Abstract
P2 × 7R is crucial in the pathogenesis of chronic inflammatory diseases, and its activation leads to the release of pro-inflammatory cytokines, exacerbating the inflammatory response. Two new series of scarce cyclic N, O-acetals (ATF 61–74) and corresponding opened N, N-aminals (CS 1–14) have been designed as novel potential P2RX7 antagonists, then synthesized and evaluated for their anti-inflammatory properties through investigating the pro-inflammatory markers and also for their antifungal activity against Candida albicans. Three compounds (ATF 64, CS 8, and CS 9) exhibited dual antifungal and anti-inflammatory properties. ATF 64, CS 8, and CS 9 reduced ROS production and IL-1β expression in macrophages and intestinal cells in a manner correlated with NF-KB expression. These compounds showed excellent antifungal activity against clinical isolates of C. albicans resistant to fluconazole and caspofungin, and reduced C. albicans biofilm formation. Treatment with CS 8 or CS 9 protected the nematode Caenorhabditis elegans against infection with C. albicans and enhanced antimicrobial gene expression. This duality of action offers a promising new pharmacological strategy to counteract inflammatory diseases and propels N, N-aminals as promising candidates for future optimization and investigation.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-92635-z.
Keywords: N,O-acetals; N,N-aminals; Inflammation; Anti-inflammatory agent; Candida albicans; Antifungal agents
Subject terms: Crohn's disease, Ulcerative colitis, Biosynthesis, Chemical modification
Introduction
A high percentage of human morbidity and mortality is caused by inflammation, which can largely be attributed to infectious diseases, but also to non-infectious pathological conditions1. A microbial component, known as pathogen-associated molecular patterns (PAMPs), triggers pattern recognition receptors (PRRs), causing infectious and inflammation, respectively2. In response to PRR activation, many inflammatory genes are expressed, including interleukin-1 (IL) cytokines3. IL-1 cytokines are among the most potent inflammation initiators4. These cytokines play a crucial role in the regulation of acute inflammatory responses, as well as contributing to chronic inflammatory responses in a number of diseases5. IL-1 is released primarily by activated monocytes and macrophages. P2 × 7R is a purinergic receptor that plays an important role in the release of IL-1 from immune cells6,7. This receptor is expressed by cells of both hemopoietic and immunological origin, including monocytes, macrophages, and microglia. A stimulation of P2 × 7R ensures a significant efflux of K+, resulting in the formation of active caspase-1, which is responsible for the production of mature IL-1β8. Therefore, P2 × 7R plays a crucial role in modulating inflammation by contributing to cytokine production6.
The PRR stimulates the release of ATP, which in turn, activates P2 × 7R and triggers a series of events that culminate in the release of mature IL-1β9. Several pathogenic microbes, including fungal pathogens, have developed sophisticated molecular strategies to evade host defences by interfering with certain compounds that are involved in inflammation signaling, which play a critical role in their survival10. Among the most significant fungal pathogens for humans is Candida albicans11,12. This opportunistic pathogen colonizes the vaginal tract, the oropharyngeal cavity, as well as the gastrointestinal tract, which may cause both mucosal and systemic infections in humans13.
The emergence of antifungal resistance among Candida species is one of the most significant threats to public health14. In recent years, drug-resistant fungi have proven to be a major health issue due to their prevalence all over the world. The use of antifungal drugs for a prolonged period can easily lead to fungal resistance. There is a strong need for new antifungal drugs which can exert effective antifungal activity and overcoming drug resistance to effectively control global fungal infections. The discovery of new antifungal drugs and targets is an important first step toward reducing the threat from drug-resistant fungi to human life, health, and safety, as well as the threat to public health15.
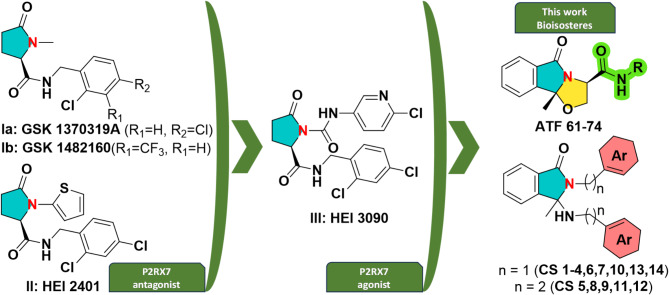
New series of molecules with anti-inflammatory potential have been designed and synthesized by our group in recent years16–19. This work is part of the continuum of our efforts to study different pharmacomodulations on the structure of the most effective compounds identified so far and aims to develop new therapeutic agents, ligands of P2RX7 receptors (P2 × 7R): agonists (e.g. compound HEI 3090, Fig. 1)20,21 and/or antagonists (e.g. compound HEI 2401, Fig. 1)17. The latter compound has a closely related structure to the reference P2RX7 antagonists developed by GSK, experimental drugs for treating inflammatory pain (for example, arthritis) and which underwent clinical trials (compounds GSK1370319A and GSK1482160, Fig. 1). The P2RX7 antagonist HEI 2401 reduced the IL-1β and ROS production in macrophages and dampened signs of colitis in a rodent model17, while the P2RX7 positive modulator HEI 3090 controlled the growth of lung tumors in mouse models and triggered a long lasting antitumor immune response20. The biologists and biochemists have shown that P2RX7 is involved in inflammatory phenomena and that P2RX7 ligands can be used in the treatment of inflammatory bowel diseases (IBD), more particularly in the treatment of Crohn’s disease, ulcerative colitis, cancers (leukemia, myelodysplastic syndromes, non-small cell lung cancer, etc.) and recently, lung fibrosis20–22.
Fig. 1.
Structure of previously developed P2RX7 ligands I-III and of newly synthesized compounds evaluated in this study ATF 61–74 and CS 1–14.
Given the importance of such ligands, we investigated the synthesis of new series of potential ligands of the P2 × 7R with improved activity. To achieve these compounds, the structures of previously identified molecules have been modulated by: (i) adding steric hindrance to the central pyrrolidine ring by replacing it with a bicyclic (compounds from the series ATF) or tricyclic (compounds from the series CS) unit, (ii) changing their amide linker with an amine (CS 1–14) or (iii) adding chemical diversity on the nitrogen atom (ATF 61–74, CS 5, and CS 8–12) (Fig. 1). The replacement of the pyrrolidine unit by bicyclic and tricyclic rings was motivated by the poor pharmacokinetic parameters of previously developed P2RX7 antagonists. For example, HEI 2401 was rapidly metabolized by h-hepatocytes, showing a half-time life (t1/2) of 1.5 min17.
To access the targeted compounds, the synthetic methodology has been redesigned compared to that needed for reference compounds.
The newly synthesized molecules (compounds ATF and CS, Fig. 1) have been evaluated for their anti-inflammatory properties through investigating the pro-inflammatory markers including NF-kB, IL-1β, and CCL-2 and also for their antifungal activity against Candida albicans.
Results
In the current study, we intend to focus on ATF 64, CS 8 and CS 9, compounds with dual actions that can attenuate the inflammatory response via P2 × 7/IL-1β and eliminate C. albicans infections. Based on our previous work on modulating the P2 × 7R through P2 × 7 antagonists, 28 novel compounds were synthesized to target the P2 × 7R. We evaluated their anti-inflammatory and antifungal properties.
Analysis of the anti-inflammatory properties of molecules from the first and second series
The THP-1 cells have been widely used as a model for studying monocyte and macrophage function, and they express the diverse cytokines, including IL-1β. NF-κB is a transcription factor which plays a key role in the release of cytokines and chemokines from macrophages.
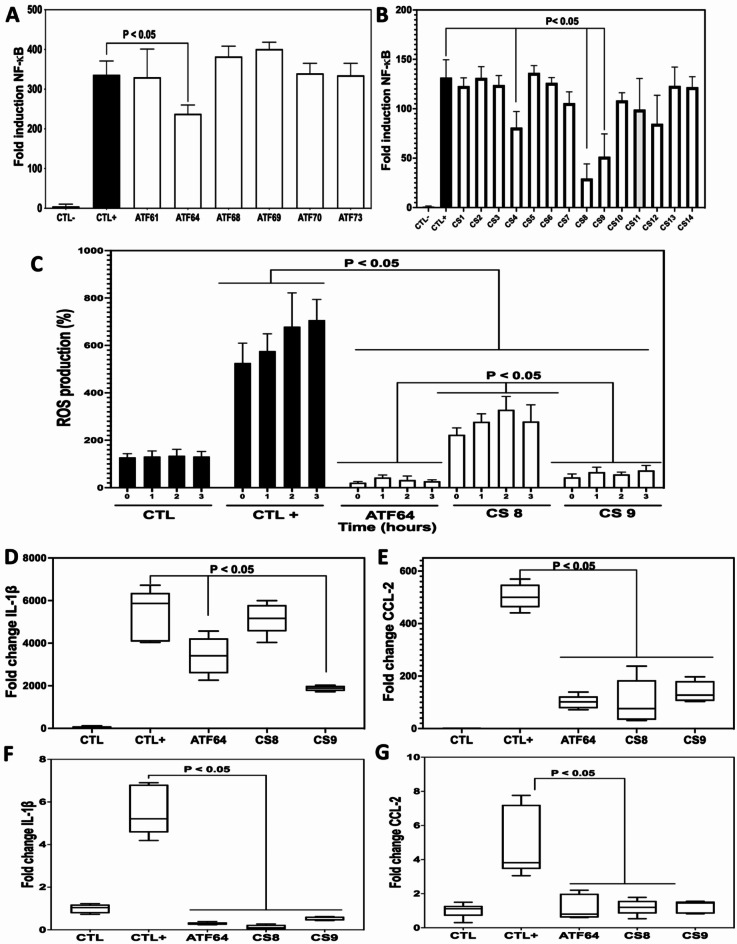
We assessed whether these compounds from this first series ATF can attenuate NF-kB expression in macrophages. Of note, the macrophages were transfected with an NF-kB-Luc reporter construct, which is very suitable for detecting NF-kB activity in macrophages (Fig. 2).
Fig. 2.
Effect of compounds ATF 64, CS 8, and CS 9 on attenuation of pro-inflammatory mediator expression in macrophages and intestinal Caco-2 cells. A and B, the effect of the first and second series of compounds on NF-κB activity in LPS-stimulated macrophage Lucia NF-kB cells. CTL- corresponds to unstimulated macrophages-Lucia NF-kB cells. CTL + corresponds to LPS-stimulated macrophage Lucia NF-kB cells. CS compounds from the first and second series were added to LPS-stimulated macrophage Lucia NF-kB cells. Luminescence intensity of the expressed luciferase protein was measured by the NF-κB reporter gene assay and calculated as the percent of treated cells over control cells (CTL+) exposed to LPS × 100 (%). C, Effect of ATF 64, CS 8, and CS 9 on LPS-induced ROS production in macrophages. The ROS production was measured in macrophages treated with ATF or CS compounds and stimulated with LPS every h for 3 h. The data shown represent the mean ± SD of three independent experiments. D, and E, proinflammatory mediators’ expression in macrophages challenged with LPS and treated with ATF and CS compounds. Relative expression levels of IL-1β (D), and CCL-2 mRNA (E), in LPS-stimulated macrophages. CTL-: control group (macrophages alone); CTL+: macrophages exposed to LPS; ATF 64, CS 8, and CS 9: macrophages challenged with LPS and treated with ATF or CS. F, and G, proinflammatory mediators’ expression in intestinal Caco-2 cells challenged with DSS and treated with ATF and CS compounds. Relative expression levels of IL-1β (F), and CCL-2 mRNA (G), in DSS-challenged Caco-2 cells. CTL-: control group (Caco-2 cells alone); CTL+: Caco-2 cells challenged with DSS; ATF 64, CS 8, and CS 9: Caco-2 cells challenged with DSS and treated with ATF or CS.
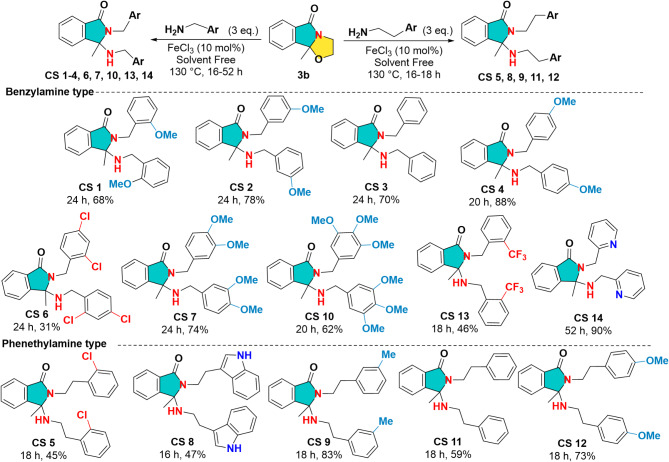
According to the analysis on the 14 compounds from the ATF series, we found that molecule ATF 64 exhibits anti-inflammatory properties. All these molecules were evaluated at a concentration of 40 µg/mL since they did not have any anti-inflammatory effects at a concentration of 4 µg/mL. Then, we synthesized a novel series of 14 derivatives (CS series) based on compound ATF 64 to improve its anti-inflammatory properties. The CS series is composed of N, N-aminals (Fig. 1, Scheme 3 and supplementary data), opened analogues of cyclic N, O-acetals composing the ATF series (Fig. 1, Scheme 2 and supplementary data). It was designed to obtain more flexible compounds compared to the ATF series, bearing a secondary amino group able to engage in hydrogen bonding both as donor and acceptor and explore its influence on the biological activity.
Scheme 3.
Scheme for the synthesis of the second series of targeted bis-aminated compounds CS 1–14.
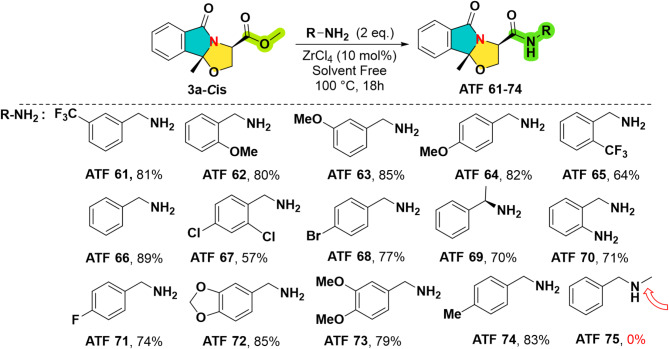
Scheme 2.
The synthesis and the structures of the first series of experimental drugs ATF 61–74.
Going further with the biological evaluation, it was found that the expression of NF-kB was significantly reduced by compounds CS 4, CS 8, CS 9, and CS 12, respectively. Of note, compounds CS 8 and CS 9 showed a greater reduction in NF-kB activity than compounds CS 4 and CS 12 (Fig. 2).
Therefore, based on these data, we were able to select compounds CS 8 and CS 9 in our following experiments to evaluate their anti-inflammatory effects with regard to ROS production and the expression of cytokines and chemokines. We compared their anti-inflammatory properties with those of the compound ATF 64 from the first series (Fig. 2). Significant reduction was observed in ROS production after treating LPS-stimulated macrophages with ATF 64, CS 8, or CS 9 compared to control LPS-stimulated macrophages. In contrast to CS 8 treatment, ROS reduction was significantly more pronounced in macrophages treated with ATF 64 or CS 9. In terms of the expression of cytokine and chemokine, CS 9 attenuated the expression of cytokine along with IL-1β and the chemokine CCL-2 in a manner similar to compound ATF 64 in LPS-stimulated macrophages. Of note, a trend towards decreased IL-1β expression was observed in LPS-stimulated macrophages treated with CS 8 (Fig. 2).
Next, we explored the effect of ATF 64, CS 8 and CS 9 on intestinal Caco-2 cells in the presence of DSS at a concentration of 2%, which disrupts the intestinal epithelial barrier. The expression of IL-1β and CCL-2 was significantly increased in intestinal cells (CTL+) exposed to DSS. Treatment of intestinal Caco-2 cells with ATF 64 or CS 9 significantly reduced the expression of IL-1β and CCL-2. In contrast, treatment with CS 8 did not significantly reduce the expression of these pro-inflammatory mediators (Fig. 2).
Assessment of the antifungal properties of ATF and CS compounds against C. albicans
Following the evaluation of molecules from the first series, we next assessed their antifungal properties. Some compounds from this first series have been found to be effective against C. albicans.
The compounds ATF 64, ATF 69, and ATF 73 showed some promise against C. albicans, with MICs of 176.19, 168.1 µg/mL, and 92.811 µg/mL, respectively. These findings suggest that these compounds possess potential antifungal properties against C. albicans. However, only compound ATF 64 was found to exhibit dual anti-inflammatory and antifungal properties (Table 1).
Table 1.
MIC determination of the first series of ATF compounds.
| Compound (CHR) | M (g/mol) | MIC (µg/mL) |
|---|---|---|
| ATF 61 | 390.36 | 975.9 |
| ATF 64 | 352.39 | 176.195 |
| ATF 68 | 401.26 | 401.26 |
| ATF 69 | 336.39 | 168.1 |
| ATF 70 | 337.38 | 506.07 |
| ATF 73 | 309.37 | 92.811 |
Aside from their anti-inflammatory effects, compounds CS 8 and CS 9 also possess moderate antifungal properties with MIC values of 224.28 µg/mL and 199.27 µg/mL respectively (Table 2). These data suggest that ATF 64, CS 8 and CS 9 have not only anti-inflammatory properties, but also antifungal properties, making them attractive candidates to be studied in the following antifungal experiments.
Table 2.
MIC determination of the second series of CS compounds.
| Compound (CHR) | Compound (HEI) | M (g/mol) | MIC (µg/mL) |
|---|---|---|---|
| CS 1 | CS081 | 402.49 | 201.245 |
| CS 2 | CS396 | 402.49 | 201.245 |
| CS 3 | CS397 | 342.44 | 171.22 |
| CS 4 | CS398 | 402.49 | 201.245 |
| CS 5 | CS433 | 439.38 | 219.69 |
| CS 6 | CS399 | 480.21 | 240.105 |
| CS 7 | CS422 | 462.55 | 231.275 |
| CS 8 | CS423 | 448.57 | 224.285 |
| CS 9 | CS434 | 398.55 | 199.275 |
| CS 10 | CS424 | 522.24 | 261.12 |
| CS 11 | CS426 | 370.5 | 185.25 |
| CS 12 | CS427 | 430.55 | 215.275 |
| CS 13 | CS430 | 478.44 | 239.22 |
| CS 14 | CS437 | 344.44 | 172.22 |
C. albicans biofilm formation
Next, we examined the effect of the molecules ATF 64, CS 8 and CS 9 on biofilm formation which is a virulence factor for C. albicans. The three compounds, ATF 64, CS 8 and CS 9 (at a concentration of 1x MIC) reduced biofilm formation by 22.7%, 27.3% and 27. 9%, respectively. From the perspective of microscopic analysis, we observed a dense and compact biofilm matrix with C. albicans challenged with PBS as a control. The biofilm matrix, however, was disturbed when C. albicans was treated with these compounds at 1x MIC (Fig. 3).
Fig. 3.
C. albicans biofilm formation after challenge with CS compounds. A, C. albicans cells formed a dense biofilm after 48 h. C. albicans biofilms were treated with CS compounds at 1x MIC for 24 h. CTL: C. albicans alone without antifungal challenge; ATF 64, CS 8, and CS 9 correspond to C. albicans biofilm challenged with compounds. B, (a) CTL: C. albicans alone without antifungal treatment, (b) b, c, and d, correspond to C. albicans challenged with ATF 64, CS 8, and CS 9 respectively. Scale bars represent 100 μm.
Drug-resistant clinical isolates of C. albicans
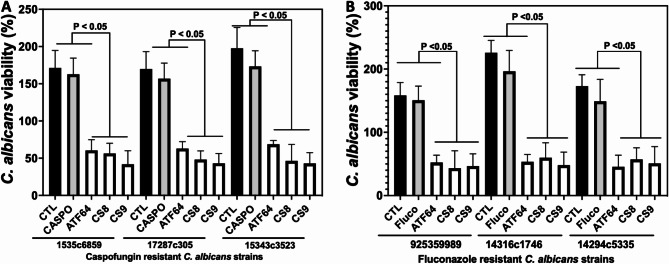
To evaluate their efficacy in eliminating the drug-resistant isolates of C. albicans, we have examined the efficacy of these compounds against fluconazole- or caspofungin-resistant strains isolated from patients. The compounds CS 8 and CS 9 showed significant reductions in C. albicans viability, which were resistant to fluconazole and caspofungin at 1x MIC (Fig. 4). These data show that the selected compounds ATF 64, CS 8 and CS 9 reduced the growth of drug-resistant isolates of C. albicans, which is considered one of the main concerns when it comes to drug resistance (Fig. 4).
Fig. 4.
CS effects on the viability of caspofungin- and fuconazole-resistant C. albicans isolates. (A) Caspofungin-resistant C. albicans strains. (B) Fluconazole-resistant C. albicans strains. C. albicans clinical isolates were challenged with CS at 1x MIC. C. albicans viability cells were determined after 24 h using Alamar Blue reagent. CTL: clinical strain of C. albicans without antifungal treatment; Fluco: C. albicans cells challenged with fluconazole at 1x MIC; Caspo: C. albicans cells challenged with caspofungin at 1x MIC.
Effect of ATF 64, CS 8 and CS 9 compounds on the survival of Caenorhabditis elegans infected with C. albicans
We then determined whether these compounds CS 8 and CS 9 were effective in vivo against C. albicans, using a model of Caenorhabditis elegans (C. elegans) infected with C. albicans. Nematodes infected with C. albicans were treated with compounds CS 8 and CS 9 at their 1x MICs. The survival rate of C. elegans was monitored daily by microscopic observation. The nematodes infected with C. albicans without any treatment were used as a control. Within 4 days of the infection, 84% of the nematodes had died. There was a significant increase in the survival rate of C. elegans when treated with compounds CS 8 and CS 9 compared to nematodes treated with ATF 64 or to an untreated control group of worms. Compounds CS 8 and CS 9 were observed to allow a 58% survival rate and a 46% survival rate for nematodes, respectively (Fig. 5). These compounds were evaluated for their effect on C. elegans’ antimicrobial response by analyzing the expression of lys-1 and fipr-22/23 (Fig. 5). CS 8 treatment of C. elegans infected with C. albicans was shown to enhance the expression of the antimicrobial genes lys-1, and fipr-22/23 when compared to untreated nematodes (CTL) or nematodes treated with ATF 64 while CS 9 enhanced the expression of lys-1.
Fig. 5.
The effect of CS on the pathogenesis of C. albicans in a C. elegans infection model. During the experiment, survival rate of nematodes infected with C. albicans was assessed every day for four consecutive days, and the percentage of worms that survived the fourth day was assessed. Worms infected with C. albicans were challenged with CS at 1x MIC. With a platinum wire pick, dead nematodes were identified that had not responded to contact. CTL: C. albicans-infected worms without antifungal treatment. ATF 64, CS 8, and CS 9 correspond to C. albicans-infected worms with CS compound treatment. Expression of antimicrobial peptides in C. elegans infected with C. albicans (lys-1 and fipr-22/23). Values are shown as mean ± SD of four independent experiments.
Discussion
Inflammation is responsible for a large percentage of human morbidity and mortality, including infectious and non-infectious diseases23. The inflammation process is accompanied by various signaling systems and the recruitment of inflammatory cells. These cells release a variety of mediators that enhance the inflammatory response and recruit more cells to the site. Among these mediators, IL-1β is one of the most potent proinflammatory cytokine that contribute to acute inflammation as well as chronic inflammation in different diseases. P2RX7 plays a crucial role in IL-1β processing and release from LPS-stimulated macrophages24. The inhibition of P2RX7 has also been shown to be effective in preventing tissue damage, cell apoptosis, cytokine production, and the activation of inflammation-related signaling pathways25.
In the present study, we aimed to identify compounds that may have a dual action that Attenuate inflammation processes by inhibiting P2RX7 and can eliminate C. albicans infections through their antifungal properties. Following our previous P2RX7 study, we have synthesized a first series of 14 new compounds (ATF) that target this receptor. We evaluated these compounds’ anti-inflammatory and antifungal properties. We found compound ATF 64 with anti-inflammatory and antifungal properties. The dual effects of this compound led to the development of a novel series of 14 compounds (CS) that are designed to potentially improve the anti-inflammatory and antifungal properties of this initial compound. The latter compounds, characterized by a bicyclic core namely isoindolin-1-one, are more flexible compared to the tricyclic 2,3-dihydrooxazolo[2,3-a]isoindol-5(9bH)-ones ATF compounds and could engage in different interactions with the biological target. Also, the bioisostere replacement of amide group in the ATF series with a secondary amine in the CS series was designed to study the impact of this linker changeset on the anti-inflammatory and anti-fungal activity.
Our second series of 14 derivatives of ATF 64 (CS) was synthesized to explore the possibility of optimizing its efficiency in combating inflammation and/or fungal infection. In this second series, we found that the CS 8 and CS 9 molecules were the most effective among these ATF 64 derivatives in reducing NF-kB in macrophages. In line with these observations, we found that ATF 64 and CS 9 reduced ROS production and the expression of the cytokine IL-1β in macrophages and intestinal cells in a manner that was correlated with the expression of NF-KB. Interestingly, CS 8 shows a significant decrease of IL-1β in intestinal cells and a tendency to decrease IL-1β expression in macrophages. Of note, these three compounds ATF 64, CS 8 and CS 9 reduced the expression of CCL-2 in macrophages and intestinal Caco-2 cells exposed to DSS. These data are in accordance with a previous study, which showed that pyroglutamic acid derivatives inhibit ROS production and IL-1β expression via P2 × 7R in macrophages17. In mice treated with P2RX7 antagonist A740003 after being infected with sepsis for three days, the inflammatory response and intestinal barrier defect were attenuated. As compared to untreated septic mice, mice treated with A740003 exhibit diminished proinflammatory cytokine production, intestinal hyperpermeability, epithelial apoptosis rates, and tight junction damage compared to mice not treated with A74000325. Thus, by blocking P2RX7, we may be able to treat sepsis-induced alterations to the intestinal barrier by targeting this potential therapeutic target25.
In terms of the antifungal aspect, all compounds from the two series were tested to determine their anti-C. albicans MICs. The three compounds ATF 64, CS 8 and CS 9 also exhibit a significant antifungal effect despite the presence of other compounds that show a more significant antifungal effect. However, we chose to keep these compounds for further biological evaluation since they have both anti-inflammatory and antifungal properties.
In addition, these three compounds significantly displayed antifungal effects against biofilm formation as well as against clinical strains resistant to fluconazole and caspofungin. This study reveals the effect of CS molecules on biofilm formation, which is an important aspect of C. albicans virulence. Biofilms are densely packed communities of cells that adhere to a surface. In addition, C. albicans biofilms are largely resistant to antimicrobial agents, but this resistance is related to the presence of the matrix that prevents drug penetration from entering by forming a diffusion barrier26,27.
One of the most successful in vivo approaches to gaining an understanding of the pathogenesis of Candida infections and the innate immune response of the host involves C. elegans model28,29. Regarding their effects on C. elegans survival, we observed that, unlike ATF 64, which has no effect on the survival of these nematodes infected with C. albicans, CS 8 and CS 9 improve substantially C. elegans survival, with CS 8 in particular exerting a stronger effect than CS 9 and providing a more potent effect on C. elegans survival. These data showed that the beneficial effects of CS 8 and CS 9 do not result only from their antifungal properties, but rather from their ability to increase the C. elegans immune response to infection. Of all the chemical diversity of electron-donating and electron-withdrawing substituents introduced in the first ATF series, the best chemical modulation for biological activity was the electron-donating p-methoxy substituent in the compound ATF 64, which gave the most active compound of this series. However, the most active compounds in this study were found to be the bicyclic compounds CS 8 and CS 9, surpassing the antifungal and anti-inflammatory potential of the entire ATF series. Interestingly, these N, N-aminals CS 8 and CS 9 are also substituted by mesomeric M + and inductive I + electron-donating substituents, respectively, the electron-withdrawing substituents resulting in reduced activity.
In conclusion, the molecules synthesized for this study are original and newly described molecules for anti-inflammatory and antifungal properties. They attenuated the inflammatory response through the reduction of NF-kB, ROS generation and pro-inflammatory mediators such as IL-1β, and CCL-2. These compounds also exhibited excellent antifungal activity against clinical isolates of C. albicans and reduced C. albicans biofilm formation. Treatment with CS 8 and CS 9 protected C. elegans against infection with C. albicans and enhanced antimicrobial gene expression. These compounds exhibit dual antifungal and anti-inflammatory properties, making them promising candidates to further development into therapeutic agents.
Materials and methods
Chemical synthesis
The targeted cyclic N, O-acetals ATF 61–74 containing amide moiety and corresponding opened N, N-aminals CS 1–14 were synthesized in two steps according to a previously reported Meyers type cyclocondensation procedure30–32 by an eco-friendly catalytic ester amidation33–35. Concisely, the synthesis of the starting materials, such as functionalized cyclic N, O-acetals 3a, b, was achieved by thermal cyclocondensation of the commercially available 2-acetylbenzoic acid (1) and (R)-serine ethyl ester hydrochloride (2a) or ethanolamine (2b) as two different model substrates (Scheme 1)36. Of interest, the synthesis of ultimate targets ATF and CS as well as all the intermediates 3a, b and 4a, b compounds has not yet been described in the literature. The full synthetic details will be reported in due course in a global paper focusing specifically on organic chemical synthesis including proposed mechanisms for these transformations.
Scheme 1.
General pathways for the synthesis of the starting materials 3a and 3b compounds.
As reported in Scheme 1, the best conditions obtained after wide screening seem to be the use of p-toluenesulfonic acid (PTSA) as a catalyst, triethylamine in a slight excess for neutralisation of the starting amino-alcohol hydrochloride, in toluene under azeotropic conditions to access 3a, b in very high isolated yields up to 99%. Under these optimised conditions, the reaction proceeded quantitatively to form cyclic N, O-acetal 3a in 70% accompanied with enamide-alcohol 4a in 30% which were separated easily by flash liquid chromatography. The structure of the cyclic N, O-acetal 3a, isolated as single diastereoisomer Cis, was secured by X-ray analysis. This interestingly suggests that the imine-alcohol cyclisation reaction, proceeding most likely via Flekin-Ahn model TS37, is the key step of the following reaction sequence which provide 3a in total stereo control. Similarly, starting with 1 and ethanolamine 2b without the need of triethylamine, the reaction provides an overall yield of 90% of the expected cyclic N, O-acetal 3b in 60% and enamide-alcohol 4b in 30%, also separable easily.
Next, the first series of compounds ATF was elaborated by solvent free reaction of cyclic N, O-acetal ester 3a and amines (R-NH2) by using a catalytic quantity of the eco-friendly ZrCl4 (10 mol%)38. After heating the reaction at 100 °C under magnetic stirring for 18 h (h), the expected products ATF 61–74 were isolated in yields ranging from 57 up to 89% (Scheme 2 and supplementary data). For the completion of the reaction, 2 equivalents of amine are needed. As we expected, the reaction failed when using the secondary amine whatever the reaction conditions changes (ATF 75).
Next, the starting material N, O-acetal 3b, without ester function, was subjected to the reaction with amines under the same conditions discussed previously. Under these conditions (ZrCl4 (10–15 mol %), 3 eq. of amines, 130 °C, 48 h), the reaction conversion was incomplete but delivered an unexpected bis-aminated compound analyzed to be CS 4 accompanied, however, with an enamide by-product in different ratios. To improve the conversion, several green Lewis acids based on calcium, bismuth and iron were tested. A complete conversion was observed in all cases with 10 mol % of these Lewis acids, with yields ranging from 62 to 88%. The optimum conditions were obtained with 10 mol % of FeCl3 since with the p-methoxybenzylamine the reaction provided after 20 h unexpected bis-aminated product (CS 4) isolated in 88% yield (Scheme 3 and supplementary data).
This particular reactivity of N, O-acetal 3b was improved successfully with substituted benzylamines to give a large panel of N, N-aminals CS 1–14 (Scheme 3 and supplementary data). This unprecedented double amination reaction seems to be tolerant to electro-withdrawing and electro-donating groups as well as to heteroaromatic amines based on pyridine (CS 14, 90%) or indole (CS 8, 47%). It is worth mentioning that the best yield of this cascade process (90%) was obtained by using with 2-(aminomethyl)-pyridine which leads to compound CS 14. Finally, the structure of these N, N-aminals CS were determined by conventional spectroscopic methods including mass spectroscopy as well as X-ray analysis of the two representatives examples CS 3 and CS 5.
Culture of C. albicans and human cell lines
C. albicans SC5314 was used in this study39. The C. albicans yeast cells were cultured on Sabouraud dextrose agar (SDA, Merck, Germany) at 37 °C for 24 h. Next, the C. albicans yeast cells were grown in Sabouraud dextrose broth (Sigma-Aldrich, France) at 37 °C for 24 h40. C. albicans yeast cells were then washed with PBS (phosphate-buffered saline) and centrifuged at 2500 rpm for 5 min before being resuspended in PBS. Regarding the culture of clinical strains, fluconazole-or caspofungin-resistant C. albicans isolates were grown on SDA for 24–48 h. Further details about these clinical strains are provided in this previous study41.
According to Pfaller et al., MIC values of these clinical strains were determined using a laboratory standard culture microdilution method developed by the Clinical and Laboratory Standard Institute (CLSI)42. C. albicans clinical strains were identified using MALDI-TOF MS analysis (Microflex-Bruker Daltonics).
In terms of THP-1 cells’ differentiation into macrophages, THP1 cells (human leukemia monocytic cell line) were treated with phorbol-12-myristate13-acetate (PMA, Sigma-Aldrich, France) for 72 h at a concentration of 200 ng/mL for macrophage differentiation. Then, macrophages were incubated in RPMI medium at a concentration of 106 cells/well for 24 h.
Macrophages were washed with PBS after exposure to lipopolysaccharide (LPS) (LPS from E. coli O111:B4; Sigma-Aldrich, France) at a concentration of 250 ng/mL and BzATP at a concentration of 70 µmol/L (Sigma-Aldrich, France) for 24 h17. LPS-exposed macrophages were treated with ATF or CS compounds at a concentration of 40 µg/mL43.
To extract mRNA from macrophages for PCR, the cells were collected, resuspended in RA1 buffer (Macherey-Nagel, Hoerdt, France). RT-PCR and q-PCR were then performed41. With PCR master mix reagent and SYBR green real-time (Applied Biosystems, Foster, CA, USA), cDNAs were amplified. The intensity of SYBR green dye in the sample was determined using a one-step software program.
For Caco-2 intestinal cells challenged with DSS (MP Biomedicals, LLC, Eschwege, Germany), in a 24-well plate, Caco-2 intestinal cells were incubated at 106 cells/mL. These intestinal cells were treated with CS 8, CS 9, or ATF 64 at a concentration of 10− 4 M with 2% DSS for 24 h at 37 °C, 5% CO2. To extract mRNA from intestinal for PCR, the cells were collected, resuspended in RA1 buffer. RT-PCR and q-PCR protocols were followed as described above.
For ROS production quantification, THP1 cells were incubated at a concentration of 105 cells/mL at 37 °C in a 96-well plate. CS 8, CS 9, or ATF 64 molecules were then added to these THP-1 cells at a concentration of 40 µg/mL. These cells were then exposed to LPS at a concentration of 250 ng/mL and BzATP at a concentration of 70 µmol/L for 3 h at 37 °C in 5% CO2. A volume of 50 µL of a mixture of 50 µg/mL horseradish peroxidase (Sigma Aldrich, France) and 50 µM luminol (Sigma Aldrich, France) were added to each well. The chemiluminescence was measured at different incubation times, 0, 1, 2 and 3 h by spectro-luminometer (FLUOstar; BMG Labtech).
THP-1 NF-κB-Luc assay
The NF-κB-Luc reporter construct was previously described44,45. The THP-1 cells were retrovirally transduced with the NF-κB-Luc construct according to the manufacture (InvivoGen, France).
For THP-1 reporter assays, after the differentiation of THP-1 NF-κB-Luc cells to NF-κB-Luc macrophages by PMA at a concentration of 200 ng/mL for 72 h. Next, NF-κB-Luc macrophages were incubated in RPMI medium for 24 h at a concentration of 7.104 cells/well.
NF-κB-Luc macrophages were washed with PBS after exposure to LPS at a concentration of 250 ng/mL and BzATP at a concentration of 70 µmol/L for 24 h17. LPS-exposed NF-κB-Luc macrophages were treated with ATF or CS compounds at a concentration of 40 µg/mL. Cells were then harvested, and luciferase expression was analyzed by spectro-luminometer (FLUOstar; BMG Labtech).
C. albicans biofilm formation
C. albicans cells at a concentration of 5 × 103 yeast cells were suspended in 200 µL RPMI medium containing 10% fetal bovine serum. The plate containing C. albicans cells was incubated for 48 h at 37 °C. Then, several PBS washes were performed to remove unattached yeast cells41,46.
ATF and CS compounds were then added to the plates at a concentration of 1x MIC for 24 h. After incubation for 24 h, the plate was washed with PBS and air dried at 37 °C. A 0.4% crystal violet solution (Fluka, India) was used to stain C. albicans biofilms for 20 min. After multiple PBS washes, 200 µL of ethanol were added to each well. A spectrophotometer (FLUOstar; BMG Labtech) was used to measure the absorbance of the destaining solution at 550 nm. In addition, C. albicans biofilms were monitored microscopically using a Zeiss AxioImager microscope.
C. elegans survival assay
A volume of 10 µL of C. albicans yeast cell suspension was spread on brain heart infusion plates containing amikacin (45 µg/mL) to grow a lawn of C. albicans SC5314. Incubation of plates was performed at 37 °C for 24 h. C. elegans N2 was grown on nematode growth medium seeded with E. coli strain OP50 at 20 °C. The populations of nematodes were synchronized and kept at 20 °C. In each experiment, 100 nematodes were selected46,47. To eliminate E. coli, M9 buffer containing 90 µg/mL amikacin was used to wash the nematodes before transfer to C. albicans lawns. The worms were washed multiple times with M9 buffer after incubation at room temperature for 6 h to remove all C. albicans yeast cells from their cuticles. C. albicans-infected worms were placed in wells of a 6-well microtiter dish containing 80% liquid M9 buffer, 90 µg/mL amikacin, and 10 µg/mL cholesterol diluted in ethanol. ATF and CS compounds at 1x MIC were added to each well. The worms were examined daily for survival for four days, and if no nematodes responded to mechanical stimulation with a pick, they were considered dead. RT-PCR was performed on nematodes after 12 h of ATF/CS compound challenge using the NucleoSpin RNA® kit (Macherey-Nagel, Hoerdt, France). With PCR master mix reagent and SYBR green real-time, cDNAs were amplified. The intensity of SYBR green dye in the sample was determined using a one-step software program. A reference gene, act-2, was used to normalize the results.
Statistical analysis
The Mann–Whitney U test was applied to evaluate the differences between the two groups. When the p value was < 0.05, < 0.01, or < 0.001, the data were considered statistically significant. GraphPad Prism 10 (GraphPad, La Jolla, CA, USA) was used for all statistical analyses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Mr. Antonino Bongiovanni for his excellent technical assistance.
Author contributions
C.S., L.C., M.O., D.L., M.B., A.M.L., A.G., A.D., and S.J. performed the experiments. C.S., L.C., M.O., D.L., M.B., A.M.L., A.G., A.D., and S.J. analyzed the data. C.S., L.C., M.O., D.L., M.B., A.M.L., A.G., A.D., and S.J. interpreted the results of experiments. A.D. and A.G. designed the chemical experiments and drafted the chemical part of the manuscript. S.J. designed the biological experiments and drafted the biological part of the manuscript.
Funding
We thank warmly Normand region, JUNIA Lille, Normand Institute ‘INC3M FR-CNRS 3038’, Le Havre Seine Métropole (LHSM) and University of Le Havre Normandie for financial support and the Scholarship attributed to one of us, Christine Safi.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Christine Safi, Louis Camaioni, Alina Ghinet, Adam Daïch and Samir Jawhara contributed equally to this work.
References
- 1.Kaczorowski, D. J., Mollen, K. P., Edmonds, R. & Billiar, T. R. Early events in the recognition of danger signals after tissue injury. J. Leukoc. Biol.83, 546–552. 10.1189/jlb.0607374 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Mogensen, T. H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev.22, 240–273 (table of contents) (2009). 10.1128/CMR.00046-08 [DOI] [PMC free article] [PubMed]
- 3.Van Den Eeckhout, B., Tavernier, J. & Gerlo, S. Interleukin-1 as innate mediator of T cell immunity. Front. Immunol.11, 621931. 10.3389/fimmu.2020.621931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello, C. A., Simon, A. & van der Meer, J. W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652. 10.1038/nrd3800 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Veerdonk, F. L. & Netea, M. G. New insights in the immunobiology of IL-1 family members. Front. Immunol.4, 167. 10.3389/fimmu.2013.00167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelegrin, P., Barroso-Gutierrez, C. & Surprenant, A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J. Immunol.180, 7147–7157. 10.4049/jimmunol.180.11.7147 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Di Virgilio, F., Ben, D., Sarti, D., Giuliani, A. C., Falzoni, S. & A. L. & The P2X7 Receptor in Infection and Inflammation. Immunity47, 15–31. 10.1016/j.immuni.2017.06.020 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Kahlenberg, J. M. & Dubyak, G. R. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K + release. Am. J. Physiol. Cell. Physiol.286, C1100–1108. 10.1152/ajpcell.00494.2003 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Mortaz, E., Adcock, I. M., Shafei, H., Masjedi, M. R. & Folkerts, G. Role of P2X7 Receptors in Release of IL-1beta: A Possible Mediator of Pulmonary Inflammation. Tanaffos11, 6–11 (2012). [PMC free article] [PubMed] [Google Scholar]
- 10.Marcos, C. M. et al. Anti-Immune strategies of pathogenic Fungi. Front. Cell. Infect. Microbiol.6, 142. 10.3389/fcimb.2016.00142 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jawhara, S. How gut bacterial dysbiosis can promote Candida albicans overgrowth during colonic inflammation. Microorganisms10. 10.3390/microorganisms10051014 (2022). [DOI] [PMC free article] [PubMed]
- 12.Jawhara, S. How fungal glycans modulate platelet activation via Toll-Like receptors contributing to the escape of Candida albicans from the immune response. Antibiotics (Basel)9. 10.3390/antibiotics9070385 (2020). [DOI] [PMC free article] [PubMed]
- 13.Poulain, D. et al. Yeasts: neglected pathogens. Dig. Dis.27 (Suppl 1), 104–110 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Vitiello, A. et al. Antifungal drug resistance: an emergent health threat. Biomedicines11. 10.3390/biomedicines11041063 (2023). [DOI] [PMC free article] [PubMed]
- 15.Fisher, M. C. et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol.20, 557–571. 10.1038/s41579-022-00720-1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homerin, G. et al. ZrCl4 as a new catalyst for ester amidation: an efficient synthesis of h-P2X7R antagonists. Tetrahedron Lett.57, 1165–1170 (2016). [Google Scholar]
- 17.Homerin, G. et al. Pyroglutamide-Based P2X7 Receptor Antagonists Targeting Inflammatory Bowel Disease. J. Med. Chem.63, 2074–2094. 10.1021/acs.jmedchem.9b00584 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Homerin, G. et al. Discovery of highly functionalized scaffolds: Pyrroloimidazolediones as P2X7 receptor antagonists. Tetrahedron73, 5327–5336 (2017). [Google Scholar]
- 19.Baudelet, D. et al. Evaluation and comparison of three different separation techniques for analysis of retroamide enantiomers and their biological evaluation against h-P2X7 receptor. J. Chromatogr. B Analyt Technol. Biomed. Life Sci.986–987, 35–43. 10.1016/j.jchromb.2015.02.001 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Douguet, L. et al. A small-molecule P2RX7 activator promotes anti-tumor immune responses and sensitizes lung tumor to immunotherapy. Nat. Commun.12, 653. 10.1038/s41467-021-20912-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janho, D. et al. Activation of the P2RX7/IL-18 pathway in immune cells attenuates lung fibrosis. Elife12. 10.7554/eLife.88138 (2024). [DOI] [PMC free article] [PubMed]
- 22.Baudelet, D., Lipka, E., Millet, R. & Ghinet, A. Involvement of the P2X7 purinergic receptor in inflammation: an update of antagonists series since 2009 and their promising therapeutic potential. Curr. Med. Chem.22, 713–729. 10.2174/0929867322666141212120926 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Chen, L. et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget9, 7204–7218. 10.18632/oncotarget.23208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrari, D. et al. The P2X7 receptor: a key player in IL-1 processing and release. J. Immunol.176, 3877–3883. 10.4049/jimmunol.176.7.3877 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Wu, X. et al. Systemic blockade of P2X7 receptor protects against sepsis-induced intestinal barrier disruption. Sci. Rep.7, 4364. 10.1038/s41598-017-04231-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira, R., Dos Santos Fontenelle, R. O., de Brito, E. H. S. & de Morais, S. M. Biofilm of Candida albicans: formation, regulation and resistance. J. Appl. Microbiol.131, 11–22. 10.1111/jam.14949 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Zarnowski, R. et al. Candida albicans biofilm-induced vesicles confer drug resistance through matrix biogenesis. PLoS Biol.16, e2006872. 10.1371/journal.pbio.2006872 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell, J. R. & Ausubel, F. M. Models of Caenorhabditis elegans infection by bacterial and fungal pathogens. Methods Mol. Biol.415, 403–427 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Shu, C., Sun, L. & Zhang, W. Thymol has antifungal activity against Candida albicans during infection and maintains the innate immune response required for function of the p38 MAPK signaling pathway in Caenorhabditis elegans. Immunol. Res.64, 1013–1024. 10.1007/s12026-016-8785-y (2016). [DOI] [PubMed] [Google Scholar]
- 30.Romo, D. & Meyers, A. Chiral non-racemic bicyclic lactams. Vehicles for the construction of natural and unnatural products containing quaternary carbon centers. Tetrahedron47, 9503–9569 (1991). [Google Scholar]
- 31.Penhoat, M. et al. Meyers’ bicyclic lactam formation under mild and highly stereoselective conditions. Tetrahedron Lett.46, 8385–8389 (2005). [Google Scholar]
- 32.Daïch, A., Ghinet, A. & Rigo, B. 2.17 Addition to N-acyliminium ions of heteroatoms such as oxygen, nitrogen, sulfur, and selenium as internal nucleophiles. Compr. Org. Synth. II 682–742 (2014).
- 33.Lundberg, H. et al. Mechanistic Elucidation of Zirconium-Catalyzed direct amidation. J. Am. Chem. Soc.139, 2286–2295. 10.1021/jacs.6b10973 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Lundberg, H., Tinnis, F. & Adolfsson, H. Zirconium catalyzed amide formation without water scavenging. Appl. Organomet. Chem.33, e5062 (2019). [Google Scholar]
- 35.Dufrénoy, P. et al. Green synthesis of a new series of pyroglutamides targeting human farnesyltransferase. Sustainable Chem. Pharm.30, 100894 (2022). [Google Scholar]
- 36.e Melo, T. M. P., Santos, C. I., Gonsalves, A. M. A. R., Paixão, J. A. & Beja, A. M. Synthesis of tricyclic isoindoles and Thiazolo [3, 2-c][1, 3] benzoxazines. Tetrahedron60, 3949–3955 (2004). [Google Scholar]
- 37.Chihab-Eddine, A., Daïch, A., Jilale, A. & Decroix, B. Synthesis and reactivity of (1S)-N-(1-phenylethyl) maleimide towards nucleophiles: an application to Preparation of chiral pyrroloisothiochroman and Pyrrolobenzo [d] thiepine based on π-cationic cyclization. Tetrahedron Lett.42, 573–576 (2001). [Google Scholar]
- 38.Nikoofar, K. & Khademi, Z. A review on green Lewis acids: zirconium (IV) oxydichloride octahydrate (ZrOCl 2· 8H 2 O) and zirconium (IV) tetrachloride (ZrCl 4) in organic chemistry. Res. Chem. Intermed.42, 3929–3977 (2016). [Google Scholar]
- 39.Gillum, A. M., Tsay, E. Y. & Kirsch, D. R. Isolation of the Candida albicans gene for orotidine-5’-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli PyrF mutations. Mol. Gen. Genet.198, 179–182. 10.1007/BF00328721 (1984). [DOI] [PubMed] [Google Scholar]
- 40.Bortolus, C. et al. A small aromatic compound has antifungal properties and potential anti-inflammatory effects against intestinal inflammation. Int. J. Mol. Sci.20 (2019). [DOI] [PMC free article] [PubMed]
- 41.Camaioni, L. et al. Natural compounds with antifungal properties against Candida albicans and identification of Hinokitiol as a promising antifungal drug. Antibiotics (Basel). 1210.3390/antibiotics12111603 (2023). [DOI] [PMC free article] [PubMed]
- 42.Pfaller, M. A., Diekema, D. J., Procop, G. W. & Rinaldi, M. G. Multicenter comparison of the VITEK 2 antifungal susceptibility test with the CLSI broth microdilution reference method for testing amphotericin B, Flucytosine, and voriconazole against Candida spp. J. Clin. Microbiol.45, 3522–3528. 10.1128/JCM.00403-07 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dufrenoy, P. et al. New efficient Eco-Friendly supported catalysts for the synthesis of amides with antioxidant and Anti-Inflammatory properties. ChemMedChem15, 459–467 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Han, J. et al. Microtubule disruption synergizes with STING signaling to show potent and broad-spectrum antiviral activity. PLoS Pathog. 20, e1012048. 10.1371/journal.ppat.1012048 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knopf, J. D. et al. RHBDL4-triggered downregulation of COPII adaptor protein TMED7 suppresses TLR4-mediated inflammatory signaling. Nat. Commun.15, 1528. 10.1038/s41467-024-45615-2 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mena, L. et al. Two new compounds containing pyridinone or triazine heterocycles have antifungal properties against Candida albicans. Antibiotics (Basel)11 (2022). [DOI] [PMC free article] [PubMed]
- 47.Camaioni, L., Lambert, D., Sendid, B., Billamboz, M. & Jawhara, S. Antifungal properties of hydrazine-based compounds against Candida albicans. Antibiotics (Basel). 12. 10.3390/antibiotics12061043 (2023). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.