Visual Abstract
Key Words: acute ischemic stroke, blood-brain barrier, endothelial cells, hypoxia/reoxygenation, inflammation
Highlights
-
•
JCAD is a membrane-associated protein, mainly expressed by endothelial cells, mediating the endothelial response to acute hypoxia in the brain through the PI3K/Akt pathway.
-
•
JCAD activation results in inhibition of cell survival and expression of proinflammatory membrane receptor, thus aggravating the ischemia/reperfusion brain damage.
-
•
JCAD knock-down by small interfering RNA reduces brain damage in a mouse model of acute brain ischemia.
-
•
Circulating JCAD can be detected in plasma of patients with acute ischemic stroke, and its values predict the 90-day risk of death.
Summary
The role of junctional protein associated with coronary artery disease (JCAD) in acute ischemic stroke (AIS) has not been investigated yet. To investigate its potential as a therapeutic target, transient middle cerebral artery occlusion was induced in JCAD knockout mice, with improvement of stroke outcome and reduced blood-brain barrier permeability and expression of vascular cell adhesion molecule (VCAM)-1. JCAD plays a deleterious role in ischemia/reperfusion cerebral damage and associates with higher 90-day mortality in patients with AIS. JCAD may thus represent a novel prognostic biomarker for patients with AIS, as well as a therapeutic target.
Acute ischemic stroke (AIS) is one of the leading causes of death and disability in industrialized countries.1 The cornerstone of AIS treatment is early reperfusion, by either systemic thrombolysis or mechanical thrombectomy.2 However, not all patients qualify for these treatments, and only 30% to 35% of patients undergoing systemic thrombolysis subsequently have a good neurological outcome.3 Therefore, despite the increasing availability of early reperfusion techniques, novel pharmacological treatments to improve the neurological outcome of patients with AIS remain an unmet clinical need.
Junctional protein associated with coronary artery disease (JCAD), also known as uncharacterized protein KIAA1462, is a recently discovered protein associated with tight junctions.4 Genetic variants of this protein were associated with an increased risk of cardiovascular diseases in different genome-wide association studies and preclinical investigations.5, 6, 7 JCAD colocalizes with cadherin 5, also known as vascular endothelial (VE)-cadherin, in intercellular junctions and is mainly expressed by endothelial cells in microvessels and arteries, where it regulates cell survival, angiogenesis, and inflammation.8, 9, 10 Mechanistically, JCAD was shown to promote vascular inflammation by inhibiting the phosphorylation of the large tumor suppressor kinase 2, in response to shear stress.8,9 Furthermore, JCAD promotes neo-angiogenesis in tumoral tissue through the activation of the mitogen-activated protein kinase kinase (MAPKK) extracellular-signal regulated kinase (ERK).10 Recently, our research group demonstrated that JCAD promotes the endothelial expression of coagulation factor III, and subsequent arterial thrombosis, through the phosphoinositide-3-kinase (PI3K)/Akt pathway.11
In line with this recent evidence, JCAD holds promise as a potential therapeutic target for the treatment and prevention of cardiovascular diseases,12 including AIS. In this paper, the potential therapeutic role of JCAD in AIS was investigated in a murine model of brain ischemia/reperfusion (I/R) damage. The underlying molecular mechanisms were then confirmed in human brain microvascular endothelial cells (HBMVECs), and finally, the translational relevance of JCAD in AIS was explored in human patients suffering from AIS.
Methods
A detailed description of the methods is provided in the Supplemental Appendix.
Animals
All experiments were conducted in accordance with the Swiss federal guidelines for the use of animals in research and were approved by the Cantonal Veterinary Office of Zurich in Switzerland (license ZH101/20). Male and female mice on a C57BL/6 background were employed. Genetically modified mice were generously donated by Prof Zheng Gen Jin, University of Rochester School of Medicine and Dentistry.5
Two lines of genetically modified mice were used: global JCAD knockout (Jcad−/−) and endothelial-specific knockout (eJCAD−/−). Controls for Jcad−/− mice were wild-type littermates (Jcad+/+) (Supplemental Figures 1A and 1B), whereas control mice for eJCAD−/− mice were Jcad “floxed” mice (Jcadfl/fl) (Supplemental Figures 1C and 1D). All experimenters were blinded to the genotype of the animals until the results were unmasked.
Transient Middle Cerebral Artery Occlusion
Acute brain I/R injury was induced by performing transient middle cerebral artery occlusion (tMCAO), as previously described.13,14 To assess the neurological status, mice were scored according to the Bederson scale before the surgery and 2, 24, and 48 hours after tMCAO.15 Motor coordination was measured by the Rota Rod test, as previously described.13,16 Stroke size was assessed postmortem by 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma-Aldrich) staining, as previously described.16,17
Cells
In vitro experiments were conducted on primary HBMVECs (Cell Systems).
In vivo and in vitro JCAD silencing
In vivo and in vitro silencing of JCAD was achieved by the administration of a small interfering RNA (siRNA) (Supplemental Figures 1E and 1F). The mouse-specific JCAD siRNA and the scramble RNA control for in vivo silencing were purchased from Santa Cruz Biotechnology (sc-146454). The human-specific JCAD siRNA and the scramble control for in vitro silencing were purchased from Dharmacon (siGENOME Human JCAD siRNA-SMARTpool). JetPEI (Polyplus) and Lipofectamine RNAiMAX (Invitrogen, Thermo Fisher Scientific) were used as transfection reagents for in vivo and in vitro silencing, respectively.
In vivo and in vitro PI3K/Akt inhibition
The PI3K/Akt inhibitor wortmannin (Enzo Life Sciences) was used to confirm the role of this molecular pathway as downstream effector of JCAD, as previously described.11
In vitro hypoxia/reoxygenation
Hypoxia/reoxygenation (H/R) injury was induced in HBMVECs as follows: cells seeded in 6-well plates (200,000 cells/plate) were incubated in a glove incubation chamber (Invivo2 400, Baker Ruskinn) under hypoxic conditions at 37 °C (0.2% O2, 5% CO2). Cells were kept under hypoxic conditions for 4 hours, then oxygen supply was restored for 4 hours.
Transendothelial electric resistance measurement
Transendothelial electric resistance (TEER) measures the resistance of a cell monolayer as an indicator of its physical and functional integrity.18 TEER was measured using the Z Theta system (Applied Biophysics). Cells were exposed to H/R injury or normoxia, as described in the previous section, “In Vitro Hypoxia/Reoxygenation.” Reoxygenation time was prolonged to 44 hours, for a total experiment duration of 48 hours.
Immunofluorescence staining on free-floating brain sections
Immunofluorescence staining of immunoglobulin G (IgG), JCAD, and vascular cell adhesion molecule 1 (VCAM1) was performed on brain sections as previously described.19
Cell death assessment
Cell death was measured as lactate dehydrogenase (LDH) concentration in the supernatant of HBMVECs, using a commercial kit (Cytotoxicity Detection Kit, Roche).
Western blot
After measuring total protein concentration in cell lysates, protein expression was assessed by Western blot analysis.
Quantitative real-time polymerase chain reaction
Total RNA was isolated from HBMVECs and the expression of JCAD messenger RNA was measured by real-time polymerase chain reaction.
Patients with AIS
In vivo and in vitro findings were validated in 2 independent cohorts of patients with AIS. Their study design and inclusion/exclusion criteria for have been previously described.13,20, 21, 22 Both cohort studies were approved by respective institutional ethics committees (Ethics Committee of the “San Raffaele” Scientific Institute, Milan, Italy, prot. STROKEMARKERS01 and Ethics Committee of the University Hospital Basel, Basel, CH, prot. EKBB#157/06) and were conducted in compliance with the 1964 Declaration of Helsinki and its later amendments. All participants provided written informed consent.
Circulating levels of JCAD were measured by enzyme-linked immunosorbent assay (MBS9317264, MyBioSource), as previously reported.11 Both inter- and intra-assay coefficients of variation were <15%.
Statistical analysis
The statistical analysis was conducted using the SPSS software package, version 29 (IBM), GraphPad Prism 8 (GraphPad Software), and R 4.1.2 (R Foundation for Statistical Computing). Statistical methods were previously described.23,24 A detailed description is provided in the Supplemental Appendix.
Results
Endothelial JCAD worsens brain ischemic damage in a murine model of AIS
The effects of acute brain ischemia and early reperfusion were modeled in vivo by tMCAO in mice. Jcad−/− mice had a smaller ischemic lesion volume compared with their littermate control mice (Figures 1A and 1B), with a comparable perilesional edema (Supplemental Figure 2A) 48 hours after tMCAO. Consistently, Jcad−/− mice had a better neurological status, as shown by the lower Bederson score, without significant differences in motor coordination, as explored by the RotaRod test (Figures 1C and 1D, Supplemental Figure 2B). Since JCAD is mainly expressed in endothelial cells,8, 9, 10 the tMCAO experiment was repeated in eJcad−/− mice to confirm the endothelial-specific effect of JCAD following brain I/R injury. In detail, the reduced ischemic volume in eJcad−/− mice, as compared with control mice, was confirmed (Figures 1E and 1F) with a significant difference in perilesional edema (Supplemental Figure 2C). In line with this, eJcad−/− mice also displayed a better neurological performance without significant differences in motor coordination (Figures 1G and 1H, Supplemental Figure 2D). Ultimately, the role of JCAD as a potential pharmacological target was tested by intravenously administering a siRNA against JCAD upon reperfusion. Postischemic JCAD silencing yielded comparable results to genetic deletion of JCAD, namely a significant reduction of ischemic volume (Figures 1I and 1J), without a significant difference in perilesional edema (Supplemental Figure 2E). Interestingly, postischemic silencing of JCAD translated into better motor coordination (Figure 1K) without a significant difference in neurological status (Figure 1L, Supplemental Figure 2F).
Figure 1.
Role of JCAD Expression in a Murine Model of Acute Brain Ischemia/Reperfusion by Transient Middle Cerebral Artery Occlusion
Effect of total JCAD knockout (ko) (Jcad−/−) on ischemic volume (A and B), motor coordination, assessed by RotaRod test (C), and neurological status, assessed by Bederson scale score (D) Wild-type (Jcad+/+) littermates were used as control mice; n = 7 Jcad−/− mice; n = 6 Jcad+/+ mice. Effect of endothelial-specific JCAD knockout (eJcad−/−) on ischemic volume (E and F), motor coordination (G), and neurological status (H). Jcad “floxed” (Jcadfl/fl) littermates were used as control mice; n = 9 eJcad−/− mice; n = 7 Jcadfl/fl mice. Effect of postischemic JCAD silencing by small interfering RNA (siJCAD) on ischemic volume (I and J), motor coordination (K), and neurological status (L). RNA scramble (siSCR)-treated littermates were used as control mice; n = 7 siJCAD mice; n = 7 siSCR mice. Data are presented as mean ± SEM. Student’s t-test (A, E, I) and 2-way repeated measures analysis of variance with Sidak’s correction for multiple comparisons (C, D, G,H, K, and L); ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001.
The aforementioned experiments were all performed in male mice, to reduce the variability due to sex differences. To confirm the detrimental role of JCAD in brain ischemia in females, tMCAO experiment was conducted in female Jcad−/− mice, reporting comparable results in terms of ischemic lesion volume (Supplemental Figures 3A and 3B), perilesional edema (Supplemental Figure 3C), motor coordination (Supplemental Figure 3D), and neurological status (Supplemental Figures 3E and 3F). Altogether, targeting JCAD in male and female mice reduces stroke-related disability and lesion volume.
JCAD promotes cell death and endothelial permeability after H/R injury
Cellular and molecular mechanisms mediated by JCAD in endothelial cells after an ischemic injury were explored in HBMVECs undergoing a H/R injury consisting of 4-hour hypoxia and then 48-hour reoxygenation. The expression of JCAD was inhibited by siRNA for 6 hours, with an 88% reduction of gene transcription (Supplemental Figure 4A) and protein expression (Supplemental Figure 4B) 24 hours after the silencing. JCAD silencing was not associated with an increased cell death (Supplemental Figure 4C).
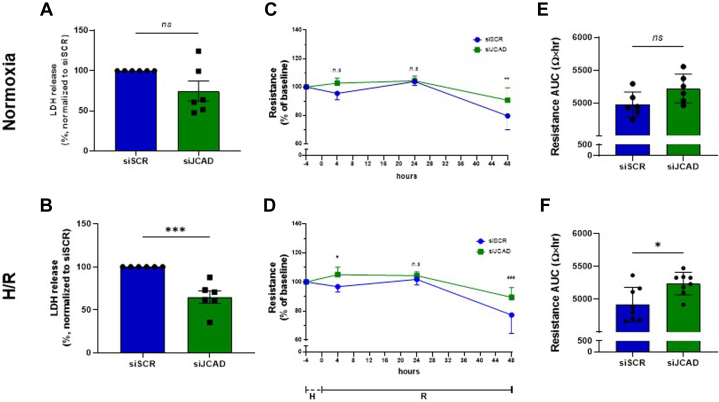
Compared with control cells, JCAD silenced cells had a lower death rate after H/R injury. This difference was not observed in stable normal O2 conditions, suggesting a promoting role for JCAD in H/R-induced endothelial damage (Figures 2A and 2B). Next, the endothelial barrier function was assessed as TEER, showing a preserved monolayer integrity after 48 hours in JCAD-silenced cells, both in H/R and in normal O2 (Figures 2C and 2D). However, when the endothelial resistance was measured throughout the entire observation time (area under the curve), the difference reached statistical significance only in cells undergoing H/R injury (Figures 2E and 2F). In summary, this finding suggests that JCAD silencing confers resistance to H/R injury in HBMVECs.
Figure 2.
Effect of H/R Injury in Human Brain Microvascular Endothelial Cells After JCAD Silencing
Cells were exposed to normoxia (A, C, and E) or hypoxia/reoxygenation (H/R) injury (B, D, and F). Cell death rate, assessed as lactic dehydrogenase (LDH) release in silenced (siJCAD) and unsilenced (siSCR) cells (A and B). Endothelial monolayer integrity, assessed as trans-endothelial electric resistance, in silenced and unsilenced cells (C and D, and E and F). Data are presented as mean ± SEM. n = 6, Student’s t-test (A, B, E, and F) and 2-way repeated measures analysis of variance with Sidak’s correction for multiple comparisons (C and D); ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001. AUC = area under the curve; other abbreviations as in Figure 1.
JCAD promotes endothelial damage by inhibiting the PI3/Akt pathway
Mechanistically, the deleterious effects of JCAD on the vascular endothelium could be either the effect of: 1) a passive leakage of the endothelial monolayer, due to a disruption of the tight junctions; or 2) actively increased permeability, mediated by the inflammation. These hypotheses were tested in vitro, and no significant difference in the expression of tight junction proteins (VE-cadherin, occludin, and claudin 5) was observed after H/R injury or in normal O2 conditions (Figures 3A to 3C). Conversely, a reduced expression of VCAM1 was observed after H/R injury in JCAD-silenced cells compared with control cells (Figure 3D). No significant difference in intercellular adhesion molecule 1 (ICAM1) expression was observed between silenced and scramble transfected cells (Figure 3E). The blood-brain barrier (BBB) permeability was explored by immunofluorescence in eJcad−/− mice and control littermates, after tMCAO. A smaller area of IgG extravasation was observed in eJcad−/− mice (Figures 4A and 4B). Based on the above-reported observations in HBMVECs, the effect on BBB permeability was attributed to a reduced expression of pro-inflammatory molecules, as confirmed by a reduced VCAM1 expression in the ischemic penumbra of eJcad−/− animals (Figures 4C and 4D).
Figure 3.
Molecular Effects of JCAD Silencing in Human Brain Microvascular Endothelial Cells Exposed to Nx or H/R Injury
Protein expression was assessed by Western Blot. Vascular endothelial cadherin (VE-cadherin) (A), occludin (B), claudin 5 (C), vascular cell adhesion molecule (VCAM)-1 (D), and intercellular adhesion molecule (ICAM)-1 (E). Data are presented as mean ± SEM. n = 6, 1-way analysis of variance with Tukey’s correction for multiple comparisons; ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001. Nx = normoxia; other abbreviations as in Figures 1 and 2.
Figure 4.
Role of Endothelial JCAD Expression in the Pathophysiology of Acute Brain Ischemia
Acute brain ischemia has been reproduced in mice with endothelial-specific JCAD deletion (eJcad−/−) by transient middle cerebral artery occlusion. Effect of endothelial JCAD deletion on blood-brain barrier integrity, assessed by extravascular IgG immunostaining (A and B) after transient middle cerebral artery occlusion. Effect of endothelial JCAD deletion on endothelial inflammation, assessed by immunostaining for the vascular endothelial cell adhesion molecule (VCAM)-1 and the endothelial marker CD31 (C and D). Jcad “floxed” (Jcadfl/fl) littermates were used as control mice. Data are presented as mean ± SEM. Student’s t-test, n = 8 eJcad−/− mice; n = 7 Jcadfl/fl mice. ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001. ko = knockout.
Potential molecular mechanisms underlying these observations were investigated with a targeted approach, based on the existing literature.10,11 Akt phosphorylation was increased in JCAD silenced cells, after both H/R and normoxia (Figure 5A), whereas no difference was observed in the p38 and ERK phosphorylation (Figures 5B and 5C). The evidence collected so far suggests that JCAD promotes cell death and inflammation upon H/R injury through the inhibition of the PI3K/Akt pathway.
Figure 5.
Role of the PI3K/Akt Signaling in Mediating the Physiological Effect of JCAD in Acute Brain Ischemia
Human brain microvascular endothelial cells were exposed to Nx or H/R injury. Protein expression was assessed by Western blot analysis. Phosphorylation of Akt (A), ERK (B), and p38 (C) in siJCAD and siSCR human brain microvascular endothelial cells; n = 6, 1-way analysis of variance. Endothelial monolayer integrity, assessed as transendothelial electric resistance, in unsilenced cells, silenced cells, and silenced cells treated with the PI3K/Akt inhibitor wortmannin (D to G); n = 6, 2-way analysis of variance (D and F) and Student’s t-test (E and G). Acute brain ischemia has been reproduced in Jcad “floxed” (Jcadfl/fl), endothelial-specific JCAD knockout mice (eJcad−/−), and eJcad−/− mice treated with wortmannin by transient middle cerebral artery occlusion (H and I). Data are presented as mean ± SEM. One-way analysis of variance with Tukey’s correction for multiple comparisons (A to C, E, G, and H) and 2-way repeated measures analysis of variance with Sidak’s correction for multiple comparisons (D and F); n = 8 eJcad−/− + wortmannin mice; n = 7 eJcad−/− + vehicle; n = 7 Jcadfl/fl mice. ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001. Abbreviations as in Figure 1, Figure 2, Figure 3.
To confirm this hypothesis, we wanted to test whether the inhibition of the PI3K/Akt pathway by wortmannin could abolish the protective effect of JCAD knock-down in H/R injury. In HBMVECs, the expression of JCAD was silenced by siRNA, as described in the preceding text. Cells were then treated with the PI3K/Akt inhibitor before undergoing H/R injury, as described in the preceding text. When measuring endothelial integrity by TEER, PI3K/Akt inhibition restored the unsilenced phenotype after both H/R and normoxia (Figures 5D to 5G). To confirm the in vivo relevance of this mechanism, wortmannin was administered to eJcad−/− mice before tMCAO. In line with results obtained in vitro, wortmannin partially restored the native wild-type phenotype in terms of ischemic volume (Figures 5H and 5I).
Circulating levels of JCAD predict 90-day mortality in patients with AIS
To confirm the translational relevance of JCAD in the pathophysiology of AIS, circulating JCAD was measured in 2 cohorts of patients presenting AIS.
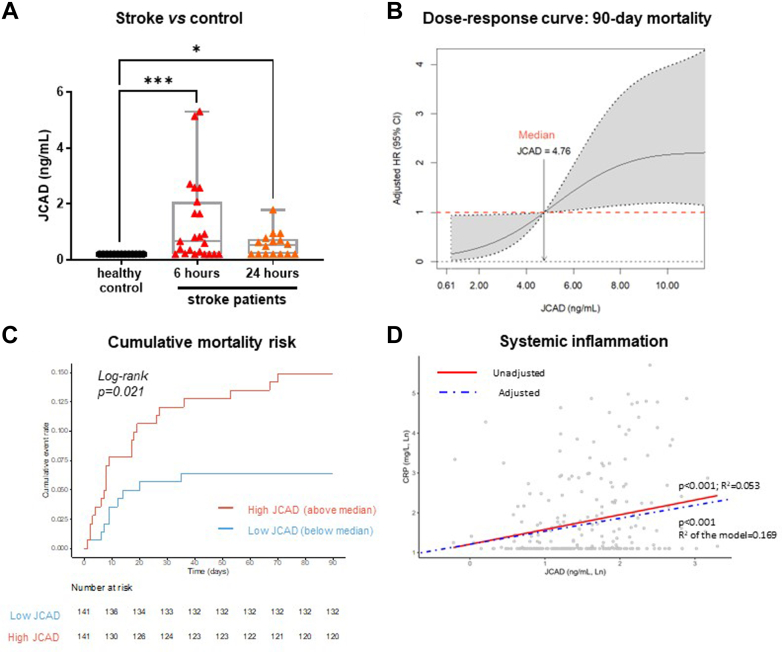
The first cohort (n = 23) was compared with a control population (n = 18) without acute neurological symptoms, matched for age, sex, and cardiovascular risk factors. The demographic, clinical, and biochemical features of patients and control subjects were previously reported.22 Whereas subjects in the control group had circulating JCAD levels below the limit of detection, patients with AIS displayed persistent elevated levels of circulating JCAD 6 and 24 hours after the onset of symptoms (Figure 6A).
Figure 6.
Circulating Levels of JCAD in Patients With Acute Ischemic Stroke
Circulating levels of JCAD assessed in control subjects (n = 18) and in patients with acute ischemic stroke (n = 23) 6 and 24 hours after the onset of symptoms. Data are presented as median, maximum, minimum, and Q1-Q3. Kruskal-Wallis test with Dunn’s correction was used for multiple comparisons; ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001 (A). Nonlinear relationship between circulating JCAD levels and 90-day mortality. The dashed horizontal line indicates no effect with median JCAD values serving as reference. For visual clarity, the dose-response curve has been truncated at the 90th percentile of the JCAD distribution. The model has been adjusted for age, National Institutes of Health Stroke Scale (NIHSS) at admission, CRP, and history of heart disease. Covariates are all dichotomized (B). Kaplan-Meier curve depicting the survival of patients with high vs low circulating levels of JCAD (C). Linear relationship between JCAD and CRP. Linear regression was adjusted for NIHSS (dichotomized), history of heart disease, and heart rate (D).
Demographic, clinical, and biochemical features of the second cohort of patients are reported in Table 1. The 90-day overall mortality in this cohort was 11.9% (30 events). A strong independent association was observed between 90-day mortality and circulating JCAD, measured within 48 hours from the onset of neurological symptoms (Figure 6B). Patients with higher values of circulating JCAD also had a higher risk of death during the follow-up (Figure 6C). The association between circulating JCAD was independent of established risk factors, namely age, clinical severity at the admission (measured as National Institute of Health Stroke Scale [NIHSS]), concomitant presence of heart diseases, and C-reactive protein (CRP) levels (Supplemental Table 1). Interestingly, circulating JCAD and CRP were significantly correlated (Table 2), and in a linear regression model including NIHSS, the concomitant presence of heart disease, heart rate, high-density lipoprotein cholesterol, and white blood cells count, JCAD was an independent predictor of circulating CRP, in line with in vivo and in vitro findings (Figure 6D, Supplemental Table 2). Because CRP is a well-established biomarker of systemic inflammation and cardiovascular risk,25 the observed association between JCAD and mortality after AIS could be mediated by systemic inflammation. This hypothesis was confirmed by a mediation analysis (Supplemental Figure 5). Altogether in AIS patients, JCAD is increased after stroke and increased JCAD levels are associated with mortality risk after stroke.
Table 1.
Demographic, Clinical, and Biochemical Features of the Population at Hospital Admission With Acute Ischemic Stroke According to Mortality Status
| Total (N = 282) | 90-Day Death |
P Value | ||
|---|---|---|---|---|
| No (n = 252) | Yes (n = 30) | |||
| Male | 110 (39.0) | 96 (38.1) | 14 (46.7) | 0.43 |
| Age, y | 75 (63-82) | 74 (61-81) | 83 (78-87) | <0.001 |
| Age quartiles, y | ||||
| 18-61 | 75 (23.7) | 74 (98.7) | 1 (0.3) | <0.001 |
| 62-75 | 76 (24.1) | 72 (94.7) | 4 (5.3) | |
| 76-81 | 76 (24.1) | 66 (86.8) | 10 (13.2) | |
| ≥82 | 89 (28.2) | 69 (77.5) | 20 (22.5) | |
| Medical history | ||||
| Smoker | 97 (34.4) | 89 (34.9) | 9 (30.0) | 0.69 |
| Hypertension | 212 (75.2) | 189 (75.0) | 23 (76.7) | 0.99 |
| Diabetes mellitus | 53 (18.8) | 47 (18.7) | 6 (20.0) | 0.81 |
| Hypercholesterolemia | 71 (25.2) | 61 (24.2) | 10 (33.3) | 0.27 |
| Heart disease | 122 (43.3) | 100 (39.7) | 22 (73.3) | <0.001 |
| Peripheral artery disease | 21 (7.4) | 19 (7.5) | 2 (6.7) | 0.99 |
| Clinical features | ||||
| NIHSS | 5 (3-10) | 4 (2-8) | 15 (8-25) | <0.001 |
| Biochemical features | ||||
| Fasting blood glucose, mmol/L | 6.1 (5.5-7.5) | 6.0 (5.4-7.4) | 6.2 (6.0-7.7) | 0.34 |
| Creatinine, μmol/L | 75.0 (62.5-88.5) | 75.0 (62.0-88.0) | 80.5 (63.5-93.8) | 0.18 |
| Total cholesterol, mmol/L | 4.4 (3.7-5.1) | 4.4 (3.8-5.2) | 4.1 (3.3-4.8) | 0.14 |
| HDL-cholesterol, mmol/L | 1.3 (1.1-1.6) | 1.3 (1.1-1.6) | 1.3 (1.0-1.7) | 0.97 |
| LDL-cholesterol, mmol/L | 2.4 (1.8-3.0) | 2.4 (1.8-3.1) | 2.0 (1.6-2.9) | 0.30 |
| C-reactive protein, mg/dL | 3.2 (3.0-8.7) | 3.1 (3.0-8.6) | 13.1 (3.8-37.5) | <0.001 |
Values are n (%) or median (Q1-Q3). Significant differences are highlighted in bold.
HDL = high-density lipoprotein; LDL = low-density lipoprotein; NIHSS = National Institute of Health Stroke Scale.
Table 2.
Demographic, Clinical, and Biochemical Features Associated With Circulating JCAD
| ρ | P Value | |
|---|---|---|
| Age | 0.072 | 0.24 |
| Sex | 0.031 | 0.61 |
| Medical history | ||
| Smoker | 0.009 | 0.88 |
| Hypertension | 0.083 | 0.18 |
| Diabetes mellitus | −0.061 | 0.32 |
| Hypercholesterolemia | −0.057 | 0.35 |
| Heart disease | 0.133 | 0.029 |
| Peripheral artery disease | 0.023 | 0.71 |
| Clinical features | ||
| NIHSS, >5 vs ≤5 | 0.136 | 0.025 |
| Heart rate | 0.209 | 0.001 |
| Systolic blood pressure | −0.025 | 0.70 |
| Diastolic blood pressure | −0.026 | 0.69 |
| Biochemical features | ||
| White blood cells | 0.199 | 0.001 |
| Fasting blood glucose | 0.118 | 0.065 |
| Creatinine | −0.034 | 0.58 |
| Total cholesterol | −0.114 | 0.075 |
| HDL-cholesterol | −0.133 | 0.037 |
| LDL-cholesterol | −0.101 | 0.12 |
| C-reactive protein | 0.223 | <0.001 |
Significant Spearman correlations are highlighted in bold.
Abbreviations as in Table 1.
DISCUSSION
The herein-described results show that JCAD plays a detrimental role in acute brain I/R injury in mice. This effect is mediated by endothelial JCAD as confirmed by the experiments in the eJcad−/− mice. This finding is in line with previous studies reporting that JCAD is mainly expressed in vascular endothelial cells.7,26 Importantly, postischemic silencing of JCAD resulted in a significant reduction of ischemic brain volume, comparable to the genetic deletion of the protein. This suggests that JCAD has an active role during acute brain ischemia and the subsequent reperfusion phase. More importantly, the deleterious effect of JCAD can be pharmacologically blocked during reperfusion, making it a potential therapeutic target to restrain reperfusion injury on top of thrombolysis or mechanical thrombectomy.
Mechanistically, the activation of JCAD upon I/R damage leads to increased permeability of the BBB, as a consequence of vascular inflammation, as suggested by the reduced expression of VCAM1 in eJcad−/− mice. This result is in line with previous evidence from transcriptomic analysis in human coronary artery endothelial cells.5
In vitro results show that JCAD impairs endothelial monolayer integrity after H/R injury, inducing cellular death and inflammation through the PI3K/Akt pathway. More in details, H/R injury activates JCAD, leading to Akt dephosphorylation. Because dephosphorylated Akt cannot translocate into the nucleus, its transcriptional activity is blocked. The PI3K/Akt pathway plays a paramount role in cell proliferation and survival by inhibiting apoptosis and promoting the cell cycle progression.27 Accordingly, inhibition of this pathway produces an imbalance between proapoptotic and antiapoptotic signals, resulting in a net prevalence of cell death. The role of Akt in inflammation is controversial, as it is proinflammatory in immune cells, such as lymphocytes and macrophages, where it induces proliferation and differentiation towards proinflammatory phenotypes.28,29 Conversely, the activation of the PI3K/Akt system has shown anti-inflammatory effects in the central nervous system.30
The herein presented results suggest that JCAD is activated in response to the H/R injury in brain endothelial cells. In turn, JCAD inhibits the PI3K/Akt signaling causing inflammation and programmed cell death, eventually leading to the increased permeability of the BBB and the progression of the H/R damage. The role of JCAD as a regulator of the PI3K/Akt pathway was previously demonstrated in the context of shear-stress mediated atherosclerosis and arterial thrombosis.8,11 In the current setting of brain I/R injury, the causal role of the PI3K/Akt pathway was confirmed by the retrieval of the unsilenced phenotype in JCAD silenced cells, as well as in eJcad−/− mice treated with the PI3K/Akt inhibitor wortmannin.
The exact molecular mechanism by which JCAD interacts with the PI3K/Akt pathway still needs to be elucidated. To the best of current knowledge, no phosphatase activity has been demonstrated for JCAD; however, we previously showed that JCAD colocalizes with Akt,11 suggesting that JCAD may prevent the phosphorylation of Akt via competitive or noncompetitive occupation of the phosphorylation site.
Similarly, the mechanism linking hypoxia to JCAD activity still needs to be further elucidated. Since no hypoxia-responsive element has been reported in the JCAD promoter, a transcriptional effect seems unlikely. To date, JCAD is described as an adaptor protein between the tight junctions and the cytoskeleton.4,10 Indeed, in its N-terminal region, JCAD presents a homology site with the adaptor proteins Rho-associated protein kinases, which confers affinity to the junctional protein VE-cadherin.7,9 Hence, the loss of interaction between the tight junctions and the cytoskeleton could be the event triggering the activation of JCAD. In other words, in healthy endothelial cells, JCAD is bound to VE-cadherin and the cytoskeleton. However, following cellular injury disrupting the cytoskeleton integrity or cell-to-cell adhesion, JCAD is released in the cytosol, where it promotes Akt dephosphorylation. Finally, the relationship between hypoxia and the PI3K/Akt pathway is quite complex. Briefly, the PI3K/Akt signaling promotes the expression of the hypoxia inducible factors, the proteins orchestrating the cellular response to hypoxia.31 Therefore, the activation of this pathway has paramount importance in response to the H/R injury. Nevertheless, the induction of the PI3K/Akt in response to hypoxia has been observed only in some cell types.32 In light of this previous evidence, JCAD may represent an endogenous modulator of the response to hypoxia in endothelial cells.
Finally, the current findings in cells and mice could be confirmed in human subjects with AIS. Circulating JCAD was increased following acute brain ischemia, in line with previous observations in patients with myocardial infarction.11 Furthermore, circulating JCAD is a predictor of mortality in patients with AIS, as high levels of JCAD were associated with a reduced 90-day survival beyond established risk factors (ie, age, clinical severity, concomitant heart disease, and systemic inflammation). The clinical data also confirmed the strong association of JCAD with neurological impairment, as assessed by the NIHSS, as well as systemic inflammation, as reflected by CRP and white blood cell count. In particular, CRP is a well-established prognostic factor in AIS,33 and the mediation analysis confirmed the association between JCAD and systemic inflammation. According to this evidence, JCAD may also have a role in clinical practice as prognostic factor, to improve risk stratification, or as a therapeutic target, to improve the prognosis of patients with AIS.
Study limitations
Any future application of JCAD as a disease biomarker or even as a therapeutic target would need appropriate validation in larger cohorts and dedicated clinical trials. Furthermore, the mechanistic explanations herein provided may be cautiously considered, because the exact biological function of JCAD still needs to be further elucidated. In particular, JCAD seems to have pleiotropic effects on multiple intracellular signaling pathways; hence, the observed effect on brain ischemia could be mediated by unaddressed mechanisms, beyond the described PI3K/Akt pathway. This is consistent with the finding of a partial phenotype rescue by the administration of wortmannin in vivo.
Conclusions
The present study shows for the first time to our knowledge that JCAD is actively involved in the endothelial response to hypoxia during acute brain ischemia, where it worsens the I/R damage by inducing cell death and inflammation. Human data confirm that circulating JCAD is a predictor of mortality in patients with AIS.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: JCAD is a membrane-associated protein, mainly expressed by endothelial cells, mediating the endothelial response to acute hypoxia in the brain through the PI3K/Akt pathway. JCAD activation results in inhibition of cell survival and expression of proinflammatory membrane receptor, thus aggravating the ischemia/reperfusion brain damage.
TRANSLATIONAL OUTLOOK: The recent introduction of siRNA technology for therapeutic uses makes JCAD a suitable pharmacological target to improve the outcome of patients with AIS, holding promise as a future add-on therapy on top of early brain reperfusion. Circulating JCAD may also represent a potential novel prognostic biomarker in AIS, for which no established prognostic biomarker has been so far implemented in clinical practice.
Funding Support and Author Disclosures
This work was funded by Swiss Heart Foundation grant FF22014/2022 (Dr Ministrini), the Novartis Foundation for Medical-Biological Research grant 21B070 (Prof Liberale), and Swiss National Science Foundation grant 501100001711-197510] (Prof Camici). Dr Puspitasari is the recipient of a Forschungskredit Candoc grant from the University of Zurich and a grant from Swiss Life Foundation for Public Health and Medical Research. Dr Kraler has received institutional research grants from the Jubiläumsstiftung SwissLife, the Lindenhof Foundation, the Novartis Foundation for Medical-Biological Research, the Swiss Heart Foundation, the Swiss Society of Cardiology, and the Theodor-Ida-Herzog-Egli Foundation; has received equipment and materials from Roche Diagnostics outside the submitted work; and has received travel support from the European Atherosclerosis Society, the European Society of Cardiology, the European Society of Clinical Investigation, Sphingotec GmbH, the 4TEEN4 Pharmaceuticals GmbH, and PAM Theragnostics GmbH. Dr Wenzl has received financial support from the Foundation for Cardiovascular Research-Zurich Heart House, the Lindenhof Foundation, the European Society of Cardiology, the Swiss Heart Foundation, the Fonds zur Förderung des akademischen Nachwuchses of the University of Zurich, the Medical University of Graz, the Theodor-Ida-Herzog-Egli Foundation, the Sphingotec GmbH, the 4TEEN4 Pharmaceuticals GmbH, and the PAM Theragnostics GmbH outside this work. Prof Katan has received funding from the Swiss National Science Foundation (Project Nr. 182267 and Project Nr. 213471), the Swiss Heart Foundation, and in kind contributions from ROCHE Diagnostics and BRHAMS Thermofisher, outside the submitted work. Prof Montecucco is the recipient of Rete CARDIOLOGICA grant RCR-2022-23682288. Work supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006) - (DN. 1553 11.10.2022) to Prof. Montecucco Prof Lüscher holds leadership positions at the European Society of Cardiology, Swiss Heart Foundation, and the Foundation for Cardiovascular Research-Zurich Heart House; has received institutional educational and research grants outside this work from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Daichi Sankyo, Eli Lilly, Novartis. Novo Nordisk, Sanofi, Servier, and Vifor; and has received consulting fees from Dacadoo, Novartis, Novo Nordisk, Pfizer, and Philips. Prof Camici and Prof Liberale are coinventors on the International Patent WO/2020/226993 filed in April 2020; the patent relates to the use of antibodies which specifically bind interleukin-1a to reduce various sequelae of ischemia-reperfusion injury to the central nervous system. Prof Camici is a consultant to Sovida Solutions limited; is the recipient of a Sheikh Khalifa’s Foundation Assistant Professorship at the Faculty of Medicine, University of Zurich; and has received financial support by the Alfred and Annemarie von Sick Grants for Translational and Clinical Research Cardiology and Oncology and by the Swiss Heart Foundation. Prof Liberale has received financial support from the Swiss Heart Foundation and the Novartis Foundation for Medical-Biological Research. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
Genetically modified mice were generously donated by Prof Zheng Gen Jin, University of Rochester School of Medicine and Dentistry (Rochester, NY, USA). Imaging was performed with equipment maintained by the Center for Microscopy and Image Analysis, University of Zurich.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section as well as supplemental figures and tables, please see the online version of this paper.
Appendix
References
- 1.Lozano R., Naghavi M., Foreman K., et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers W.J., Rabinstein A.A., Ackerson T., et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 3.Emberson J., Lees K.R., Lyden P., et al. Stroke Thrombolysis Trialists’ Collaborative Group Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Q8P266 – JCAD_HUMAN. UniProt. Accessed December 5, 2023 https://www.uniprot.org/uniprotkb/Q9P266/entry
- 5.Xu S., Xu Y., Liu P., et al. The novel coronary artery disease risk gene JCAD/KIAA1462 promotes endothelial dysfunction and atherosclerosis. Eur Heart J. 2019;40:2398–2408. doi: 10.1093/eurheartj/ehz303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erdmann J., Willenborg C., Nahrstaedt J., et al. Genome-wide association study identifies a new locus for coronary artery disease on chromosome 10p11.23. Eur Heart J. 2011;32:158–168. doi: 10.1093/eurheartj/ehq405. [DOI] [PubMed] [Google Scholar]
- 7.Akashi M., Higashi T., Masuda S., Komori T., Furuse M. A coronary artery disease-associated gene product, JCAD/KIAA1462, is a novel component of endothelial cell-cell junctions. Biochem Biophys Res Commun. 2011;413:224–229. doi: 10.1016/j.bbrc.2011.08.073. [DOI] [PubMed] [Google Scholar]
- 8.Douglas G., Mehta V., Al Haj Zen A., et al. A key role for the novel coronary artery disease gene JCAD in atherosclerosis via shear stress mechanotransduction. Cardiovasc Res. 2020;116:1863–1874. doi: 10.1093/cvr/cvz263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones P.D., Kaiser M.A., Ghaderi Najafabadi M., et al. JCAD, a gene at the 10p11 coronary artery disease locus, regulates Hippo signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2018;38:1711–1722. doi: 10.1161/ATVBAHA.118.310976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hara T., Monguchi T., Iwamoto N., et al. Targeted disruption of JCAD (Junctional Protein Associated With Coronary Artery Disease)/KIAA1462, a coronary artery disease-associated gene product, inhibits angiogenic processes in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2017;37:1667–1673. doi: 10.1161/ATVBAHA.117.309721. [DOI] [PubMed] [Google Scholar]
- 11.Liberale L., Puspitasari Y.M., Ministrini S., et al. JCAD promotes arterial thrombosis through PI3K/Akt modulation: a translational study. Eur Heart J. 2023;44(20):1818–1833. doi: 10.1093/eurheartj/ehac641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzik T.J., Channon K.M. JCAD: a new GWAS target to reduce residual cardiovascular risk? Eur Heart J. 2023;44:1834–1836. doi: 10.1093/eurheartj/ehac708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberale L., Gaul D.S., Akhmedov A., et al. Endothelial SIRT6 blunts stroke size and neurological deficit by preserving blood-brain barrier integrity: a translational study. Eur Heart J. 2020;41:1575–1587. doi: 10.1093/eurheartj/ehz712. [DOI] [PubMed] [Google Scholar]
- 14.Liberale L., Bonetti N.R., Puspitasari Y.M., et al. Postischemic administration of IL-1α neutralizing antibody reduces brain damage and neurological deficit in experimental stroke. Circulation. 2020;142:187–189. doi: 10.1161/CIRCULATIONAHA.120.046301. [DOI] [PubMed] [Google Scholar]
- 15.Bederson J.B., Pitts L.H., Tsuji M., Nishimura M.C., Davis R.L., Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 16.Spescha R.D., Klohs J., Semerano A., et al. Post-ischaemic silencing of p66Shc reduces ischaemia/reperfusion brain injury and its expression correlates to clinical outcome in stroke. Eur Heart J. 2015;36:1590–1600. doi: 10.1093/eurheartj/ehv140. [DOI] [PubMed] [Google Scholar]
- 17.Liberale L., Bonetti N.R., Puspitasari Y.M., et al. TNF-α antagonism rescues the effect of ageing on stroke: perspectives for targeting inflamm-ageing. Eur J Clin Invest. 2021;51 doi: 10.1111/eci.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivasan B., Kolli A.R., Esch M.B., Abaci H.E., Shuler M.L., Hickman J.J. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 2015;20:107–126. doi: 10.1177/2211068214561025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz-Cañestro C., Merlini M., Bonetti N.R., et al. Sirtuin 5 as a novel target to blunt blood-brain barrier damage induced by cerebral ischemia/reperfusion injury. Int J Cardiol. 2018;260:148–155. doi: 10.1016/j.ijcard.2017.12.060. [DOI] [PubMed] [Google Scholar]
- 20.Katan M., Fluri F., Schuetz P., et al. Midregional pro-atrial natriuretic peptide and outcome in patients with acute ischemic stroke. J Am Coll Cardiol. 2010;56:1045–1053. doi: 10.1016/j.jacc.2010.02.071. [DOI] [PubMed] [Google Scholar]
- 21.Liberale L., Ministrini S., Arnold M., et al. Serum circulating sirtuin 6 as a novel predictor of mortality after acute ischemic stroke. Sci Rep. 2022;12 doi: 10.1038/s41598-022-23211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akhmedov A., Bonetti N.R., Reiner M.F., et al. Deleterious role of endothelial lectin-like oxidized low-density lipoprotein receptor-1 in ischaemia/reperfusion cerebral injury. J Cereb Blood Flow Metab. 2019;39:2233–2245. doi: 10.1177/0271678X18793266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vo T.-T., Superchi C., Boutron I., Vansteelandt S. The conduct and reporting of mediation analysis in recently published randomized controlled trials: results from a methodological systematic review. J Clin Epidemiol. 2020;117:78–88. doi: 10.1016/j.jclinepi.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Wenzl F.A., Bruno F., Kraler S., et al. Dipeptidyl peptidase 3 plasma levels predict cardiogenic shock and mortality in acute coronary syndromes. Eur Heart J. 2023;44:3859–3871. doi: 10.1093/eurheartj/ehad545. [DOI] [PubMed] [Google Scholar]
- 25.Tracy R.P., Lemaitre R.N., Psaty B.M., et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17:1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 26.Shigeoka M., Arimoto S., Akashi M. JCAD expression and localization in human blood endothelial cells. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noorolyai S., Shajari N., Baghbani E., Sadreddini S., Baradaran B. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–128. doi: 10.1016/j.gene.2019.02.076. [DOI] [PubMed] [Google Scholar]
- 28.Fallone L., Walzer T., Marçais A. Signaling pathways leading to mTOR activation downstream cytokine receptors in lymphocytes in health and disease. Int J Mol Sci. 2023;24 doi: 10.3390/ijms241612736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acosta-Martinez M., Cabail M.Z. The PI3K/Akt pathway in meta-inflammation. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232315330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He X., Li Y., Deng B., et al. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: mechanisms and therapeutic opportunities. Cell Proliferation. 2022;55 doi: 10.1111/cpr.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Y., Shi X., Sheng K., et al. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review) Mol Med Rep. 2019;19:783–791. doi: 10.3892/mmr.2018.9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez-Tejado M., Alfranca A., Aragonés J., Vara A., Landázuri M.O., del Peso L. Lack of evidence for the involvement of the phosphoinositide 3-kinase/Akt pathway in the activation of hypoxia-inducible factors by low oxygen tension. J Biol Chem. 2002;277:13508–13517. doi: 10.1074/jbc.M200017200. [DOI] [PubMed] [Google Scholar]
- 33.VanGilder R.L., Davidov D.M., Stinehart K.R., et al. C-reactive protein and long-term ischemic stroke prognosis. J Clin Neurosci. 2014;21:547–553. doi: 10.1016/j.jocn.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.