Abstract
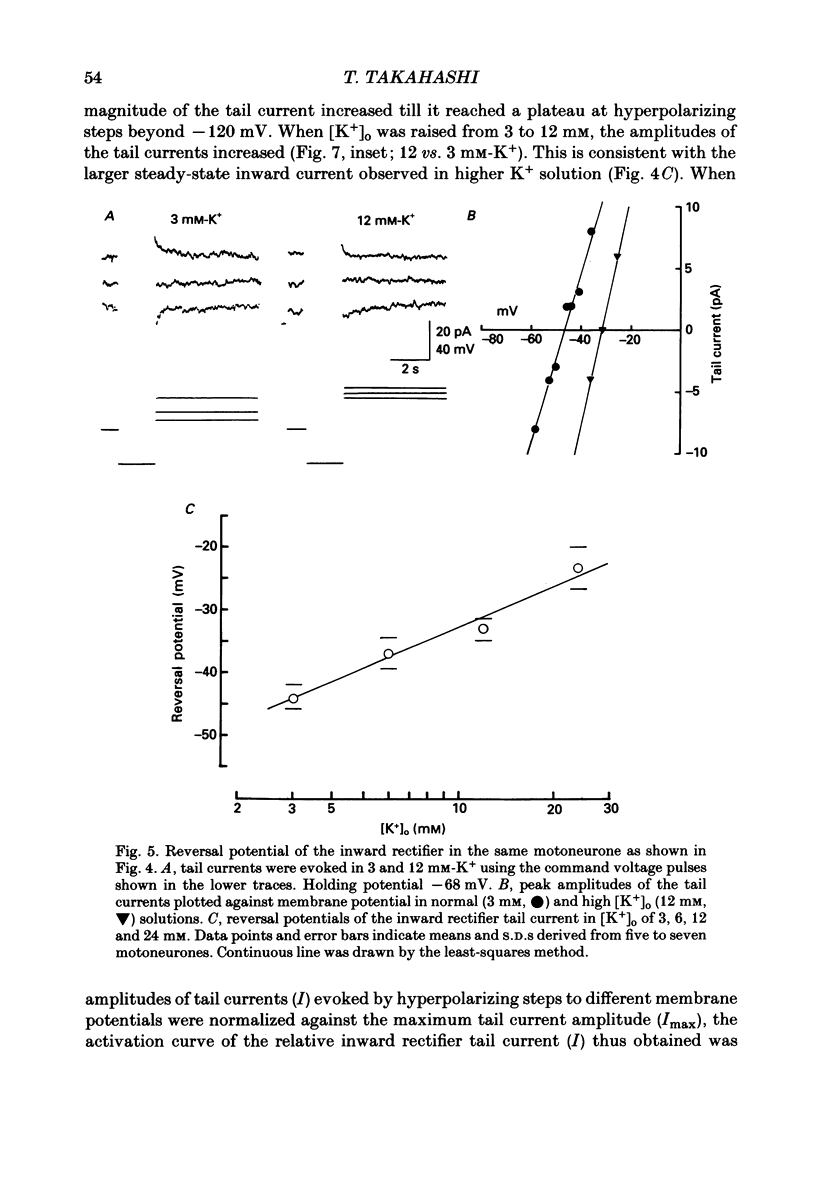
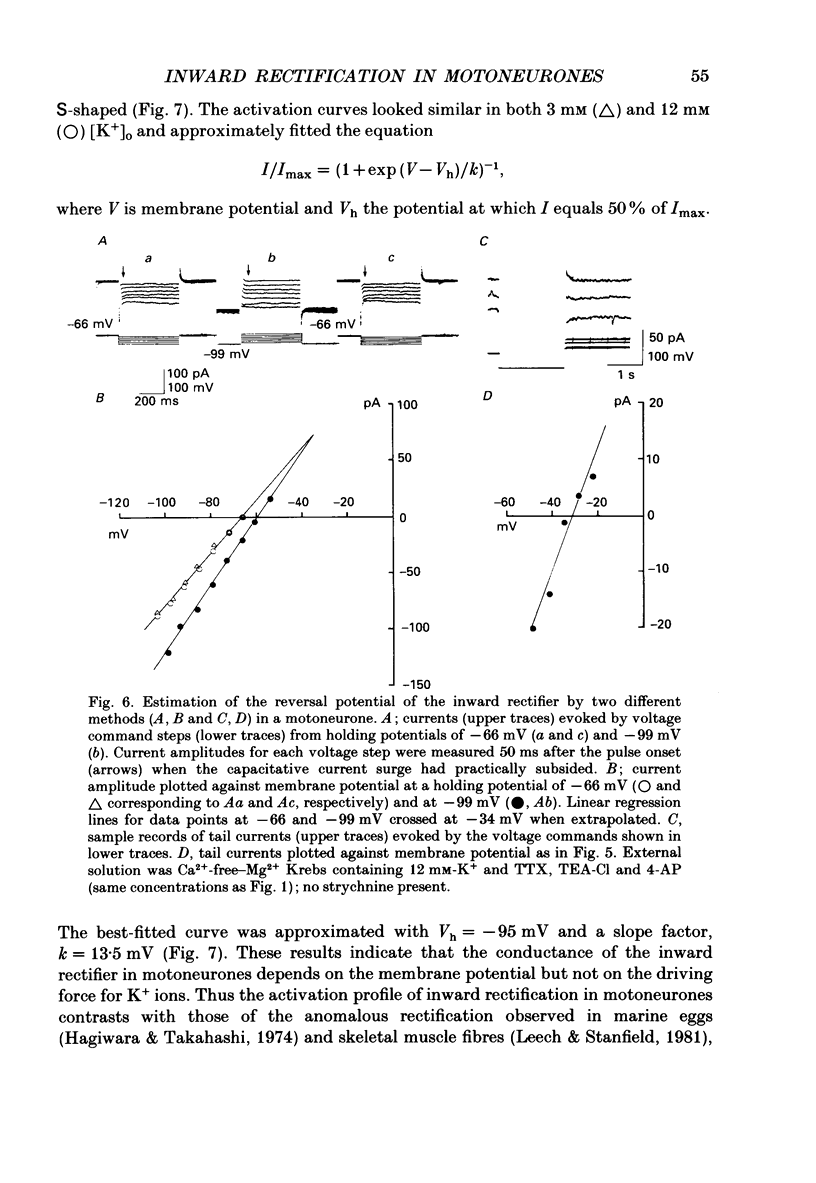
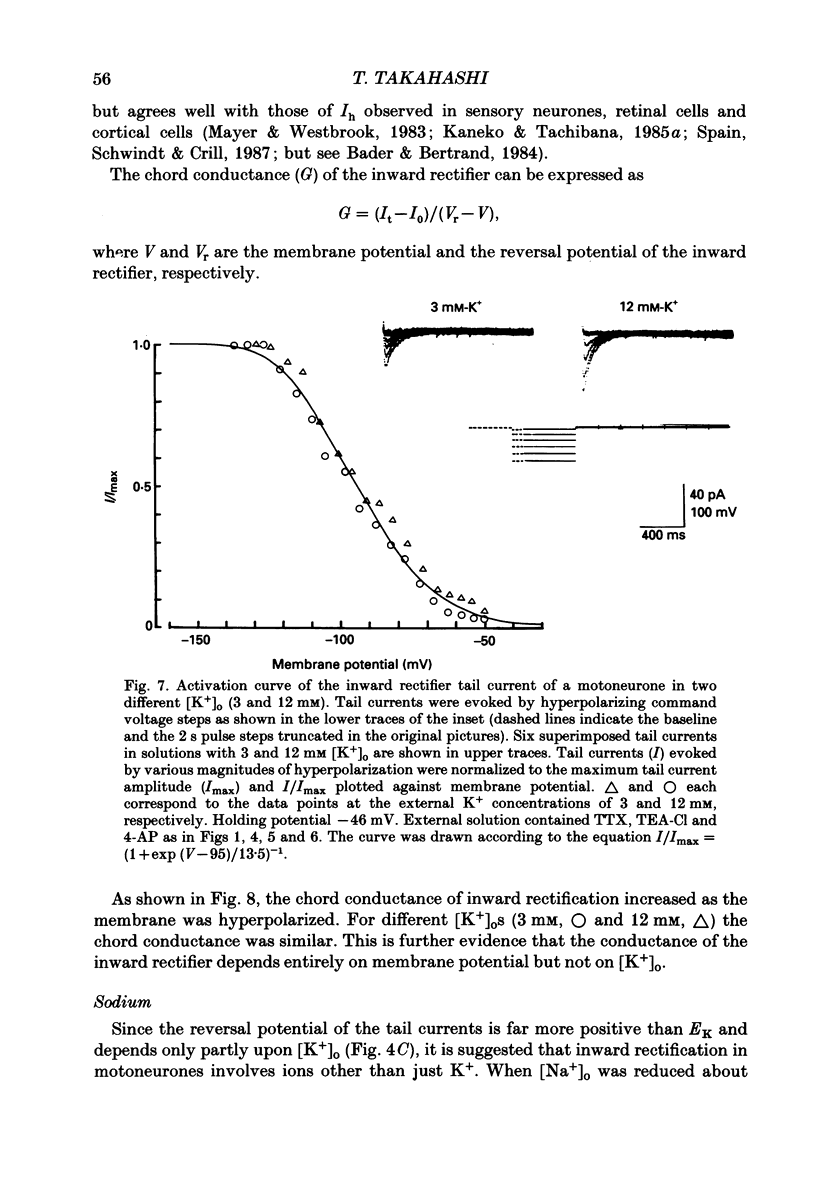
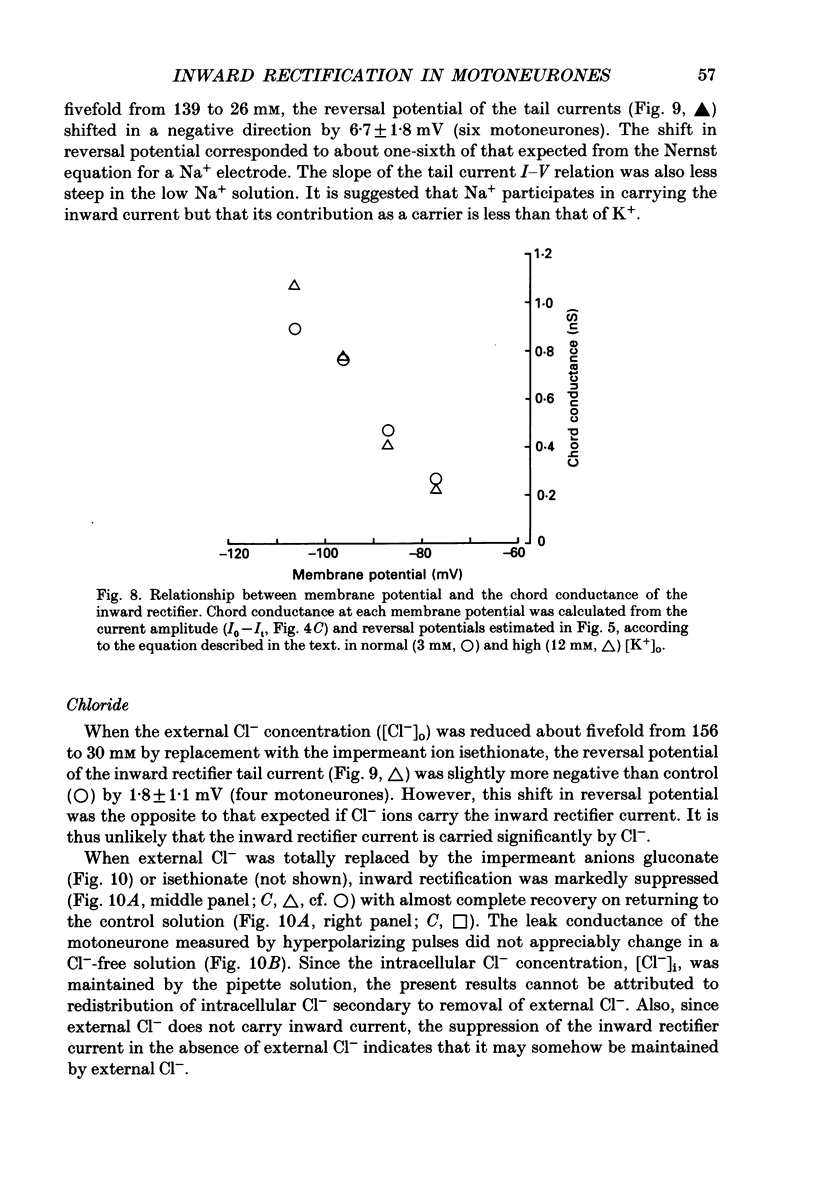
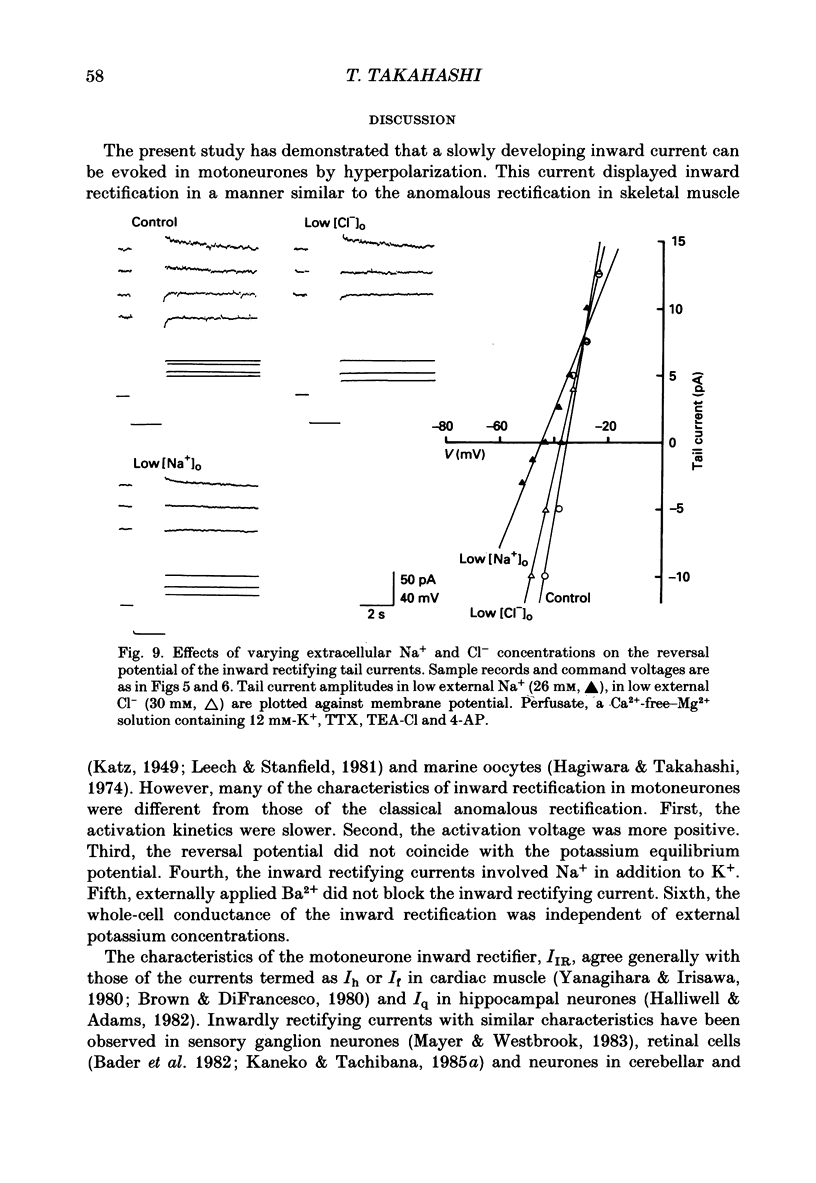
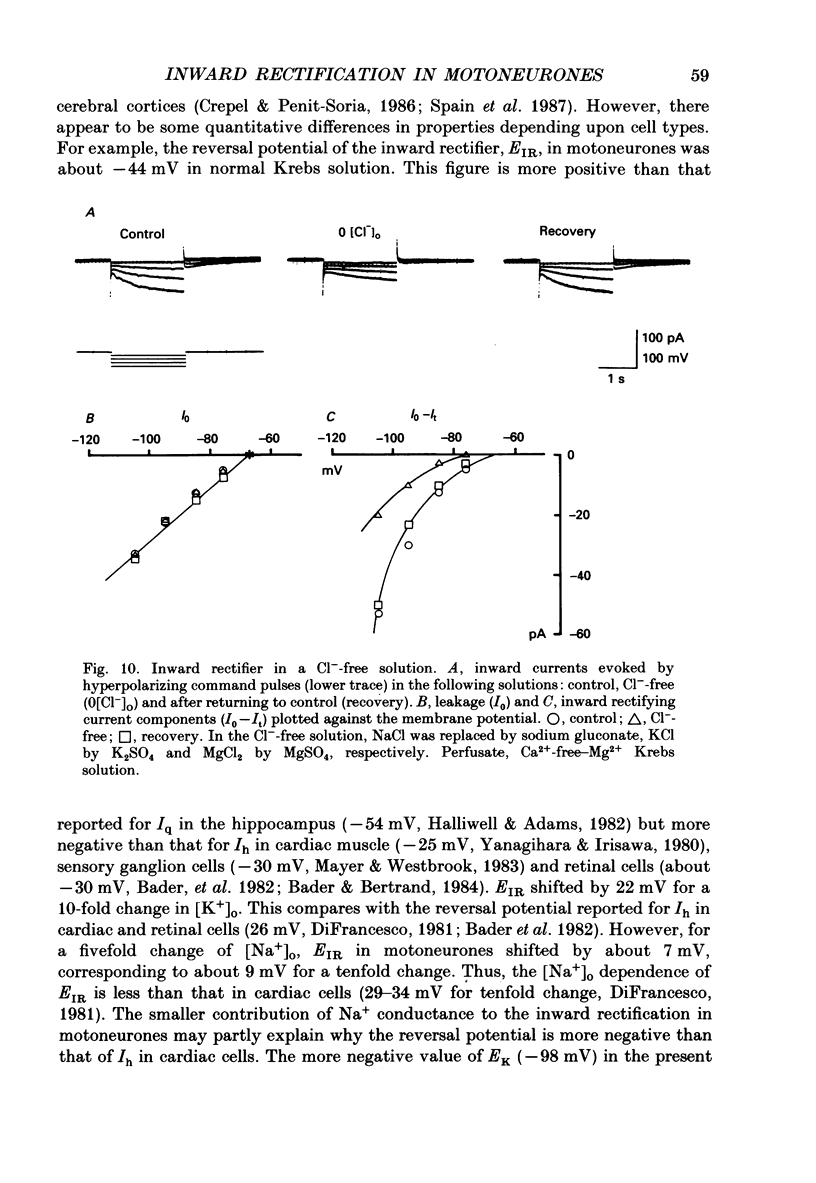
1. Inward rectifying currents were recorded, using tight-seal, whole-cell voltage-clamp methods, from motoneurones visually identified in thin slices of neonatal rat spinal cord. 2. When motoneurones were hyperpolarized from holding potentials near the resting potential (-60 to -70 mV), a slow inward-going current was recorded. After the hyperpolarizing command pulses, inward tail currents were recorded. Amplitudes of the inward current at the end of hyperpolarizing pulses as well as those of the tail current increased non-linearly with the membrane hyperpolarization, showing an inward rectification in the current-voltage relation. 3. Neither the amplitude nor the kinetics of the inward rectifying current (IIR) was appreciably affected by replacement of extracellularly Ca2+ with Mg2+ combined with the application of tetrodotoxin (1 microM), tetraethylammonium (30 mM), and 4-aminopyridine (4 mM). The current was relatively resistant to Ba2+, being only slightly suppressed at 2 but not at 0.2 mM. However, it was completely and reversibly abolished by Cs+ (2 mM). 4. When the external K+ concentration was raised, IIR was augmented. However, the activation curve of IIR constructed from relative tail current amplitudes in high K+ solutions was indistinguishable from that in normal solution. The chord conductance of IIR at various membrane potentials was similar for both normal and high K+ solutions. Thus the whole-cell conductance of inward rectification in motoneurones depends on the membrane potential but not appreciably on the external K+ concentration ([K+]o). 5. The reversal potential of IIR was estimated by measuring the tail currents. In standard solution ([K+]o = 3 mM), the reversal potential was about -44 mV. Increasing [K+]o shifted the reversal potential toward positive potentials by 22 mV for a tenfold change in potassium concentration. 6. A fivefold reduction in the external Na+ concentration shifted the reversal potential of IIR in a negative direction by about 7 mV, suggesting that Na+ may carry part of IIR. A fivefold reduction in external Cl- concentration shifted the reversal potential by about 2 mV but in a negative direction, the opposite of the expected shift in the Cl- equilibrium potential. 7. When external Cl- was substituted with isethionate or gluconate, IIR was markedly and reversibly suppressed. 8. It is concluded that in spinal motoneurones, IIR is carried by both K+ and Na+ ions and that external Cl- might be required to maintain the inward rectifier current.
Full text
PDF


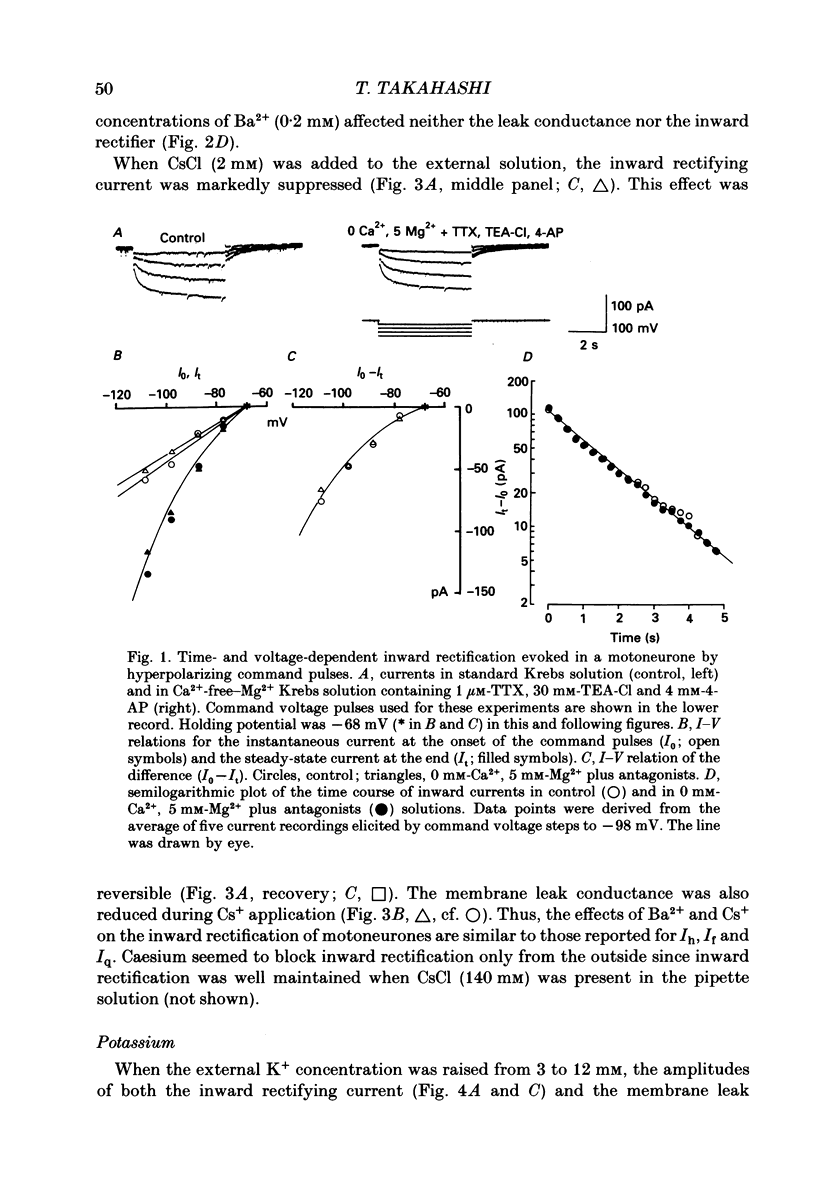
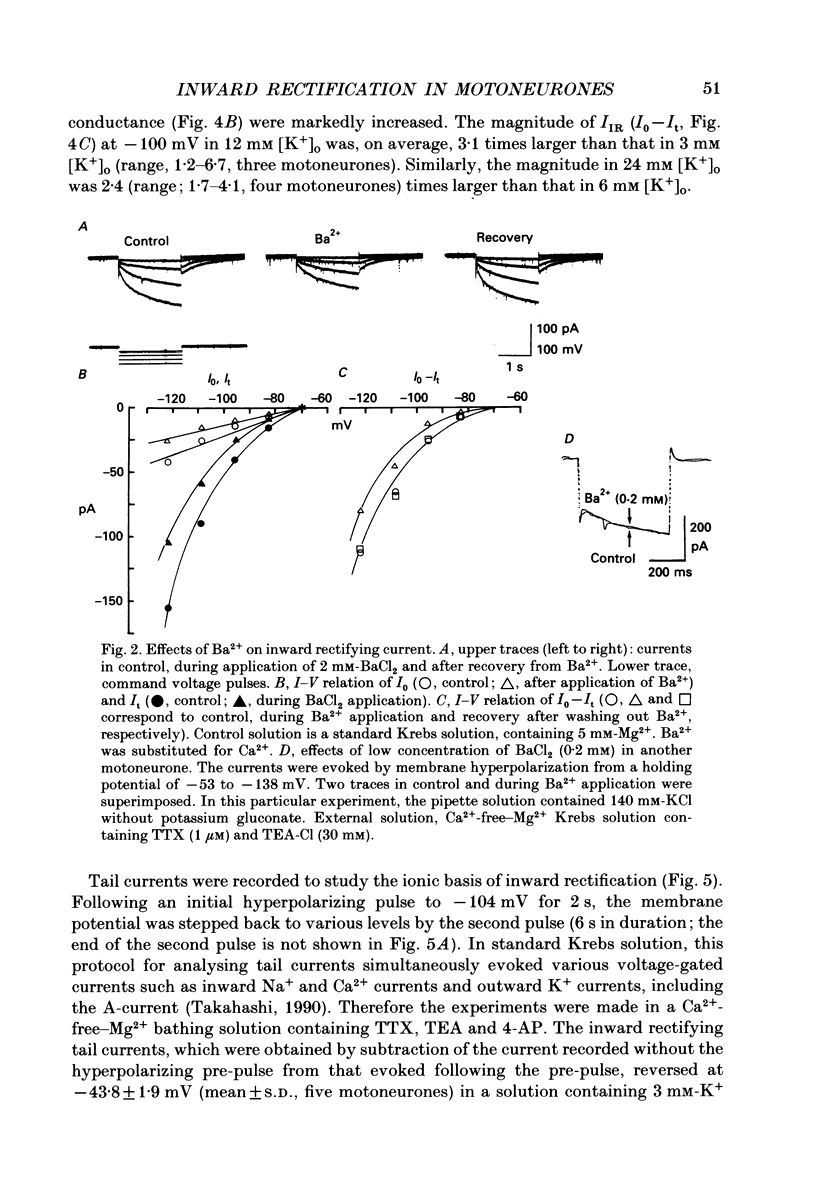
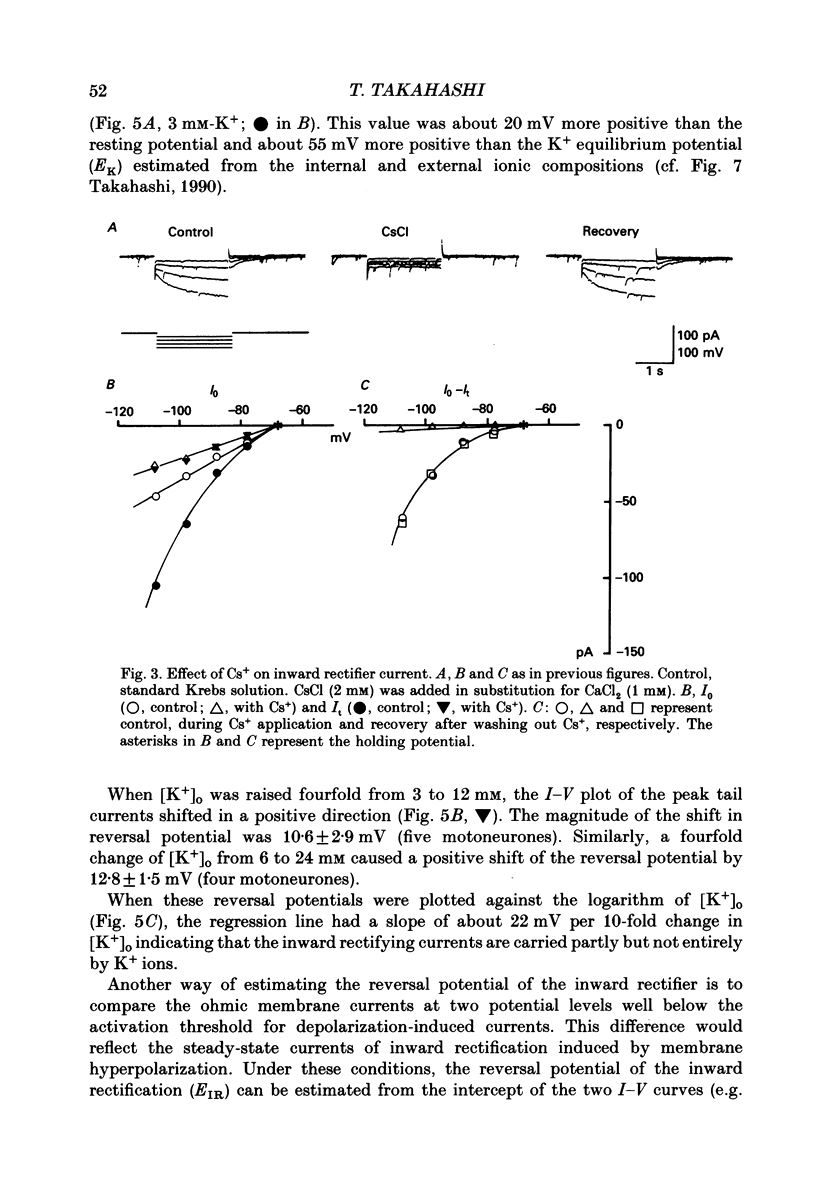
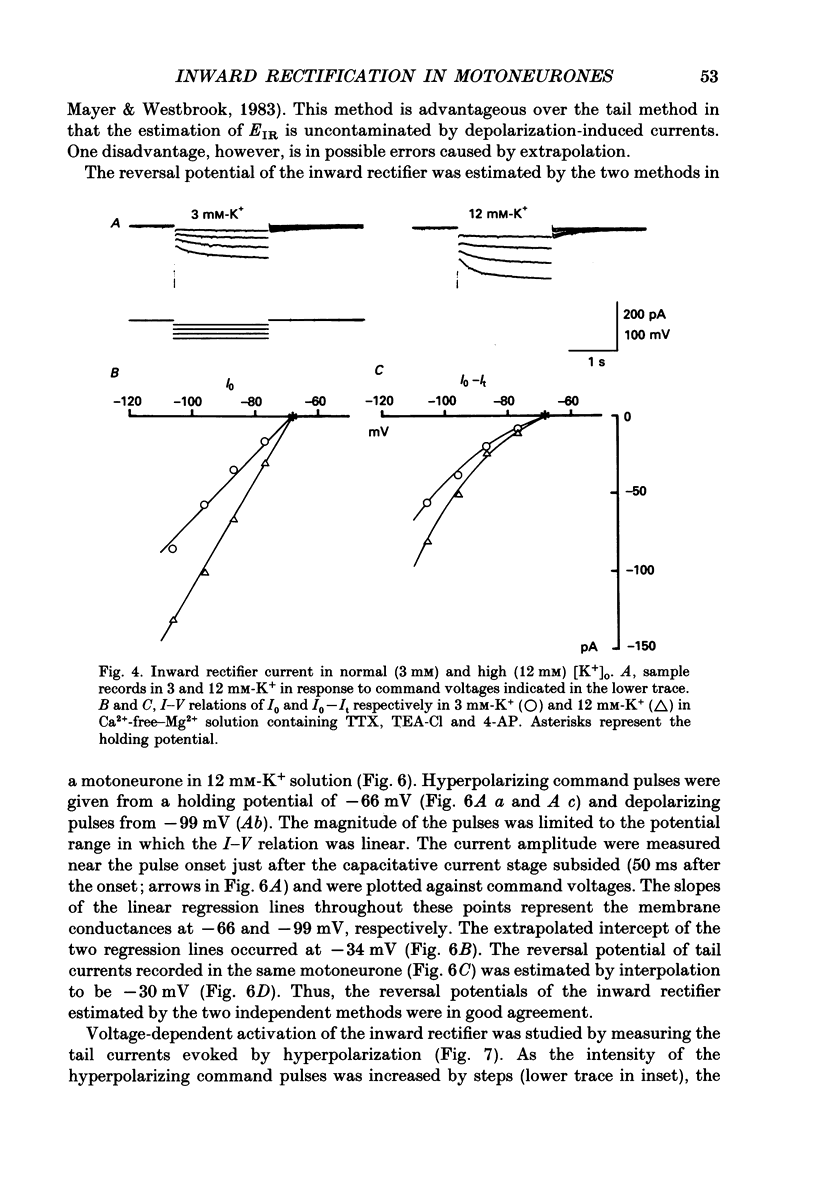



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Bertrand D. Effect of changes in intra- and extracellular sodium on the inward (anomalous) rectification in salamander photoreceptors. J Physiol. 1984 Feb;347:611–631. doi: 10.1113/jphysiol.1984.sp015086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schwartz E. A. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982 Oct;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Crill W. E. Voltage-sensitive outward currents in cat motoneurones. J Physiol. 1980 Jul;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H., Difrancesco D. Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol. 1980 Nov;308:331–351. doi: 10.1113/jphysiol.1980.sp013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoy-Marchais D. A Cl- conductance activated by hyperpolarization in Aplysia neurones. Nature. 1982 Sep 23;299(5881):359–361. doi: 10.1038/299359a0. [DOI] [PubMed] [Google Scholar]
- Constanti A., Galvan M. Fast inward-rectifying current accounts for anomalous rectification in olfactory cortex neurones. J Physiol. 1983 Feb;335:153–178. doi: 10.1113/jphysiol.1983.sp014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Penit-Soria J. Inward rectification and low threshold calcium conductance in rat cerebellar Purkinje cells. An in vitro study. J Physiol. 1986 Mar;372:1–23. doi: 10.1113/jphysiol.1986.sp015993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ojeda C. Properties of the current if in the sino-atrial node of the rabbit compared with those of the current iK, in Purkinje fibres. J Physiol. 1980 Nov;308:353–367. doi: 10.1113/jphysiol.1980.sp013475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Redman S. J. The dependence of motoneurone membrane potential on extracellular ion concentrations studied in isolated rat spinal cord. J Physiol. 1988 Oct;404:83–99. doi: 10.1113/jphysiol.1988.sp017280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL A. E., HUTTER O. F., NOBLE D. Current-voltage relations of Purkinje fibres in sodium-deficient solutions. J Physiol. 1963 Apr;166:225–240. doi: 10.1113/jphysiol.1963.sp007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Ito M., Oshima T. Electrical behaviour of the motoneurone membrane during intracellularly applied current steps. J Physiol. 1965 Oct;180(3):607–635. doi: 10.1113/jphysiol.1965.sp007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. A voltage-clamp analysis of membrane currents in solitary bipolar cells dissociated from Carassius auratus. J Physiol. 1985 Jan;358:131–152. doi: 10.1113/jphysiol.1985.sp015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Effects of L-glutamate on the anomalous rectifier potassium current in horizontal cells of Carassius auratus retina. J Physiol. 1985 Jan;358:169–182. doi: 10.1113/jphysiol.1985.sp015546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech C. A., Stanfield P. R. Inward rectification in frog skeletal muscle fibres and its dependence on membrane potential and external potassium. J Physiol. 1981;319:295–309. doi: 10.1113/jphysiol.1981.sp013909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Malenka R. C., Nicoll R. A. Phorbol esters block a voltage-sensitive chloride current in hippocampal pyramidal cells. Nature. 1986 Jun 12;321(6071):695–697. doi: 10.1038/321695a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G., Frank K. Anomalous rectification in cat spinal motoneurons and effect of polarizing currents on excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1097–1113. doi: 10.1152/jn.1967.30.5.1097. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. A calcium-independent chloride current activated by hyperpolarization in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1988 Mar 22;233(1271):191–199. doi: 10.1098/rspb.1988.0018. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., Standen N. B., Stanfield P. R. The voltage-dependent block of ATP-sensitive potassium channels of frog skeletal muscle by caesium and barium ions. J Physiol. 1988 Nov;405:677–697. doi: 10.1113/jphysiol.1988.sp017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyama I. Characteristics of the anion channel in the sino-atrial node cell of the rabbit. J Physiol. 1979 Sep;294:447–460. doi: 10.1113/jphysiol.1979.sp012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain W. J., Schwindt P. C., Crill W. E. Anomalous rectification in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1987 May;57(5):1555–1576. doi: 10.1152/jn.1987.57.5.1555. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R., Nakajima Y., Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985 Jun 6;315(6019):498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Berger A. J. Direct excitation of rat spinal motoneurones by serotonin. J Physiol. 1990 Apr;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T. Inhibitory miniature synaptic potentials in rat motoneurons. Proc R Soc Lond B Biol Sci. 1984 Mar 22;221(1222):103–109. doi: 10.1098/rspb.1984.0025. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. J Physiol. 1990 Apr;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. T., Colmers W. F., Pan Z. Z. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. J Neurosci. 1988 Sep;8(9):3499–3506. doi: 10.1523/JNEUROSCI.08-09-03499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]


