenium- and heme-free peroxidases involved in the detoxification of 90% of cellular peroxides including hydrogen peroxide, peroxynitrite and hydroperoxides due to its high abundance in a wide range of cells and its high catalytic efficiencies in comparison to other peroxidases such as catalase, horseradish peroxidase (HRP) and glutathione peroxidase (GPx) etc1–6; thus, Prdxs help to protect cellular components such as other proteins, lipids, and DNA from oxidative damage.
Prdxs have been categorized broadly into different isoforms (Prdx1-6) based on their structural characteristics and specific functions2,7,8. These include: 1. Typical 2-Cys Prdxs (Prdx1–4) that contain two cysteine residues: a peroxidatic cysteine (Cp) and a resolving cysteine (Cr). They form dimers arranged in antiparallel fashion with Cp in one monomer facing Cr in the other. Upon peroxide binding, Cp forms a sulfenic acid intermediate (Cp-SOH), which then creates an intermolecular disulfide bond with Cr. This oxidized dimer can then be reduced back to its active form by thioredoxin (Trx). 2. Atypical 2-Cys Prdxs (Prdx5) also has Cp and Cr but differs from the typical 2-Cys Prdxs as its dimers are not antiparallel; instead, it forms an intramolecular disulfide bond between Cp and Cr within the same molecule. Reduction of this bond is also carried out by Trx. 3. The third class of Prdx is 1-Cys Prdx (Prdx6) that has only one cysteine (Cp) and lacks Cr. It forms disulfide bonds within the same molecule and is reduced by glutathione in presence of π glutathione S-transferase (π-GST) rather than Trx9,10.
Prdx6 is called a “moonlighting protein” with both GSH peroxidase and calcium2+-independent phospholipase A2 activity (aiPLA2)11,12. In contrast to other Prdxs, Prdx6 has two functionally independent active sites: one for peroxidase activity (involving Cys47, His39, and Arg132) and another for its aiPLA2 activity (involving His26, Ser32, and Asp140). This separation of activities means that loss of function in one site does not impact the function of the other. The Cys47 residue at active site of Prx6 forms a sulfenic acid intermediate during peroxide reduction, with His39 stabilizing the intermediate and assisting in proton transfer. In contrast, aiPLA2 activity is generated by Ser32 as the nucleophile that attacks the ester bond in phospholipids, His26 as the base that facilitates the nucleophilic attack, and Asp140 to stabilize the transition state and to support the hydrolysis reaction. Both activities are pH dependent, i.e., at neutral (pH 7.4), Prdx6 exhibits optimal peroxidase activity, while at low pH (pH 4.0), it exhibits maximal aiPLA2 activity13–15. This pH dependent switch in activity is believed to involve formation of different oligomeric species (monomer or higher oligomer in case of peroxidase and aiPLA2 activity, respectively).
To date, there has been no molecular level understanding of how changes in the pH can help to regulate the peroxidase or aiPLA2 functions in Prdx6. It has been shown that Prdx6 undergoes pH dependent oligomerization, i.e., at neutral pH it exists as monomer while it forms dimer or higher oligomers and gain aiPLA2 activity at low pH16. However, to date, the role of pH in modulating the oligomeric status of Prdx6 has not been explored sufficiently. It has previously been reported that in case of Prdx1, His113 is protonated at low pH and forms electrostatic interaction with Asp7617. Formation of this electrostatic pair results in the generation of high order oligomeric species that help to gain other additional functions including chaperone activity. Indeed, similar to Prdx1, there is existence of well conserved His79 and Asp42 in the Prdx6 (Fig. 1). Therefore, we postulated that protonation or deprotonation of certain Asp or His residues might be responsible for the oligomerization of Prdx6, leading to the acquisition of aiPLA2 activity. This has led us to investigate the roles of His79 and Asp42 on the aiPLA2 function and oligomerization status of Prdx6. Using two different mutants, His79-Ala and Asp42-Ala, we systematically investigated the role of both His and Asp by analysing the oligomeric status and characterizing the functional activity of the proteins. We discovered that both His and Asp are responsible for the formation of higher oligomers at pH 4.0 and, hence, in acquiring the aiPLA2 function in Prdx6.
Fig. 1.
Primary structure of 1-Cys Prdx from different species. Consensus sequences from NCBI aligned using T-coffee multiple sequence alignment of EMBL-EBI. The red and yellow shading indicates regions of fully-conserved and highly-conserved regions respectively among the species. Residue numbering follows the residues of Prdx6. Black vertical box shows the fully conserved Asp residue. Red box represents the highly conserved His residue throughout the species.
Results
Mutant Prdx6s (D42A and H79A) exhibit peroxidase activity but not aiPLA2 activity
Wild type (Wt) Prdx6 has been known to exhibit pH-dependent activity, i.e., peroxidase activity at neutral pH, while aiPLa2 at low pH14,18–20. Therefore, we investigated if the mutant Prdx6s possess respective activities. Overnight incubation with excessive amount of DTT was followed by its removal via dialysis against the standard buffer (pH 7.4). The thiol content of the recombinant Wt Prdx6 and its mutant Asp42A (D42A) and His79A (H79A) Prdx6s was measured using DTNB assay to check the oxidation state of Prdx6’s. More than 90% of the Prdx6 remained in the reduced state for 6 h after DTT removal (see supplementary Table 1).
The time-dependent Prdx6 mediated hydrolysis of H2O2 (shown in Fig. 2A) shows that H2O2 exhibits self-degradation potential in the absence of the enzymes and the rate of this degradation is enhanced significantly upon addition of the Wt Prdx6. Mutant Prdx6s also exhibit this hydrolytic potential towards H2O2, albeit at a slower rate than that of Wt Prdx6 (Fig. 2B and Table 1). The peroxidase activity of Prdx6 and its mutants calculated as 2nd order rate constant of Prdx6 was also determined using HRP competitive assay (supplementary Fig. 1). The activity parameters measured using the competitive assay are independent of variability in Prdx6 turnover rate due to varied concentrations of Prdx6. The estimated values of specific activity and 2nd order rate constant of Prdx6 (Table 1) agree well with earlier reports21.
Fig. 2.
(A) Determination of Peroxidase activity of Prdx6 and its mutant, D42A and H79A using H2O2 decay assay as the extent of consumption of H2O2 upon reacting with Prdx6 is monitored by the decrease in absorbance at 240 nm (ε240 for H2O2 being 43.6 M−1 cm−1). (B) The enzymatic activity estimated for Prdx6 and its mutant is expressed as nmol of reduced H2O2/min. mg of Prdx6. Estimation of calcium independent Phospholipase activity (aiPLA2) of Prdx6 and its mutant as fluorescence emission plot (against time) (C) of EnzChek® Phospholipase A2 substrate incorporated in liposomes (prepared by mixing of 30 μl 10 mM Dioleoylphosphatidylcholine, 30 μl 10 mM Dioleoylphosphatidylglycerol, and 30 μl 1 mM PLA2 substrate) with addition of Prdx6 at temperature, 25 °C and (D) its fluorescence ratio (515/575).
Table 1.
Peroxidase Activity of Prdx6 and its mutant D42A and H79A at pH 7.4.
| Protein | Peroxidase activity (nmol/min/mg of protein) |
2nd order constant, k (M−1 s−1) |
|---|---|---|
| Wt Prdx6 | 660 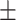 30 30 |
3.85 × 107 |
| D42APrdx6 | 540  20 20 |
2.56 × 107 |
| H79APrdx6 | 600 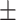 20 20 |
3.69 × 107 |
In terms of aiPLA2 functions, there is large difference between the time-dependent binding affinity of the phospholipid with the Wt and mutant Prdx6s. At the end of the 2 h incubation, Wt Prdx6 exhibit a relative fluorescence intensity of 5 while the mutant proteins exhibit only around 1.5 ( Fig. 2C). The distinct fluorescence resonance energy transfer (FRET) emission of Wt Prdx6 at 515/575 nm shows , reports higher fluorescence ratio at pH 4.0 (Fig. 2D and Table 2), consistent with previous reports, but this is minimal for the mutant. The results indicate that catalytically, the mutant proteins fail to exhibit aiPLA2 activity but exhibits only the peroxidase function and show that the formation of electrostatic interaction at pH 4.0 is required for aiPLA2 activity.
Table 2.
Phospholipase Activity comparison of Prdx6 and its mutant, D42A and H79A at pH 4.
| Protein | Fluorescence ratio (515/575) | Fluorescence at 515 nm (104) |
|---|---|---|
| Wt Prdx6 | 4.23 ± 0.34 | 632.52 |
| D42APrdx6 | 0.45 ± 0.12 | 153.86 |
| H79APrdx6 | 0.62 ± 0.15 | 159.32 |
Wt Prdx6 forms higher oligomer while mutant Prdxs are dimeric
To probe for the oligomeric status of the Wt and mutant Prdxs, we have measured the hydrodynamic diameter (Hd) of the proteins at both pH 7.4 and 4.0. Raw data representing the size vs volume reaction are shown in Fig. 3A and B and the estimated hydrodynamic diameter along with the percent volume fractions is shown in Table 3. Wt Prdx6 at pH 7.4 remain dimeric with hydrodynamic size around 5 nm, while, upon transfer to low pH medium, higher oligomers are formed with hydrodynamic size almost double to that of the Wt. On the other hand, mutant Prdx6s remain dimeric at both the neutral and low pH, with hydrodynamic size ranging from 5.5–5.8, as shown (Table 3).
Fig. 3.
Dynamic light scattering (DLS) measurement as the size distribution by volume of Prdx6 and its mutant D42A and H79A at (A) pH 7.4 and (B) pH 4.0. Measurement of elution volume of Prdx6 and its mutant as absorbance at 280 nm through Size Exclusion Chromatography at pH 7.4 (C) and 4.0 (D).
Table 3.
Hydrodynamic size of Prdx6 and its mutant, D42A and H79A under different pH 7.4 and 4.0 measured by DLS.
| Protein | pH 7.4 | pH 4.0 | ||
|---|---|---|---|---|
| Hydrodynamic size, Hd (nm) | Volume fraction (%) | Hydrodynamic size, Hd (nm) | Volume fraction (%) | |
| Wt Prdx6 | 5.35 ± 0.13 | 98.2 | 12.30 ± 0.81 | 98.5 |
| D42APrdx6 | 5.56 ± 0.21 | 97.4 | 5.96 ± 0.26 | 98.8 |
| H79APrdx6 | 5.88 ± 0.15 | 98.6 | 5.92 ± 0.31 | 99.2 |
Size exclusion column (SEC) of the Wt and mutant proteins depicts the collected elution profiles of the Wt and mutant proteins at pH 7.4 and 4.0 (Fig. 3C and D). The apparent molecular weight of Prdx6 at pH 7.4 and 4.0 using the calibration curve from the elution volume obtained after passing through SEC were determined under different physiological pH (Table 4). Both Wt and mutant proteins exhibit similar elution profiles at neutral pH but not at low pH. At low pH, Wt Prdx6 elutes as dimer and becomes a higher oligomer with an elution volume that is nearly three times greater than that at pH 7.4. The oligomeric form of Wt Prdx6 at pH 4.0 has an apparent molecular weight of approximately 158 kD, which is consistent with the elution profile of yeast alcohol dehydrogenase (150 kD) (Fig. 3D). However, for the mutant proteins, the elution profiles at pH 7.4 and 4.0 almost are identical. The oligomeric states of Wt Prdx6 and its mutants, D42A and H79A, were also investigated under varying pH conditions using native-PAGE as shown in supplementary Fig. 2. The proteins were dialyzed against the standard buffer at pH 7.4 and 4.0 and then were electrophoresed in a continuous 7.5% PAGE under non-reducing and non-denaturing conditions in presence of two markers, bovine serum albumin (BSA, Mr 68 kD, pI 4.7–4.9) and high molecular weight horse ferritin (Mr 480 kD, pI 5.5). We found that Wt Prdx6 exhibits nearly identical molecular weight to that of the D42APrdx6 and H79APrdx6 at neutral pH comparable to the size of dimeric BSA (i.e. Mr 100–130 kD) (supplementary Fig. 2A). As expected at low pH the molecular weights of both the mutant Prdx6s also correspond to the dimeric BSA (supplementary Fig. 2B) and their electrophoretic mobility is different from the Wt Prdx6. It is also seen in this figure that the molecular weight of Wt Prdx6 at low pH 4.0 is largely different from that appeared at pH 7.4 indicating different oligomeric status at both the pH conditions.
Table 4.
Measurement of elution volume and apparent molecular weight of Prdx6 and its mutant, D42A and H79A under different pH 7.4 and pH 4.0 using FPLC.
| Protein | pH 7.4 | pH 4.0 | ||
|---|---|---|---|---|
| Elution volume (ml) | Molecular weight (kD) | Elution volume (ml) | Molecular weight (kD) | |
| Wt Prdx6 | 60.3 ± 0.08 | 47 | 43.3 ± 0.09 | 158 |
| D42APrdx6 | 59.2 ± 0.21 | 51 | 57.2 ± 0.18 | 59 |
| H79APrdx6 | 61.1 ± 0.05 | 45 | 57.1 ± 58 | 58 |
Wt and mutant Prdx6 exhibit different thermodynamic stabilities
We also measured the thermodynamic stability of both the Wt and mutant Prdx6s by evaluating heat-induced denaturation of the proteins at both pH 7.4 and 4.0. Representative heat-induced denaturation profiles and the estimated Tm and △Hm are shown in Fig. 4. We observed no significant difference between the Tms of the Wt and mutant proteins at neutral pH. However, at low pH, Wt Prdx6 could not undergo any transition within the measurable temperature range. In contrast, the mutant proteins could exhibit complete transitions with defined pre- and post-transition baselines and their Tms that were nearly identical to that of the Wt.
Fig. 4.
Thermal-induced denaturation of Prdx6 and its mutant D42A and H79A as recorded following changes in [θ]220 from 20 °C to 80 °C at a rate of 1 °C/min using Circular Dichroism spectroscopy at pH 7.4 (A) and 4.0 (B).
Structural studies of Wt and mutant Prdx6s
We performed structural studies of the Wt and mutant proteins by using various spectroscopic techniques so as to gain molecular insight for the formation of species of higher oligomers by Wt but not by the mutant proteins at low pH. Far-UV CD of Prdx6 and its mutant observed at pH 7.4 from the Fig. 5A shows no apparent change on the secondary structure content of Wt Prdx6 and its mutants. This is confirmed by the content of alpha-helix content obtained by measuring MRE at θ222 (supplementary Table 2). We also monitored the effect of pH on the secondary structure of the proteins Wt Prdx6 and its mutant under low pH as shown in Fig. 5B. Far-UV CD measurements reveal that there is an apparent change in the secondary structural contents of the proteins as the spectral properties of the mutants show a decreased MRE around 208 as compared to the Wt Prdx6.
Fig. 5.
Conformational studies of Prdx6 and its mutant, D42A and H79A. Far-UV CD measurements at pH 7.4 (A) and pH .4.0 (B). Tryptophan fluorescence at pH 7.4 (C) and pH 4.0 (D); and ANS measurements at pH 7.4 (E) and pH 4.0 (F) of Prdx6 and its mutant were measured at standard buffer Na-Acetate buffer, pH 4.0 and 50 mM Tris–HCl, 100 mM NaCl, pH 7.4 at 25 °C. All spectra are mean of three independent experiments.
The tryptophan fluorescence was scanned in the wavelength 310–450 nm after excitation at 295 nm to evaluate the effect of mutation of Prdx6 in the local environment of tryptophan (Trp) residues of Prdx6. The mutant Prdx6 shows decrease in fluorescence in comparison to Wt Prdx6 at pH 7.4 (Fig. 5C). We also measured the tryptophan fluorescence of Wt Prdx6 and its mutant at pH 4.0 (Fig. 5D). Although the mutant Prdx6 shows decrease in fluorescence, the Trp micro-environment have been blue shifted relative to the Wt Prdx6. Thus, the Trp chromophores are apparently shifted to the more non polar environments that may help to increase the structural integrity of the mutants as a consequence of the mutation. Such conformational reshuffling can bring about large alterations in the internal packing of the proteins, resulting in the exposure vs burial of many of the hydrophobic groups to the solvent.
ANS fluorescence measurements of Wt Prdx6 and its mutants D42A and H79A at neutral pH have been evaluated to detect accessible hydrophobic surfaces on proteins (Fig. 5E). There is no increase in the ANS fluorescence of the proteins at the neutral pH, indicating that ANS does not bind to Prdx6 after mutation. However, Wt Prdx6 exhibits a large degree of ANS binding as exemplified by blue shift and increase in fluorescence relative to ANS alone (control) at low pH (Fig. 5F). On the other hand, mutant Prdx6 fails to exhibit binding to ANS, as observed by a decrease in fluorescence along with no blue shift. These results indicate that higher oligomer formation of the Prdx6 at low pH might involve hydrophobic contacts which is absent in the mutant proteins.
Discussion
Prdx6 and functionally related proteins essentially exhibit pH dependent activities by changing the oligomeric status. However, it is not yet known how a low pH induces the oligomerization process in these proteins. Speculatively, previous studies have reported the pH induced formation of an electrostatic interaction due to protonation of His113 is primarily responsible for the formation of high order oligomers in Prdx117. Since, Prdx6 has also been known to generate dimers or other higher oligomeric species to gain of aiPLA2 function at low pH values, we investigated the necessity for formation of such electrostatic interaction in the process. Prdx6 does not have a conserve His or Asp residues at 113 or 76 positions (Fig. 1). However, a well conserved His and Asp residues are present at the position 79 and 42 respectively. The Asp42 and His79 residues of Prdx6 align with corresponding residues in Prdx1 and Prdx2 and exhibit a similar conformation based on the overlapping of their crystal structures. The distance between the oxygen atom of conserved Asp and the nitrogen atom of conserved His is consistent across different members of the Prdxs family (illustrated in Fig. 6). Such distance between Asp and His is appropriate for the formation of electrostatic interaction once His is protonated. Furthermore, theoretical pKa predictions of the His79 and Asp42 side chains in the crystal structure of Prdx6, using the PROPKA 3.0 program, are 8.18–8.40 and 1.21–1.80, respectively22. At the low pH , where there is maximimum aiPLA2 activity, His79 remains protonated while Asp42 is negatively charged. This charge difference can result in electrostatic interactions between the different subunits of Prdx6, promoting its oligomerization. Thus, we investigated if Asp42 and His79 residues are (i) essential for oligomerization and (ii) responsible for aiPLA2 function.
Fig. 6.
Illustration of structural forms of Asp42 and His79 in different Peroxiredoxin family. Intramolecular distance between Oxygen atom of Asp42 and Nitrogen atom of His 79 in different Peroxiredoxin family. (A) Prdx6 (represented from PDB ID: 1PRDX) (B) Prdx1 (represented from PDB ID: 2Z9S (C) Prdx2 (represented from PDB ID: 7KJ0 (D) Overlapped view of Prdx6, Prdx1 and Prdx2. Both the residues of Asp42 and His79 of Prdx6 and corresponding residues of Prdx1 and Prdx2 are aligned and have similar conformation.
In the present study, we investigated the hypothesis that the formation of electrostatic interaction driven by the protonation of His79 and Asp42 because of lowering pH is necessary for the oligomerization process of Prdx6. Thus, we have intentionally generated two different mutants by substituting His79 by Ala or Asp42 by Ala. We presumed that if the proteins fail to form the pH driven electrostatic interaction, oligomer formation would not be initiated. Our results on the measurements of hydrodynamic radius, size exclusion chromatography and native-PAGE indicate that change in pH does not influence the nature of oligomeric status of the mutant proteins as compared with the behaviour of Wt Prdx6. Interestingly, loss of oligomer formation at low pH corresponds well with the loss of the aiPLA2 function of Prdx6. Mutations do not largely affect the peroxidase function of the proteins. Therefore, the results are similar to the previous report on the behaviour of Prdx1. It is also worth noting that the catalytic pocket of the peroxidatic site comprises 3 important residues, Cys47, His39, and Arg132, which are far away from the conserved sites of Asp42 and His79 and do not impact the catalytic core after substitutions by Ala.
It has also been reported previously that such low pH induced oligomeric Prdx6 is highly stable and thermotolerant 16. Therefore, it was also important to verify, if the mutant Prdx6 also exhibit thermotolerance at pH 4.0. We investigated this possibility with heat-induced denaturation of the Wt and mutant Prdx6 at both pH 7.4 and 4.0. As expected, mutant proteins at pH 4.0 were found to undergo thermal melting and the Tm values are nearly identical to that of the Wt Prdx6 (at pH 7.4). The results further led us to believe that the formation of low pH driven electrostatic interaction might be in part responsible for the thermotolerance of Wt Prdx6. Thus, the electrostatic interaction is not only required for the oligomer generation but also for the gain thermotolerance.
We further investigated the structural consequences of the mutations at both the neutral and low pH values. It is evident in Fig. 5A that at neutral pH , there is not much significant difference in the secondary structure of the mutant proteins relative to the Wt Prdx6 at neutral pH . However, there is apparent alterations in the micro-environments of the Trp residues of the mutant proteins as revealed by the observed decrease in Trp fluorescence as compared to the Wt fluorescence. Such alterations in the Trp microenvironment of the mutant proteins do not appear to affect the overall packing of the hydrophobic groups as the ANS binding behaviour is almost identical between the Wt and mutant proteins. Thus, there is little structural consequences to the mutations at neutral pH.
In contrast to the relatively small structural changes of the mutant Prdx6 that are observed at neutral pH, relatively large changes in terms of the secondary structural content of the proteins are found at low pH. Indeed, the alpha helical content at neutral pH is decreased by around 20% relative to the Wt Prdx6 (Fig. 5B). As a result, there occur concomitant changes in the microenvironment of the Trp residues relative to the Wt. However, there is no significant binding of ANS to the mutant proteins indicating that solvent exposed hydrophobic clusters at the protein surface does not exist. On the other hand, Wt Prdx6 exhibits large binding behaviour of ANS suggesting the presence of large number of solvent exposed hydrophobic groups. Thus, it is imperative that formation of electrostatic contact results in large structural consequences leading to the exposition of hydrophobic clusters that might favour the assembly of Prdx6 with higher oligomer. On the other hand, in the mutant forms, in the absence of the electrostatic interaction, the native protein cannot initiate sufficient structural rearrangement that is required for oligomer formation. Thus, it is confirmed that formation of electrostatic interaction results in large structural reshufling that involves the exposure of hydrophobic groups and hence favor the assembly of hydrophobic contacts between two Prdx6 monomers. In support, it has been reported that hydrophobic residues (Leu 145, Ile 147, Leu 148 and Tyr 149) between β-strands (β7 strands from each monomer) may play an important role in stabilizing the protein dimer23. Additionally, the hydrophobic amino acids L145 and L148 play an important role in homodimerization of Prdx6 as well as in its heterodimerization with πGST24.
There is no concrete explanation of how His79 and Asp42 exclusive interaction or its accompanying micro-environment induces oligomer formation in Wt Prdx6. However, it can be explained that in Prdx6, at a pH of 4.0, His79 becomes protonated while Asp42 retains its negative charge, resulting in electrostatic interactions between these residues. Structure of Prdx6 formed at pH 4.0 is very complex not a conventional type as the protein gains thermotolerance at this condition (see Fig. 4). Further examination of the local residues and structures in and around the residues revealed that Asp42 is located within the loop that connects β-strand 3 to α-helix 2 (α2) (where His79 is present). It has been already reported that this loop plays a critical role in peroxidase activity and A-type dimerization, where α-helices from two monomers interact and form dimer. Additionally, glutathionylation of a cysteine residue in α-helix 2 has been shown to promote helix unwinding and disassembly of the homodimer25. Therefore, it is possible that formation of His79-Asp42 electrostatic interaction impact the position of the loop that helps in repositioning the orientation of the helices in one monomer so that it could interact favorably with another monomer to form dimer.
Since protein folding or unfolding is a cooperative process wherein all forces stabilizing the native structure act in a concerted manner, acquision of an electrostatic interaction at pH 4.0 in the Wt enzyme might help to facilitate formation of other additional cooperative bonds/forces bringing about accompanying structural alterations exemplified by the exposition of hydrophobic groups in the monomers leading to oligomer formation. Thus, formation of electrostatic interaction is not the exclusive event toward the whole structural consequences.
Conclusion
We are sure that formation of electrostatic interaction between His79 and Asp42 is responsible for the gain of aiPLA2 function of Prdx6. Such a formation of electrostatic interaction also helps not only in oligomer formation essential for aiPLA2 activity but also in gaining thermotolerant conformation in Prdx6 at pH 4.0. On structural basis, the formation of electrostatic interaction results in large structural reshufling including the exposition of hydrophobic groups that eventually helps in the thermodynamically favorable hydrophobic contact formation. From the thermodynamic perspectives, at low pH, native protein is expected to experience conformational tension as a result of the two opposing forces (repulsive forces contributed by positive charges and electrostatic interaction between His79-Asp42). Eventually, such conformational reshuffling results in the exposition of hydrophobic clusters in the monomeric protein which is thermodynamically unfavorable. However, this is followed by a hydrophobic contact formation between two monomers leading to the assembly of the two monomers making the thermodynamically unfavorable process favorable in the dimer.
Materials and methods
Isopropyl β-d-1-thiogalactopyranoside (IPTG), Horse peroxidase, DTT (DL-Dithiothreitol), yeast alcohol dehydrogenase, Sodium acetate, analytical grade Tris–HCl, bovine serum albumin (BSA), Ferritin and 8-Anilinonaphthalene-1-sulfonate (ANS) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Amicon protein concentrator having 10 kD cut off were purchased from Millipore Corporation (Burlington, Massachusetts, United States). Dialysis tubing with a molecular weight cut-off of 6–8 kD was purchased from Sigma-Aldrich (St. Louis, MO, USA). Plasmid isolation kit was obtained from Qiagen, Hilden, Germany while the EnzChek® Phospholipase A2 Assay Kit, restriction enzymes such as Nde1 and sap1 were from Invitrogen, USA. The pH 4 and pH 7 standard buffer solutions used for calibrating the pH meter at 25 °C were purchased from Merck (India). Chitin resin beads were obtained from New England Biolabs (NEB) (Ipswich, MA, USA). Ethylenediaminetetraacetic acid (EDTA) and sodium bicarbonate were sourced from Glaxo Laboratories (India). All the above chemicals were of analytical grade reagents that can be used without further purifications.
Methods
Preparation of recombinant protein
Recombinant rat wild-type was cloned between the NdeI and SapI sites of the pTYB1 vector (New England BioLabs), as described earlier21,26. The mutants Asp43Ala and His79Ala mutants of Prdx6 were created using QuikChange™ site-directed mutagenesis (Stratagene).
Protein expression of the recombinant Wt and mutant proteins and its purification using chitin resin beads was performed as reported earlier27. Subsequent size-exclusion chromatography yielded a homogenous protein product, confirmed by SDS-PAGE (see Supplementary Fig. 3).
Hydrogen peroxide decay assay and horseradish peroxidase (HRP) competitive assay
Measuring consumption of hydrogen peroxide in the presence of GSH as an electron dono can be used to assay the glutathione peroxidase activity of Prdx615,28. The standard reaction buffer at different pH values were (a) 10 mM Tris–HCl, 0.1 mM EDTA, 2 mM NaN3, and 0.2 mM GSH (pH 7.0) and (b) 40 mM Na acetate, 0.1 mM EDTA, 2 mM NaN3, and 0.2 mM GSH (pH 4.0) . The specific activity of Prdx6 is computed using the equation:
 |
where, ΔAbs/min as change in the OD240 per minute, and [E] as the concentration of enzyme used in ‘mg/ml’. Enzymatic activity was reprented as nmol of H₂O₂ reduced per minute per mg of protein. Data for multiple replications are represented as mean ± S.E. The rate constant for reaction of Prdx6s with H2O2 was determined by competition with HRP after estimation of protein sulfhydryl (-SH) group using DTNB assay as described earlier21,29.
Ca2+ independent PLA2 activity (aiPLA2)
Beside its peroxidase activity, Prdx6 exhibits Ca2+ independent intracellular phospholipase, aiPLA2 activity, that hydrolyze the sn-2 ester linkage of phospholipids maximally at pH 4.0. The PLA2 activity of Prdx6 in standard buffer 10 mM sodium acetate buffer (pH 4.0) was quantified using the EnzChek® Phospholipase A2 Assay as described previously16.
Dynamic light scattering measurements
Size distribution of particles present in the protein sample was obtained using a Zetasizer Micro V/ZMV 2000 (Malvern, UK) as decrebed previously16. Each sample was measured fifteen times with an acquisition time of 30 s at sensitivity of 10%. The data were analysed using Zetasizer software provided by the manufacturer to determine the hydrodynamic diameters of the particles and the volume fraction corresponding to each diameter. The protein concentration was 2 mg/ml in standard buffer at pH 7.4 and 4.0 and all measurements were carried out at 25 °C.
Gel permeation chromatography
Analytical SEC experiments at pH 7.4 and 4.0 were performed using a Hi-Prep 26/60 Sephacryl S-200 HR GL column (GE Healthcare) with a flow rate of 0.5 ml min-1. After purification with a chitin affinity column, rat Prdx6 was dialyzed against the standard buffers at pH 7.4 and pH 4.0. The dialysed protein, after concentration, was loaded onto the pre-equilibrated column with the corresponding standard buffer after filtering the protein using a 0.1 mm pore-sized filter (Millipore, Burlington, MA) and degassed thoroughly. The SEC column was calibrated using low and high molecular weight markers (GE Healthcare). A calibration curve was plotted using the gel phase distribution coefficient (Kav) versus logarithm of the molecular weight (Log Mw) of the markers usindg the formulae:
 |
1 |
where Ve, elution volume; Vo, column void volume (39.26) and Vc, geometric column volume (120 ml).
Native-PAGE
Native-PAGE at neutral pH was performed in the standard buffer i.e., 50 mM Tris–HCl, 100 mM NaCl pH 7.4 with running buffer 35 mM Hepes and 43 mM Imidazole, pH 7.4 at 120 v for 1.30 h with normal polarity while native-PAGE at low pH was done in 40 mM Na-acetate, pH 4, using running buffer of 30 mM beta alanine and 20 mM lactic acid (85–90%), pH 3.8, at a constant voltage (180 V) for 90 min with reverse polarity. Protein samples were electrophoresed in a continuous 7.5% polyacrylamide gel under non-reducing and non-denaturing condition using two markers, bovine albumin (Mr 68 kD, pI 4.7–4.9) and high molecular weight horse ferritin (Mr 480 kD, pI 5.5). Coomassie brilliant blue G-250 was used for the detection of the proteins on the gel.
Thermal-induced denaturation
Heat-induced denaturation of Prdx6 was monitored using a Jasco-750 CD Spectropolarimeter equipped with a Peltier-type temperature controller as described earlier27. Each thermal denaturation curve was analyzred using a two-state unfolding model in Sigma Plot 13.0 software, from Systat Software, Inc., San Jose, CA, USA as shown earlier30,31 after assuming a linear temperature dependence for both pre-and post-transition baselines using the following equation
 |
2 |
y(T)is the observed mean residue ellipticity at a given temperature, mN and mD are slopes of the native and denatured baselines, while yN and yD are the intercept of the native and denatured baselines, respectively. T is the temperature in Kelvin, Tm is the melting temperature in Kelvin and ΔHm is the enthalpy change of denaturation at melting temperature and R is the universal gas constant.
Circular dichroism (CD)
CD measurements were recorded in standard buffers using Jasco-750 CD spectropolarimeter at 25 °C as performed earlier27,32. The change in the far-UV CD is quantitated by measuring the alpha-helix content using the formula: alpha-helix (%) = [(-[θ]222 + 3000) / 36,000 + 3000] × 100, where [θ]222 is the mean residual ellipticity at 222 nm33.
Fluorescence spectroscopy
Tryptophan fluorescence and ANS fluorescence measurementswere conducted using a PTI spectrofluorometer (Photon Technology International, Lawrenceville, NJ) with a single photon counting system, and dual fluorescence and absorbance channels using excitation and emission slits of 1 nm each as described earlier27,32.
Statistical analysis
Results are expressed as mean ± SD. Data points for thermal-induced denaturation were curve-fitted using dynamic fit function in Sigma Plot 13 software .
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Financial support was provided by HL19737 and HL102016 from the National Heart, Lung and Blood Institute. We are also thankful to the Department of Biotechnology, Govt. of India for support as grant (Ref No.: BT/359/NE/TBP/2012). N.A.S thanks the Researchers Supporting Project number (RSPD2025R981), King Saud University, Riyadh, Saudi Arabia for the support.
Author contributions
Conceptualization: H.R.; Methodology: H.R., S.S. & P.K.; Resources: H.R.; Data curation: S.S. & P.K.; Writing—Original draft: H.R., S.S. & P.K.; Writing—Review and Editing: S.S., P. K., A.K., J.L., W.A.N., K.H.S., N. A. S., L.R.S., A.B.F. & H.R.; Supervision: H.R. & L.R.S.; Project administration: H.R., L.R.S. & A.B.F.; Funding acquisition: H.R., L.R.S. & A.B.F.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wood, Z. A., Poole, L. B. & Karplus, P. A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science300, 650–653 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Sue, G. R., Ho, Z. C. & Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med.38, 1543–1552. 10.1016/j.freeradbiomed.2005.02.026 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Mizohata, E. et al. Crystal structure of an archaeal peroxiredoxin from the aerobic hyperthermophilic crenarchaeon Aeropyrum pernix K1. J. Mol. Biol.354, 317–329 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Perkins, A., Nelson, K. J., Parsonage, D., Poole, L. B. & Karplus, P. A. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci.40, 435–445. 10.1016/j.tibs.2015.05.001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee, S. G., Woo, H. A., Kil, I. S. & Bae, S. H. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J. Biol. Chem.287, 4403–4410. 10.1074/jbc.R111.283432 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang, H. H. et al. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell117, 625–635 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Fujii, J. & Ikeda, Y. Advances in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep.7, 123–130. 10.1179/135100002125000352 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Seo, M. S. et al. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J. Biol. Chem.275, 20346–20354 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Manevich, Y., Reddy, K. S., Shuvaeva, T., Feinstein, S. I. & Fisher, A. B. Structure and phospholipase function of peroxiredoxin 6: Identification of the catalytic triad and its role in phospholipid substrate binding. J. Lipid Res.48, 2306–2318 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Ralat, L. A., Misquitta, S. A., Manevich, Y., Fisher, A. B. & Colman, R. F. Characterization of the complex of glutathione S-transferase pi and 1-cysteine peroxiredoxin. Arch. Biochem. Biophys.474, 109–118 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher, A. B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys.617, 68–83. 10.1016/j.abb.2016.12.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher, A. B. The phospholipase A2 activity of peroxiredoxin 6. J. Lipid Res.59(7), 1132–1147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiba, S., Dodia, C., Chen, X. & Fisher, A. B. Characterization of acidic Ca2+ independent phospholipase A2 of bovine lung. Comp. Biochem. Physiol. B120, 393–404 (1998). [DOI] [PubMed] [Google Scholar]
- 14.Kim, T. S. et al. Identification of a human CDNA clone for lysosomal type Ca2+ independent phospholipase A2 and properties of the expressed protein. J. Biol. Chem.272(4), 2542–2550 (1997). [DOI] [PubMed] [Google Scholar]
- 15.Fisher, A. B., Dodia, C., Manevich, Y., Chen, J.-W. & Feinstein, S. I. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J. Biol. Chem.274(30), 21326–21334 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Chowhan, R. K., Hotumalani, S., Rahaman, H. & Singh, L. R. pH induced conformational alteration in human peroxiredoxin 6 might be responsible for its resistance against lysosomal pH or high temperature. Sci. Rep.11, 9657. 10.1038/s41598-021-89093-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morais, M. A. B. et al. How pH modulates the dimer-decamer interconversion of 2-cys peroxiredoxins from the Prx1 subfamily. J. Biol. Chem.290, 8582–8590 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen, J. W., Dodia, C., Feinstein, S. I., Jain, M. K. & Fisher, A. B. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J. Biol. Chem.275, 28421–28427 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Fisher, A. B. Peroxiredoxin 6: A bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid. Redox. Signal.15, 831–844 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahaman, H., Herojit, K., Singh, L. R. & Fisher, A. B. Structural and functional diversity of the peroxiredoxin 6 enzyme family. Antioxid. Redox Signal.40, 13–15 (2024). [DOI] [PubMed] [Google Scholar]
- 21.Shahnaj, S. et al. The anti-oxidant enzyme, Prdx6 might have cis-acting regulatory sequence(s). Int. J. Biol. Macromol.149, 1139–1150 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Olsson, M. H. M., SØndergaard, C. R., Rostkowski, M. & Jensen, J. H. PROPKA3: Consistent treatment of internal and surface residues in empirical p K a predictions. J. Chem. Theory Comput.7, 525–537 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Kim, K. H., Lee, W. & Kim, E. E. K. Crystal structures of human peroxiredoxin 6 in different oxidation states. Biochem. Biophys. Res. Commun477, 717–722 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Zhou, S. et al. Peroxiredoxin 6 homodimerization and heterodimerization with glutathione S-transferase pi are required for its peroxidase but not phospholipase A2 activity. Free Radic. Biol. Med.94, 145–156 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noguera-Mazon, V. et al. Glutathionylation induces the dissociation of 1-Cys D-peroxiredoxin non-covalent homodimer. J. Biol. Chem.281, 31736–31742 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Chong, S. et al. Single-column purification of free recombinant proteins using a self-cleavable affinity tag derived from a protein splicing element. Gene192(2), 271–281 (1997). [DOI] [PubMed] [Google Scholar]
- 27.Shahnaj, S. et al. Hyperoxidation of peroxiredoxin 6 induces alteration from dimeric to oligomeric state. Antioxidants8, 33. 10.3390/antiox8020033 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang, S. W., Baines, I. C. & Rhee, S. G. Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J. Biol. Chem.273, 6303–6311 (1998). [DOI] [PubMed] [Google Scholar]
- 29.Ellman, G. L. Tissue sulfhydryl groups. Arch. Biochem. Biophys.82, 70–77 (1959). [DOI] [PubMed] [Google Scholar]
- 30.Santoro, M. M. & Bolen, D. W. Unfolding free energy changes determined by the linear extrapolation method. 1. Unfolding of phenylmethanesulfonyl alpha-chymotrypsin using different denaturants. Biochemistry27(21), 8063–8068 (1988). [DOI] [PubMed] [Google Scholar]
- 31.Khurshid Alam Khan, M. et al. A single mutation induces molten globule formation and a drastic destabilization of wild-type cytochrome c at pH 60. J. Biol. Inorg. Chem.14, 751–760 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Rahaman, H. et al. Increased phospholipase A2 activity with phosphorylation of peroxiredoxin 6 requires a conformational change in the protein. Biochemistry51, 5521–5530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen, Y.-H., Yang, J. T. & Chaus, K. H. Determination of the helix and p form of proteins in aqueous solution by circular dichroism. Biochemistry13(16), 3350–3359 (1974). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.








