Abstract
Populations are continually adapting to their environment. Knowledge of which populations and individuals harbor unique and agriculturally useful variations has the potential to accelerate crop adaptation to the increasingly challenging environments predicted for the coming century. Landscape genomics, which identifies associations between environmental and genomic variation, provides a means for obtaining this knowledge. However, despite extensive efforts to assemble and characterize ex situ collections of crops and their wild relatives, gaps remain in the genomic and environmental datasets needed to robustly implement this approach. This article outlines the history of landscape genomics, which, to date, has mainly been used in conservation and evolutionary studies, provides an overview of crops and wild relative collections that have the necessary data for implementation and identifies areas where new data generation is needed. We find that 60% of the crops covered by the International Treaty on Plant Genetic Resources for Food and Agriculture lack the data necessary to conduct this kind of analysis, necessitating identification of crops in need of more collections, sequencing, or phenotyping. By highlighting these aspects, we aim to help develop agricultural landscape genomics as a sub-discipline that brings together evolutionary genetics, landscape ecology, and plant breeding, ultimately enhancing the development of resilient and adaptable crops for future environmental challenges.
Key words: Crop wild relatives, genome–environment association, local adaptation, plant breeding
Populations are continually adapting to their environment, and knowing which ones contain unique and agriculturally useful variations can help adapt crops to an increasingly stochastic climate. This review shows that agricultural landscape genomics holds promise for guiding the creation of resilient and sustainable food systems by highlighting an approach that allows people to harness the power of natural variation and local adaptation.
Introduction
Given the challenges of climate change, increasing competition for land and water, and concerns about the environmental footprint of agriculture, new approaches are needed to accelerate the breeding of environmentally resilient cultivars (Benitez-Alfonso et al., 2023). One such approach, known as landscape genomics, studies the relationship between genetic and environmental variation. Landscape genomics derives from the fields of evolutionary biology and landscape ecology and leverages the adaptation of natural plant populations to their local environments (Box 1; Table 1). Genetic signatures of local adaptation can be identified by searching for associations between environmental and genomic variation (Joost et al., 2007). In this method, environmental variables (e.g., climate, soil, and topography) from collection sites of the study organism represent the selection pressures driving local adaptation. Sequence or structural variants that are significantly associated with one or more environmental variables are therefore candidate loci for local adaptation (Hancock et al., 2011).
Box 1. History of tension between ecology and breeding when exploring population fitness.
Most natural populations of plants are adapted to their local environments (Leimu and Fischer, 2008). Local adaptation has been studied by evolutionary biologists for more than a century using a variety of experimental approaches, including common garden studies, reciprocal transplants, selection experiments, and provenance trials (Clausen, 1951; Kawecki and Ebert, 2004; Savolainen et al., 2007; Lowry, 2012). The latter, which have mainly been applied to trees, are similar to common garden experiments, except that seeds from different sources (i.e., provenances) are planted in multiple environments (Savolainen et al., 2007). The rationale underlying each of these approaches is that, under local adaptation, organisms are predicted to perform better in their local environments than in a novel environment (Kawecki and Ebert, 2004)
Agronomists and plant breeders have also long recognized that the relative performance of different crop varieties varies according to the environment in which they are grown. Such genotype-by-environment interactions are especially strong in crop landraces, which are often adapted to the local region in which they evolved (Mercer et al., 2008; Allaby et al., 2015). In contrast, modern cultivars are bred to maximize their performance across many environments and to minimize local adaptation (Simmonds, 1991; Gao et al., 2023). Evidence of crop local adaptation (or lack thereof) is mainly derived from multi-environment varietal trials, which are similar in principle to the methods employed for studying local adaptation in natural populations (DeLacey et al., 1996).
Classic ecological and evolutionary theory has often focused on identifying the ideal population for maximizing fitness in local environments (e.g., Levins, 1962). In contrast, modern plant breeding aims to maximize fitness across broad geographic regions and widely varying environments (e.g., Finlay and Wilkinson, 1963). Stated another way, a major goal of plant breeding has been stability, which can be defined either as having the same productivity across a range of diverse environments or having a predictably good/bad performance in known environments (Becker and Leon, 1988). This approach inherently treats the spatial aspects of an environment as a categorical variable, which is beneficial for choosing the best cultivar to grow in a particular location (Elias et al., 2016). In contrast, ecology/evolution typically views the environment as a continuous variable that impacts fitness in predictable ways (e.g., Etterson and Shaw, 2001), which aids our understanding of how natural selection optimizes the fitness of local populations. This tension, created by the different disciplinary goals, has led to similar analytical approaches being adopted at different rates and scales across these allied disciplines. Additionally, it has occasionally hindered the development of interdisciplinary approaches because of different assumptions or vocabularies. Techniques that borrow from both of these disciplines permit exploration of similar questions with different objectives and, in the case of agricultural landscape genomics, offer a useful tool for developing resilient crop populations.
Table 1.
Glossary of important terms for agricultural landscape genomics.
| Term | Definition |
|---|---|
| Ex situ conservation | conservation of biological diversity outside of natural habitats (seed bank, botanical garden) |
| GEA | a class of analyses where environmental characteristics (e.g., average temperature) of collection sites are used as the response and genetic information is used as a predictor, thus associating allelic variation with environmental variation |
| Landrace | a crop variety/population that is adapted to a local area having a recognizable identity and geographic origin (Khoury et al., 2022) |
| Primary germplasm | plants that can cross with a focal crop without meiotic abnormalities or other reproductive barriers (Harlan and de Wet, 1971; Harlan, 1976) |
| Secondary germplasm | plants that can cross with a focal crop with some meiotic abnormalities or other reproductive barriers, such as a reduction in F1 viability (Harlan and de Wet, 1971; Harlan, 1976) |
| Tertiary germplasm | plants that require technical interventions from humans to enable successful crosses with a focal crop, such as embryo rescue or tissue culture; such crosses typically result in meiotic abnormalities with low fertility in the F1 generation (Harlan and de Wet, 1971; Harlan, 1976) |
| Quaternary germplasm | organisms that contribute to a focal crop gene pool via genetic/genome engineering (e.g., Agrobacterium, CRISPR-Cas9, zinc-finger nuclease) |
| Germplasm genomics | genomic characterization of germplasm collections and subsequent analyses, including associations of sequence variation with phenotypes or environmental variables |
| Domestication | process by which humans select desirable qualities in plants or animals to make them more useful to humans; this results in dependency on human intervention for persistence |
| Wild progenitor | species from which a domesticated species was selected |
| Accession | genetically related plant germplasm from a single species that is collected at one time from a specific location |
| Minor crop/underutilized crop | a crop that is largely regional, grown on small acreages, and with limited international trade and has thus received less attention from researchers than major crops |
| Pre-breeding | the development of improved populations/lines from wild or semi-wild germplasm that would then be suitable for use in breeding programs designed to release finished varieties for farmers |
| Cultural diffusion | human dispersal of technology across a wide geographic area |
| Natural selection | process by which heritable traits that enhance reproductive success increase in frequency |
| Artificial selection | process where organisms containing favorable characteristics are chosen by humans to establish subsequent generations (Falconer and Mackay 1996) |
| Genetic drift | Changes in allele frequencies over time due to random sampling and not due to selection |
| Linkage disequilibrium | nonrandom association of alleles at two or more loci (Falconer and Mackay, 1996). |
| Genetic bottleneck | when alleles are lost from populations, usually due to a single sharp decline in population size rather than continual decreases in population size |
| Gene flow | transfer of genes between natural populations |
| Effective population size | the size of an idealized population experiencing the same amount of genetic drift as the focal population; it is impacted by the number and relatedness of parents as well as variance in parental contributions |
| Introgression | transmission via hybridization of genetic material between genetic backgrounds or species |
| QTL(s) | region(s) of the genome that control a continuously varying trait |
| Selective sweep | the process of a mutation and linked sites going from rare to fixation within a population due to natural selection |
| Linkage drag | reduction in fitness due to maladapted alleles introduced along with the target allele during breeding |
| Landscape genomics | a suite of methods used to identify genetic variation associated with environmental factors and local adaptation |
| Agricultural landscape genomics | using landscape genomics to increase crop resilience |
| Top-down approach/forward genetics | approaches where unique phenotypes are found and then, afterward, their genetic basis is identified |
| Bottom-up approach/reverse genetics | approach where gene sequence variation is identified first and then, later, the phenotypes that sequence variation causes |
Genome–environment association (GEA) analysis can be used to detect such associations. GEA is similar to standard genome-wide association studies (GWASs), but it uses environmental variables as the response variable instead of phenotype trait values (Hancock et al., 2011; Yeaman et al., 2016; Ferrero-Serrano and Assmann, 2019). Because alleles associated with the challenging environments experienced by natural populations are also likely to be useful for crop improvement, landscape genomics holds significant promise for agile development and deployment of cultivars resilient to future climates (reviewed in Cortés et al., 2022; Lasky et al., 2023; Gao et al., 2023). While landscape genomics can be useful to study climate change adaptation of any crop (e.g., Lasky et al., 2015), it is especially promising for minor crops, which have historically received limited investment in breeding (Cortés et al., 2022; Neyhart et al., 2022).
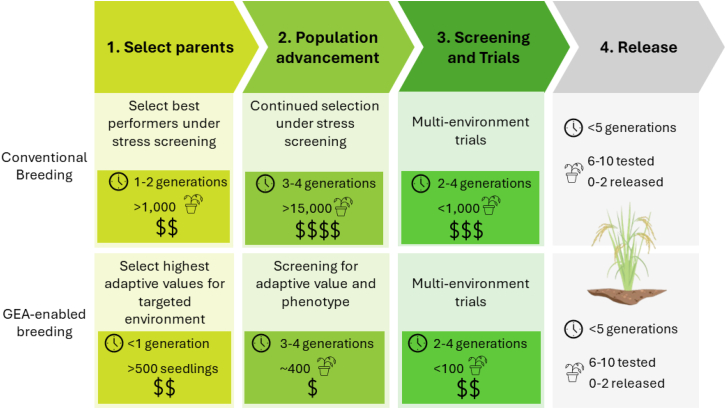
Local adaptation is required for successful GEA. Therefore, the method will be mostly applicable to crop wild relatives (CWRs) and landraces found across diverse environments but generally not to modern cultivars, which are shaped by the contemporary breeding practices and geographic redistribution that reduce local adaptation (Box 1). In this paper, we explore the extent to which landscape genomic approaches have been applied to species that are relevant to food production. We specifically show how landscape genomic approaches can be employed, describe data gaps that are present, and explore where future work could be focused to improve food security (Figure 1). We refer to this sub-discipline as agricultural landscape genomics.
Figure 1.
Comparison of GEA-enabled breeding to a general traditional breeding scheme.
Breeding timeline, population size, and relative cost of activities are considered here. Basic breeding timelines are generally formulated in a species-specific way to account for calendar time; they can also be conceptualized as the number of generations. With the ease and reduced costs of high-throughput sequencing and genotyping platforms, phenotyping is generally considered the primary bottleneck that limits the identification and validation of lines, traits, and QTL due to the high costs (Bazakos et al., 2017; Langstroff et al., 2022). Whether an individual breeding program can use GEA-enabled breeding depends on many factors, including generation time, ploidy, ability to use tissue culture methods, transformation potential, relationship of experimental lines to elite material, and trait genetic architecture. However, cultivar turnover is often slow, particularly when moving material between geographies (Lucier, 1991; Singh et al., 2020). Implications of yield protection from climate resilience breeding imply that there is a need to make decisions now to ensure that cultivars will thrive under the projected climate regimens of the 21st century.
Using agricultural landscape genomics to make crops more resilient
Agricultural landscape genomics uses GEA methods to identify potentially adaptive loci in CWRs and landraces (reviewed in Bragg et al., 2015; Rellstab et al., 2015; Henry, 2016; Landridge and Waugh, 2019; Cortés et al., 2022; Lasky et al., 2023). CWRs and landraces are often adapted to a diverse array of habitats; they therefore represent a promising source of adaptive alleles that can be deployed for enhancing the resilience of their crop relatives (Savolainen et al., 2013; Dempewolf et al., 2017). Introducing exotic germplasm into breeding programs can be challenging (Box 2); however, landscape genomics could contribute to making this process accessible to the global plant breeding community. A first and major point of access is that landscape genomics does not require phenotyping. It can readily help identify genomic regions or haplotypes that contribute to abiotic (Todesco et al., 2020; Cortés et al., 2022; Lasky et al., 2023) and biotic stress resistance by using modeled environmental (Pais et al., 2020) and pathogen distribution and occurrence data (Vajana et al., 2018). Second, landscape genomics can guide the selection of relevant germplasm once resilience alleles of interest have been identified. These alleles can be incorporated into breeding programs, targeting genotypes that contain the highest number of stress tolerance alleles (e.g., Neyhart et al., 2022). This can be extended to the use of genomic selection (GS) to efficiently move such alleles into breeding lines and to prioritize parental selection for breeding climate-resilient crops. Finally, landscape genomics can guide conservation efforts of accessions with the greatest adaptive potential so resilience panels are prioritized for conservation and for use as parents in breeding (e.g., Campbell et al., 2024; Halpin-McCormick et al., 2024).
Box 2. Breeding with exotic (wild and landrace) material.
Plant breeding with unadapted (exotic) material that is either wild or landrace is a common practice, but this process may lengthen the breeding cycle. A frequent problem is linkage drag, in which maladaptive alleles are introduced along with the favored trait. Plant breeders typically employ MAS to minimize the introduction of unwanted genomic regions. MAS is an indirect selection technique where a genetic marker known to co-occur with a desirable phenotype is used to select that trait before its phenotypic expression. MAS is particularly useful when introducing traits of interest from a donor parent into an elite or adapted recurrent parent (marker-assisted backcrossing). This technique can reduce the number of generations of backcrossing needed to introduce a new trait (while minimizing linkage drag) in half (Iftekharuddaula et al., 2011). Beyond MAS, GS and the genomic estimated breeding value can be estimated from genome-wide genotyping data (Newell and Jannick, 2014), thereby taking into account minor loci contributing to the trait(s) of interest and not only the major QTL utilized in MAS. GS can also consider the relationship between genotypes and traits in multiple environments (Oakey et al., 2016), offering significant potential to identify individuals with the greatest likelihood of generating the ideal phenotype. One potential solution that has been proposed is to encourage breeders to maintain two breeding pipelines: one for elite lines and one for improving diversity (Sanchez, et al., 2023).
Rapid phenotyping using radar, imaging, and spectral analysis, alongside advances in AI/machine learning approaches, is helping to enhance the breeder’s ability to sample greater numbers of plants over a short period (Chen et al., 2014; Vlaminck et al., 2020; Fan et al., 2021). Additionally, “speed breeding” approaches shorten crop cycles (i.e., Gudi et al., 2022). In controlled environment agriculture, this can involve optimizing the temperature and photoperiod to break dormancy, which, coupled with single-seed descent and phenotyping large numbers of seedlings, can result in inbred line development up to two-fold faster than with conventional methods (Watson et al., 2018). These new data-intensive rapid breeding techniques are currently being deployed in many public and private-sector breeding programs (Bernardo 2008, 2020; Cobb et al., 2013; Rahaman et al., 2015; Pauli et al., 2016). Notable successes include, for example, the production of salt-tolerant rice cultivars (Rana et al., 2019). Germplasm collections, while a treasure trove of unique diversity, often do not have the combination of traits necessary to be immediately grown by farmers but are excellent parents for future breeding.
Discovering and using alleles that contribute to plant resilience
At the beginning of this century, a top-down, phenotype-first paradigm for discovering alleles contributing to crop resilience dominated the field (Ross-Ibarra et al., 2007). Starting with a phenotype of interest and identifying candidate alleles was a laborious process, often involving quantitative trait locus (QTL) mapping in biparental populations, phenotyping complex traits, map-based cloning, bacterial artifical chromsome (BAC) sequencing, and functional validation using reverse genetics (e.g., Yan et al., 2003; 2004; Ren et al., 2005; Xu et al., 2006). Recently, advances in genome sequencing technologies (Sun et al., 2022), the creation of crop diversity panels that are amenable to GWAS (Rafalski, 2010), and access to functional tools (Gaj et al., 2013) have transformed and accelerated the isolation of adaptive alleles. Nonetheless, the accurate phenotyping of large populations for complex traits related to abiotic stress resistance remains expensive and time consuming.
Bottom-up approaches, which use population genetic analyses to identify candidate genes followed by the use of reverse genetics tools to connect genes to phenotype, have been offered as an alternative by evolutionary biologists. This is based on the observation that the action of natural selection leaves characteristic footprints on the genome (Ross-Ibarra et al., 2007). Under directional selection, for example, a favorable mutation (along with linked neutral sites) is expected to sweep to high frequency, resulting in reduced variation in the swept region, increased linkage disequilibrium, and greater differentiation between populations (Nielsen et al., 2005). While this approach is effective in identifying adaptive alleles, the selective forces that drove their evolution are often unknown, and therefore it is difficult to know whether their genes play a role in environmental resilience. Despite the inherent lack of knowledge about the functional nature of many of the adaptive alleles identified though bottom-up approaches, scans for selective sweeps have proven effective for detecting alleles underlying the resistance or avoidance of abiotic stress, especially when the groups being compared differ in the environments with which they are associated (e.g., Zhou et al., 2015; Lu et al., 2019; Calfee et al., 2021; Shan et al., 2022; Ahmadi et al., 2023).
GEA represents a recently developed workaround for the lack of data on the selective pressures affecting populations (Lasky et al., 2023). In GEA studies, selection pressures underlying adaptive alleles can be tentatively inferred from environmental correlations (Booker et al., 2024). Like other bottom-up approaches, it retains the downside of not knowing the phenotype associated with the alleles, which could lead to faulty conclusions. For example, consider an allele associated with high summer temperatures. This allele might either contribute to heat avoidance (e.g., via early maturation leading to lower seed production) or to heat resistance (e.g., via increased production of osmoprotectants). Misinterpreting the allele as promoting heat resistance when it actually promotes heat avoidance could result in selecting plants that mature early, thus reducing crop yields and undermining breeding goals. It is therefore important to experimentally validate such inferences via follow-up phenotypic or physiological analyses (e.g., Lasky et al., 2015). The applicability of GEA methodologies depends on the characteristics of the species, samples, and environments targeted for analysis (Lotterhos and Whitlock, 2015; reviewed in Lasky et al., 2023; Lotterhos, 2023). As Lasky et al. (2023) noted, the power of GEA to detect genomic regions underlying local adaptation relies on the distinctiveness of environmental conditions, the extent of local adaptation, potential confounding variables, the polygenic nature of traits, and the influence of population structure and linkage disequilibrium. Additionally, in situations with high genomic redundancy (i.e., when many different genotypes can achieve the same phenotype) and low levels of gene flow, adaptive allele frequency changes may not co-vary with environmental clines, an underlying assumption for GEA (Lotterhos, 2023). Although gene flow typically increases the power of GEA by reducing population structure and genomic redundancy (reviewed in Lotterhos, 2023), high levels of gene flow can erode signatures of local adaptation (Kirkpatrick and Barton, 1997).
In the context of agricultural species, this means that the wild progenitors of crops or landraces that have a long history of association with specific geographic locations and, thus, specific environmental conditions are the best samples for this kind of analysis. However, GEA is unlikely to be successful in crop relatives or landraces that reproduce mainly asexually or that have an obligate self-fertilization system. With respect to crops, any variation that is outside the major cultivated environment has the potential to be useful and can be assessed with the range of tools developed for GEA analysis (e.g., Capblancq et al., 2018; Qin et al., 2022).
One challenge of adopting GEA approaches is the typically large number of samples required for sufficient power to detect robust associations, especially if genome sequencing is employed. Poolseq approaches, in which 10–100 individuals per collection site are combined into a single sample prior to sequencing, can increase power at low cost (Schlötterer et al., 2014), thus overcoming standard GEA requirements. Although the power to detect genomic regions underlying local adaptation increases with sample size, minor-allele frequency, and heritability, power also increases with both the number of collection sites and the number of individuals per collection site sampled (Lotterhos and Whitlock, 2015). Thus, if funds are limited, then researchers could focus on increasing the number of sampled populations across the environment and adopt Poolseq approaches. This pathway will maximize the pool of adaptive alleles that are potentially detectable.
In general, considerations related to population size and structure, the extent of environmental variation across collection sites, sampling scheme across a wide environmental gradient, and access to samples in extreme environmental conditions will determine the utility of GEA models. Although GEA methods are ideally positioned to identify the most promising parents or specific loci that may provide the most benefit for specific local breeding programs, key validation experiments should be considered immediately after the initial GEA study. The first step is to evaluate the prevalence of any identified variant (or combination of variants) in elite breeding material and then grow these lines in both a controlled environment and field trials to see whether the expected directional effects are observed. The second step is to cross potential parents with elite varieties to create segregating breeding populations and place these populations into standard breeding pipelines. This will enable an opportunity to observe whether the material has the expected impacts. As a final step, natural experiments offer a means for validating predictions from GEA. For example, imagine that natural populations are polymorphic for a GEA allele that is predicted to contribute to drought tolerance. If there were a drought event, then we would expect the allele to rise in frequency, creating more drought-tolerant populations. Also, if the allele has a major effect, then demographic responses of populations to drought might be correlated with the frequency of the beneficial allele.
What does a successful GEA look like?
To understand what the output of a successful GEA study should look like, we briefly explore a GEA study of wild sunflowers (Helianthus) that accounts for the above considerations in its design (Todesco et al., 2020). Wild sunflowers thrive in extreme environments in the central and southwestern US, including deserts (heat and drought stress), sand dunes (low nutrient stress), and salt marsh habitats (Kantar et al., 2015). Todesco et al. (2020) generated whole-genome sequence data for 1506 samples representing three wild species, including the progenitor of the domesticated sunflower (Helianthus annuus) and two close relatives. Samples were collected across independent environmental transects, employing the paired sample strategy described by Lotterhos and Whitlock (2015) to decouple (as far as possible) environmental variation and evolutionary history. Testing for associations between sequence variants and several climate and soil characteristics detected strong associations for most of the focal environmental variables. For example, one of the top associations with several temperature-related climate variables in H. annuus is a sunflower ortholog of HEAT-INTOLERANT 1 (HIT1), which mediates resistance to heat stress by regulating plasma membrane thermo-tolerance in Arabidopsis thaliana (Figure 2A, top). In another example, large haplotype blocks were detected for cation exchange capacity, a measure of soil fertility, in the prairie sunflower, H. petiolaris (Figure 2B, bottom). Such haplotype blocks were common in all three species and are mainly caused by chromosomal inversions, which are thought to contribute to local adaptation in the presence of gene flow by reducing recombination between co-selected alleles (Huang and Rieseberg, 2020).
Figure 2.
GEA analyses in sunflower.
(A and B) Here, we see the expected output of a GEA study, a Manhattan plot showing associations between SNPs and (A) degree days below 18°C (DD < 18) for H. annuus, with HaHIT1, a sunflower ortholog of HEAT-INTOLERANT 1 (HIT1), as one of the top associations, and (B) cation exchange capacity, a measure of soil fertility, in H. petiolaris ssp. fallax. The purple line represents a Bayes factor (BFis) of 20 deciban (dB). Modified with permission from Todesco et al. (2020).
Utilizing germplasm collections for landscape genomics
Conservation of plant diversity has long been part of the global economy, with one of the earliest examples being the Hanging Gardens of Babylon and then in the classical/medieval world with a focus on medicinal plants (Stafleu, 1969). In the 18th and 19th centuries, there was a concerted effort by national governments to expand collections to include other economically valuable plants, especially crops. While connected to the colonial goal of creating commodity-based export economies, it also led to widespread efforts to characterize species distributions, diversity, and agronomic potential (Britton, 1896; Blakeslee, 1910; Raven, 1981; Brockway, 2002).
Germplasm collections were key to overcoming many challenges to agriculture in the 20th century (Hajjar and Hodgkin, 2007), and the use of landraces and CWRs has been substantial, with collections being valued in the billions of dollars (Smale and Jamora, 2020) for a relatively small cost of upkeep (∼150 million dollars globally for the entire 21st century; Koo et al., 2003a; 2003b). However, as these collections have ballooned to millions of accessions (Wambugu et al., 2018), it has become logistically impractical for programs or institutes to phenotype all of them in multi-environment trials. This logistical difficulty comes both from the side of obtaining seed from repositories and conducting field trials with large numbers of entries and environmental scenarios. Many techniques are emerging that leverage genomics to practically explore these large collections; these techniques have been labeled germplasm genomics (Yu et al., 2016; Langridge and Waugh, 2019).
Despite being less costly than phenotyping hundreds or thousands of accessions, landscape genomics techniques still require substantial resource development. Specifically, they require large (e.g., hundreds) georeferenced collections (to extract environmental data) from a wide range of environments along with genomic data; generating these datasets de novo is costly. Because many historic collections are convenience samples, with plant explorers collecting samples when the opportunity arose without regard to environmental gradients or population structure, the use of landscape genomics techniques may be limited by the extent of historic sampling. The potential for discovering valuable diversity in these collections is also highly dependent upon the pool of diversity of landraces and wild relatives, the availability of this diversity in accessible germplasm collections, and the relatedness (and ease of introgression) of landraces and wild relatives to the crop species (Supplemental Figure 1).
Despite these limitations, the information necessary to conduct GEA analysis in crops and CWRs is often present in germplasm repositories. Recently, there has been extensive sequencing of germplasm collections of both landraces and wild relatives (e.g., rye, Schreiber et al., 2019; barley, Milner et al., 2019; sorghum, Lasky et al., 2015; maize, Gouesnard et al., 2017; soybean, Song et al., 2013; and sunflower, Todesco et al., 2020). Since many accessions stored in these collections have global positioning system (GPS) coordinates, it is possible to easily access information on climate, soil, and topography from public databases, such as the WorldClim Database (https://www.worldclim.org/) and the Harmonized World Soil Database (https://iiasa.ac.at/models-tools-data/hwsd). Species locally adapted to North America or Asia–Pacific can also utilize climate resources such as Climate North America (https://climatena.ca), land-holding farmers and communities in developing Climate Asia–Pacific (https://web.climateap.net), and general climate data from the PRISM (Parameter-elevation Regressions on Independent Slopes Model) Climate Group (prism.oregonstate.edu). Such databases offer hundreds of monthly, seasonal, and annual climate variables that are scale free for specific spatial locations, providing increased precision over grid-averaged climate data. Further, the fact that these collections have recorded collection times offers the opportunity to explore allele frequency shifts over time to observe adaptation in action (Spear et al., 2023).
Making the best use of landscape genomics in the pre-breeding process
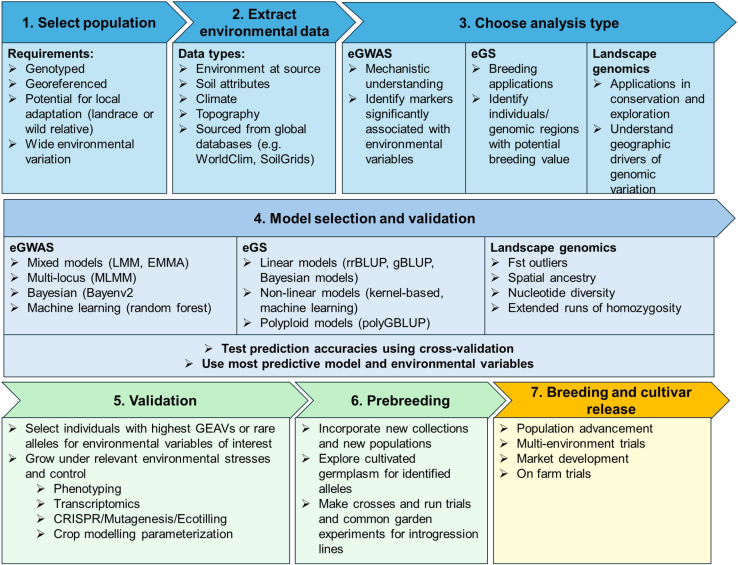
Landscape genomics can provide candidate genes, genomic regions, and parents that have the potential to be used in breeding (Neyhart et al., 2022; Bedford et al., 2023; Wang et al., 2023; Campbell et al., 2024). Screening of exotic germplasm for use in plant breeding has traditionally been a lengthy and costly endeavor often leading plant breeders to operate closed programs only using “good” parents (Rasmusson and Phillips, 1997). However, recent work has suggested that the strategic use of technology could make exotic germplasm more accessible (Box 2; Figure 3; Wang et al., 2017). Despite efforts to increase the use of germplasm resources, there has been limited discussion of how specific techniques can be integrated into breeding timelines or used to alter such timelines (Figure 1).
Figure 3.
Exploration of the workflow for the agricultural landscape genomics process.
Different parts of the analysis are shown in different colors.
Germplasm collections contain landrace and heritage varieties as well as historic types generally associated with both local geographies and markets (Khoury et al., 2022). The long connection with specific locations allows for the assumptions of GEA to be maintained and in less exotic material than wild counterparts (Box 2). This means that using this subset of diversity has the advantage of having desirable characteristics (agronomic/quality/flowering) in addition to stress tolerance in a genetic background that is likely to have less linkage drag versus that of wild populations when used for crossing to elite material. GEA on collections can facilitate the use of landrace and heritage varieties by providing a clear framework for identifying potential parents that can be used in pre-breeding (see Box 3 for use cases). Specifically, GEA offers a theoretical framework for the creation of a climate-resistant breeding program. This includes the ability to (1) determine the best accessions (lines/populations stored in gene banks) to be used as parents, (2) identify a subset of accessions for phenotypic validation, and (3) reduce the number of populations that need to be created to minimize the use of resources in pre-breeding. These activities can occur in parallel, leveraging interdisciplinary teams and multiple environments to rapidly address climate change (Figure 1).
Box 3. Successfully incorporating GEA into breeding programs: cranberry and barley.
Cranberry production and breeding are very new relative to other crops. Large-scale intentional cultivation started in eastern North America in the mid-1800s, and initial varieties were landraces selected as the best from native stands (Eck, 1990). Due to the long-lived nature of this species and the initiation of breeding efforts only in the 1930s, current cultivars are only three to four generations removed from wild material (Vorsa and Zalapa, 2019). The long length of the breeding cycle along with a strong investment in genomic resources (e.g., sequenced germplasm collection from wild samples) has made cranberry an excellent test case for using landscape genomics to speed up the breeding process and search for genetic variation for adaptability or tolerance to pressing environmental stresses such as heat, cold, drought, and soil quality. Recent work analyzed available wild cranberry germplasm collections (n = 111 samples from 17 different sampling locations) and used a landscape genomics approach to identify putative allelic variation for climate and soil adaptation (Neyhart et al., 2022). Among the most promising genotypes were those from disjunct northern populations in the northern US and maritime eastern Canada with potentially cold-adaptive alleles. These genotypes, along with those from contrasting southern climes with potential heat tolerance, have been used in breeding crosses by the United States Department of Agriculture (USDA) with the goal of introgressing environmental adaptability into elevated productivity and fruit quality backgrounds. Despite not having the ideal collection where (1) sampling would cover the entire species range (e.g., containing all of the extreme environments), (2) the collection would include multiple individuals from all collection locations (permitting estimation of allele frequencies), (3) each georeferenced site would have weather and soil data collected in real time for the site, and (4) would have been phenotyped for important agronomic/quality traits, there is still tremendous practical value for plant breeders.
Landraces make up a large proportion of all barley germplasm conserved in gene banks (Schmidt et al., 2023); however, they are typically underutilized in breeding programs (Monteagudo et al., 2019). Hordeum spp. are distributed across a wide range of environments, from near-arctic to semi-desert regions, making them exemplars for understanding environmental variation and adaptation (Dawson et al., 2015). While environmental adaptation is important, market class (feed, malt, and food) and quality also greatly influence the adoption of barley varieties (Kumar et al., 2020). Thus, landraces, which have higher quality than wild germplasm, provide a better starting point for breeding. Landscape genomic techniques have been used extensively to explore environmental variation in barley (Russell et al., 2016; Lu et al., 2019; Milner et al., 2019; Chang et al., 2022; Chen et al., 2023; Schmit et al., 2023; Zhou et al., 2024). These studies use a bottom-up approach where identifying candidate genes based on proximity to molecular markers and predicted function provides insight into which genotypes and alleles have potential utility for breeding for abiotic stress tolerance. In each of these studies, candidate genes were associated with specific environmental variables, and the specific wild or landrace accessions that have the beneficial allele are described. These traits often co-localize with physiologically relevant traits, such as root growth and stay green (Williams et al., 2022). Further work has been done to explore how different alleles have changed over time in different breeding programs using this framework (Sharma et al., 2022). As these techniques become more common, heritage collections that are adapted to local conditions (e.g., soil type) to which modern cultivars are not adapted are being incorporated into breeding programs (Martin et al., 2023). In barley, GEA analysis has been strategically employed by many breeding programs to leverage identified lines and alleles. This has led to a better understanding of why certain cultivated lines perform better in different environments. This dual use, gaining understanding and being able to plan crosses for the future, makes such analyses very useful.
Untapped opportunities for adoption of landscape genomic techniques in crops
Climate change is threatening food production. Techniques that were pioneered in evolutionary biology are now becoming relevant and tractable in applied plant breeding (Box 1). As described above, landscape genomic approaches have great potential to identify important accessions and loci associated with climate adaptation. However, it is unclear how prevalent the techniques from evolutionary biology are in the crop science literature and whether opportunities exist to harness relevant information from landscape genomic studies into the agile production of more resilient crops. To address this question, we first conducted an analysis of the crop science literature on the use of landscape genomics and environmental association. We used several key terms to extract the most relevant studies indexed in Web of Science (“Crop Wild Relative AND environmental association,” “Crop Wild Relative AND landscape genomics,” “Crop AND Environmental AND Association,” “Crop AND Landscape AND Genomics,” “Crop AND Germplasm AND Sequencing,” “Crop AND Domestication AND sequencing,” “Wild Relative of Crop AND Breeding Trials,” “Germplasm AND Genomics AND screen,” and “Crop wild relative AND GWAS”). We filtered for research articles published between 2000 and 2023. This resulted in a total of 15 981 papers (Supplemental Table 1), of which 11.6% were relevant based on keyword searches and Web-of-Science (WOS) category. Studies were explored for high marker coverage and georeferences for the biological material and focused on species that were readily crossable with crops without specialized techniques. We found 271 articles (1.6% of all articles) stating an explicit genetic–environmental association in the landraces of a specific crop or a wild relative of a specific crop.
We found that the most common study goal in the database (271 articles) was to characterize the genetic diversity of a germplasm collection (46%), followed by those that focused on the relationship between phylogenetics and geography (25%). Studies that used germplasm collections to explore variation in abiotic agronomic traits (19%) and those that correlated genetic variation with specific landscape features (e.g., soil, temperature, and precipitation) (10%) were also present in the database. These results highlight that the use of landscape genomic approaches to leverage information on local adaptation in crops and CWRs remains rare. And while most of the studies provide useful information on different genetic and environmental aspects of a crop species, such as population structure, genetic diversity, and some environment–trait association, they rarely provide a path forward for the adoption of local adaptation information in a breeding pipeline.
However, it is possible that most relevant studies on landscape genomics were conducted on globally important crops. We therefore conducted a second analysis to explore the use of landscape genomics in the list of 64 crops associated with the Annex of the multilateral International Treaty on Plant Genetic Resources for Food and Agriculture (ITPGRFA). We first explored the total number of accessions available (Supplemental Figure 2). We then looked at the overlap between genome availability, phenotype status, genotype status, and geo-referenced data sets (Supplemental Figure 3; Supplemental Table 2). Of 64 species, 26 systems had detailed information on the number of accessions per collection and georeferenced genomic and phenotypic resources and therefore had everything needed for GEA (Supplemental Figures 3 and 4; Supplemental Table 2). Only 12 of these have had GEA analyses conducted, providing breeders and researchers with useful information for breeding and conservation.
Our analysis reveals that, while these approaches are increasingly being applied to crop species and their wild relatives, their use remains limited in scope and uneven across different crop groups. One of the most striking findings was that only 1.6% of the studies in our database explicitly tested for genetic–environmental associations in crop landraces or wild relatives. This suggests that there is still significant untapped potential for using these approaches to identify adaptive genetic variation in these important genetic resources. Given the urgent need to develop more resilient and sustainable crop varieties in the face of climate change, increasing the application of landscape genomics to these underutilized populations should be a high priority. Another key insight from our survey was the importance of having comprehensive and well-characterized germplasm collections for the success of landscape genomics studies. We found that the crop species with the most extensive and diverse collections, such as wheat, maize, and rice, were also the ones with the highest number of published studies. This highlights the need to continue investing in the development and maintenance of high-quality germplasm collections, particularly for minor and understudied crops that may hold valuable adaptive variation. Our analysis also revealed some promising trends in the use of landscape genomics in crop research. For example, we found that studies focusing on abiotic stress tolerance, such as drought and heat stress, were among the most common applications. Additionally, we observed an increasing number of studies that combined landscape genomics with other approaches, such as GWAS and genomic prediction, demonstrating the potential for integrating these methods to accelerate crop improvement.
Our survey also identified several gaps and challenges that need to be addressed to fully realize the potential of landscape genomics in agriculture. One major challenge is the lack of consistent and standardized methods across studies. This can make it difficult to compare results across different crop species and regions, limiting our ability to draw general conclusions about the genetic basis of adaptation. Developing common protocols and data standards for environmental data collection and analysis should be a priority for the field. Another challenge is the need for more diverse and representative sampling strategies in landscape genomics studies. Many of the studies in our database focused on a limited number of populations or environments, which can limit the generalizability of their findings. Future studies should aim to sample across a wider range of environments and genetic backgrounds to capture the full spectrum of adaptive variation. This may require collaboration among researchers working in different regions and with different crop species.
Need for interdisciplinary and international collaboration
Our survey also highlighted the need for greater interdisciplinary collaboration and capacity building to advance the use of landscape genomics in agriculture. Many of the studies in our database were conducted by researchers in the fields of evolution, genomics, and conservation, with limited involvement from crop breeders, agronomists, and social scientists. However, as discussed earlier (Box 1), the approaches and strategies employed in evolution, conservation, and agriculture often overlap conceptually. For example, assisted migration, which is becoming increasingly important as a conservation strategy, is similar conceptually to crop replacement. Both approaches have been greatly facilitated by species distribution modeling, but the use of GEA may improve success even more, by simultaneously improving genetics and matching future environments (Isabel et al., 2020; Rising and Devineni, 2020; Sandercock et al., 2024). Agricultural scientists can also learn from landscape ecologists who, in addition to documenting associations between organisms and their environment, may also identify ongoing change in these associations. The latter can be viewed as natural experiments that can inform both climate change ecology and agriculture. Examples include natural experiments underway in forest trees such as black ash, in which range changes are tracking range shifts in pest populations (Iverson et al., 2016) or changes in the synchrony of plant–pollinator interactions (Freimuth et al., 2022). Much can be learned from such experiments, in particular with respect to orchard crops (Fraga et al., 2019; Parker et al., 2021).
Operationalizing these interdisciplinary partnerships can be accomplished in many different ways. For example, they could be based on the creation of new competitive grant programs that are specifically targeted toward the utilization of gene bank collections. There may also be opportunities to foster public/private partnerships to test specific parental combinations for underutilized crops. Fostering collaborations among these different disciplines will be critical for translating the insights from landscape genomics into practical applications for crop improvement and ensuring that these approaches benefit farmers and communities on the ground. Considered together, there is a need for the community to coordinate at a larger scale in order to develop best practices so that potential synergies between collections and genomics can be operationalized. This will require integration across governmental, academic and industry groups and the development of easy to access databases integrating many forms of data and metadata (Goff et al., 2011; Morales et al., 2022; Runck et al., 2022).
Concluding remarks and future directions
The development of landscape genomics techniques over the past decade has the potential to improve our understanding of local adaptation and enhance the resilience of our agricultural systems in the face of climate change (Yeaman, 2022; Lotterhos 2023; Whiting et al., 2024). There are several major takeaways from our reflections and review of the literature that we now discuss.
The first lesson is the importance of building comprehensive and diverse germplasm collections for the success of landscape genomics approaches in agriculture. These collections are foundational for identifying genetic variation associated with environmental pressures and ensuring that the full range of variation underlying climate adaptation is conserved and readily available for use in future breeding efforts. However, many of the current collections are incomplete or biased, which limits our ability to capture the genetic architecture of local adaptation. This is especially true for orphan (minor) crops, which play important roles in local or regional food security (Ye and Fan 2021; Cortés et al., 2022; Wu et al., 2022). Landscape genomics hold promise for speeding up climate change adaptation in such crops (Figure 1). Thus, expanding and diversifying these collections should be a high priority. However, this will require close collaboration between researchers and local communities to ensure that collections are representative and that the benefits derived are equitably shared (Box 4).
Box 4. Ethical use of germplasm collections.
Many wild relatives of crops have cultural and traditional significance. Conserving these species respects and preserves the cultural heritage of local communities and indigenous peoples who may rely on these plants for various purposes. Developing and implementing policies and legislation that support the conservation of wild relatives is essential. This includes regulations on access and benefit sharing to ensure fair and equitable use of genetic resources. Raising awareness of the importance of wild relatives of crops and their conservation is crucial for garnering support from policymakers, farmers, and the general public. Education programs can highlight the value of biodiversity for sustainable agriculture and food security. It is important that this is done with intention, as historically there has been asymmetric unidirectional transfer of benefits between nation states and non-nation formerly sovereign actors who retain rights to specific knowledge and territory.
There has been movement over the last 40 years to globally codify appropriate behavior––these mechanisms are The Convention on Biological Diversity (CBD) and the ITPGRFA. Enforcement mechanisms for these agreements involve a combination of legal, institutional, and cooperative measures. Countries that are parties to the CBD are expected to develop and implement national biodiversity strategies and action plans. This often involves enacting and enforcing domestic legislation. Parties to the convention must show compliance through national reports. The Nagoya Protocol, a supplementary agreement to the CBD, also provides a framework for access and benefit sharing of genetic resources. Moreover, the ITPGRFA establishes a multilateral system for facilitated access to plant genetic resources for food and agriculture, which further expands on how to appropriately conduct benefit sharing, including the sharing of monetary benefits derived from the commercialization and the use of standard material transfer agreements. The ITPGRFA includes a mechanism for the settlement of disputes, allowing parties to resolve conflicts related to the interpretation or application of the treaty. It’s important to note that enforcement mechanisms for international agreements often rely on the willingness of countries to cooperate and fulfill their obligations. Additionally, the development of national legislation and institutional frameworks is crucial for translating international commitments into tangible actions at the national level. Many countries with a history of colonial extraction of genetic resources still do not benefit from the products developed from their historic resources, with many of these countries being at the greatest risk from climate change (Pomeranz, 2000; Mignolo, 2011; Lipper et al., 2014; Moore, 2015; Connolly-Boutin and Smit, 2016; Patnaik, 2018). Creating accessible methods so that anyone can use these resources, allowing for equitable use of germplasm, will be essential to ensure proper benefit sharing.
Another insight from our review is the need to integrate landscape genomic approaches such as GEA with other forward genetic approaches like transcriptomics, QTL analyses, GWASs, and genomic prediction (Yeaman, et al., 2016; Gao et al., 2023). While GEA is a powerful tool for identifying putative adaptive genetic regions, it does not provide direct information on the phenotypic effects of these regions, which can sometimes be necessary to guide simpler phenotypic selection efforts. Moreover, combining GEA with GWASs and genomic prediction can help validate the functional significance of GEA-identified regions, which can then guide the targeted introgression of beneficial alleles into crop breeding programs or implementation of GS. This integration can also help mitigate potential negative associations between resilience genes and agronomically important traits by enabling the selection of optimal combinations of alleles. Another important consideration is that the genetic architecture of local adaptation is likely to vary across populations and species due to differences in the evolutionary histories and selective pressures populations/species have experienced. The species-specific history of genome duplication, intraspecific hybridization, and geographic ploidal variation will also impact selective outcomes and local adaptation. In some cases, adaptation may be driven by a few large-effect loci, while in others, it may involve many small-effect loci. These differences will affect the decisions when designing effective GEA studies, interpreting their results, and designing breeding strategies. For example, if major genes are discovered, then marker-assisted selection (MAS) might be appropriate, while genomic prediction/selection is likely to be more successful if local adaptation is highly polygenic (Bernardo and Yu, 2007). However, we must keep in mind that evolutionary adaptation will lag behind environmental change, especially for species with long generation times or if environmental change is rapid. Such a mismatch could weaken genomic signatures of adaptation. Thus, we recommend testing against climate conditions at multiple time points.
There are also conceptual challenges associated with using contemporary environmental gradients to identify adaptations for future climates (Gao et al., 2023). One promising approach involves the identification of climate analogs (e.g., Fitzpatrick and Dunn, 2019), in which the predicted future climate at a given site is matched with the current climate at another location. However, it is unclear how accurately this will predict future environments and the adaptations required for cultivars to thrive in such locations. Other possible methods include a focus on extreme environments that represent expected future conditions, integration of paleoclimate records with adaptations predicted from archaeological DNA, and controlled environment validation of current material using future projected climate ranges (Krieg et al., 2024). Building analysis and breeding pipelines that incorporate these approaches will help ensure that the best germplasm is being used to protect the food supply. Future work will also benefit from comparative studies across diverse crop species and their wild relatives, as they can reveal the general patterns of adaptation in plants and may therefore inform strategies for harnessing resilience variation for breeding programs.
GEA can make diverse germplasm more accessible to plant breeding programs by creating a shortlist of promising parents or to molecular geneticists by finding specific alleles. There is great potential to conduct validation experiments within the context of ongoing breeding work. By choosing appropriate parents, it is possible to generate more direct information about line performance. By using multi-environment trials within breeding programs, the advantages of MAS (Iftekharuddaula et al., 2011) and GS (Bernardo and Yu, 2007) can be fully embraced, and difficult phenotypes (e.g., heat and drought; Tubersosa, 2012) can be examined with levels of precision that matter for food production. Combining validation with population creation allows for both a predictive and retrospective approach to understanding the genetic control of traits. Crop species are particularly well suited to validation of these concepts (e.g., large number of individuals, large geographic ranges, and modifiable generation length). Validation through exposing new populations to stress during the breeding process creates a fertile space to track adaptation to climate. The success of landscape genomics in agriculture will depend not only on scientific advances but also on social, economic, and political factors. Ensuring that these approaches benefit small land-holding farmers and communities in developing countries, who are often the most vulnerable to climate change, will require concerted efforts to build local capacity, strengthen seed systems, and promote participatory breeding approaches (Mastretta-Yanes et al., 2024). Engaging with policymakers, funding agencies, and the private sector to prioritize and invest in this work will also be critical. In addition, there is a need for greater interdisciplinary collaboration and data sharing among researchers working in the fields of ecology, evolution, and agriculture. Historical tensions between these fields, stemming from different goals and approaches, have sometimes hindered progress. However, the challenges posed by climate change necessitate a more integrated and collaborative approach. Establishing common data standards, databases, and analytical frameworks could greatly facilitate this integration, thereby accelerating the pace of discovery and application. Increased efforts in education will be crucial for the successful use of these methods and successful adaptation to climate change.
Our review shows that agricultural landscape genomics holds promise for guiding the creation of resilient and sustainable food systems, particularly in the face of climate change. This approach allows us to harness the power of natural variation and local adaptation. By doing so, we can develop crops that can better withstand the challenges of a changing and often unpredictable environment. A concerted effort involving multiple research disciplines and industry sectors, transcending geographic and political boundaries, will be required to realize this promise.
Acknowledgments
The authors declare no competing interests.
Published: January 22, 2025
Footnotes
Supplemental information is available at Plant Communications Online.
Supplemental information
References
- Allaby R.G., Gutaker R., Clarke A.C., Pearson N., Ware R., Palmer S.A., Kitchen J.L., Smith O., Smith O. Using archaeogenomic and computational approaches to unravel the history of local adaptation in crops. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015;370 doi: 10.1098/rstb.2013.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi N., Barry M.B., Frouin J., de Navascués M., Toure M.A. Genome Scan of Rice Landrace Populations Collected Across Time Revealed Climate Changes' Selective Footprints in the Genes Network Regulating Flowering Time. Rice. 2023;16:15. doi: 10.1186/s12284-023-00633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H.C., Leon J.I. Stability analysis in plant breeding. Plant Breed. 1988;101:1–23. [Google Scholar]
- Bedford J.A., Carine M., Chapman M.A. Detection of locally adapted genomic regions in wild rice (Oryza rufipogon) using environmental association analysis. G3 (Bethesda). 2023;13:jkad194. doi: 10.1093/g3journal/jkad194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Alfonso Y., Soanes B.K., Zimba S., Sinanaj B., German L., Sharma V., Bohra A., Kolesnikova A., Dunn J.A., Martin A.C., et al. Enhancing climate change resilience in agricultural crops. Curr. Biol. 2023;33:R1246–R1261. doi: 10.1016/j.cub.2023.10.028. [DOI] [PubMed] [Google Scholar]
- Bernardo R., Yu J. Prospects for genomewide selection for quantitative traits in maize. Crop Sci. 2007;47:1082–1090. [Google Scholar]
- Bernardo R. Molecular markers and selection for complex traits in plants: learning from the last 20 years. Crop Sci. 2008;48:1649–1664. [Google Scholar]
- Bernardo R. Reinventing quantitative genetics for plant breeding: something old, something new, something borrowed, something BlUe. Heredity. 2020;125:375–385. doi: 10.1038/s41437-020-0312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker T.R., Yeaman S., Whiting J.R., Whitlock M.C. The WZA: A window-based method for characterizing genotype–environment associations. Mol. Ecol. Resour. 2024;24 doi: 10.1111/1755-0998.13768. [DOI] [PubMed] [Google Scholar]
- Bragg J.G., Supple M.A., Andrew R.L., Borevitz J.O. Genomic variation across landscapes: insights and applications. New. Phytol. 2015;207:953–967. doi: 10.1111/nph.13410. [DOI] [PubMed] [Google Scholar]
- Brockway L.H. Yale Univ. Press; 2002. Science and Colonial Expansion: The Role of the British Royal Botanical Garden. [Google Scholar]
- Britton N.L. Botanical gardens. Science. 1896;4:284–293. doi: 10.1126/science.4.88.284. [DOI] [PubMed] [Google Scholar]
- Blakeslee A.F. The botanic garden as a field museum of agriculture. Science. 1910;31:685–688. doi: 10.1126/science.31.801.685. [DOI] [PubMed] [Google Scholar]
- Bazakos C., Hanemian M., Trontin C., Jiménez-Gómez J.M., Loudet O. New strategies and tools in quantitative genetics: how to go from the phenotype to the genotype. Annu. Rev. Plant Biol. 2017;68:435–455. doi: 10.1146/annurev-arplant-042916-040820. [DOI] [PubMed] [Google Scholar]
- Calfee E., Gates D., Lorant A., Perkins M.T., Coop G., Ross-Ibarra J. Selective sorting of ancestral introgression in maize and teosinte along an elevational cline. PLoS Genet. 2021;17 doi: 10.1371/journal.pgen.1009810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell Q., Castaneda-Alvarez N., Domingo R., Bishop-von Wettberg E., Runck B. Climate resilience conserved in global germplasm repositories: Picking the most promising parents for agile plant breeding. bioRxiv. 2024 doi: 10.1101/2024.05.11.593573. Preprint at. [DOI] [Google Scholar]
- Capblancq T., Luu K., Blum M.G.B., Bazin E. Evaluation of redundancy analysis to identify signatures of local adaptation. Mol. Ecol. Resour. 2018;18:1223–1233. doi: 10.1111/1755-0998.12906. [DOI] [PubMed] [Google Scholar]
- Chang C.W., Fridman E., Mascher M., Himmelbach A., Schmid K. Physical geography, isolation by distance and environmental variables shape genomic variation of wild barley (Hordeum vulgare L. ssp. spontaneum) in the Southern Levant. Heredity. 2022;128:107–119. doi: 10.1038/s41437-021-00494-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Xu J., Wang L., Wang H., You E., Deng C., Bian H., Shen Y. Landscape genomics reveals adaptive genetic differentiation driven by multiple environmental variables in naked barley on the Qinghai-Tibetan Plateau. Heredity. 2023;131:316–326. doi: 10.1038/s41437-023-00647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Neumann K., Friedel S., Kilian B., Chen M., Altmann T., Klukas C. Dissecting the phenotypic components of crop plant growth and drought responses based on high-throughput image analysis. Plant Cell. 2014;26:4636–4655. doi: 10.1105/tpc.114.129601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen, J. (1951). Stages in the evolution of plant species.
- Cobb J.N., DeClerck G., Greenberg A., Clark R., McCouch S. Next-generation phenotyping: requirements and strategies for enhancing our understanding of genotype– phenotype relationships and its relevance to crop improvement. Theor. Appl. Genet. 2013;126:867–887. doi: 10.1007/s00122-013-2066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly-Boutin L., Smit B. Climate change, food security, and livelihoods in sub-Saharan Africa. Reg. Environ. Change. 2016;16:385–399. doi: 10.1007/s10113-015-0761-x. [DOI] [Google Scholar]
- Cortés A.J., López-Hernández F., Blair M.W. Genome–environment associations, an innovative tool for studying heritable evolutionary adaptation in orphan crops and wild relatives. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.910386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson I.K., Russell J., Powell W., Steffenson B., Thomas W.T.B., Waugh R. Barley: a translational model for adaptation to climate change. New Phytol. 2015;206:913–931. doi: 10.1111/nph.13266. [DOI] [PubMed] [Google Scholar]
- DeLacy I.H., Basford K.E., Cooper M., Bull J.K., McLaren C.G. Analysis of multi-environment trials–an historical perspective. Plant adaptation and crop improvement. 1996;39124:39–124. [Google Scholar]
- Dempewolf H., Baute G., Anderson J., Kilian B., Smith C., Guarino L. Past and Future Use of Wild Relatives in Crop Breeding. Crop Sci. 2017;57:1070–1082. doi: 10.2135/cropsci2016.10.0885. [DOI] [Google Scholar]
- Eck P. The American cranberry. Rutgers University Press; New Brunswick, NJ: 1990. [Google Scholar]
- Elias A.A., Robbins K.R., Doerge R.W., Tuinstra M.R. Half a century of studying genotype× environment interactions in plant breeding experiments. Crop Sci. 2016;56:2090–2105. [Google Scholar]
- Etterson J.R., Shaw R.G. Constraint to adaptive evolution in response to global warming. Science. 2001;294:151–154. doi: 10.1126/science.1063656. [DOI] [PubMed] [Google Scholar]
- Falconer D.S., Makay T.F.C. Longman Scientific & Technical; 1996. Introduction to Quantitative Genetics. [Google Scholar]
- Fan J., Zhang Y., Wen W., Gu S., Lu X., Guo X. The future of Internet of Things in agriculture: Plant high-throughput phenotypic platform. J. Clean. Prod. 2021;280 [Google Scholar]
- Ferrero-Serrano Á., Assmann S.M. Phenotypic and genome-wide association with the local environment of Arabidopsis. Nat. Ecol. Evol. 2019;3:274–285. doi: 10.1038/s41559-018-0754-5. [DOI] [PubMed] [Google Scholar]
- Fraga H., Pinto J.G., Santos J.A. Climate change projections for chilling and heat forcing conditions in European vineyards and olive orchards: A multi-model assessment. Climatic Change. 2019;152:179–193. [Google Scholar]
- Freimuth J., Bossdorf O., Scheepens J.F., Willems F.M. Climate warming changes synchrony of plants and pollinators. Proc. Biol. Sci. 2022;289 doi: 10.1098/rspb.2021.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T., Gersbach C.A., Barbas C.F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends. Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Kantar M.B., Moxley D., Ortiz-Barrientos D., Rieseberg L.H. Crop Adaptation to Climate Change: An Evolutionary Perspective. Mol. Plant. 2023;16:1518–1546. doi: 10.1016/j.molp.2023.07.011. [DOI] [PubMed] [Google Scholar]
- Goff S.A., Vaughn M., McKay S., Lyons E., Stapleton A.E., Gessler D., Matasci N., Wang L., Hanlon M., Lenards A., et al. The iPlant collaborative: cyberinfrastructure for plant biology. Front. Plant Sci. 2011;2:34. doi: 10.3389/fpls.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouesnard B., Negro S., Laffray A., Glaubitz J., Melchinger A., Revilla P., Moreno-Gonzalez J., Madur D., Combes V., Tollon-Cordet C., et al. Genotyping-by-sequencing highlights original diversity patterns within a European collection of 1191 maize flint lines, as compared to the maize USDA genebank. Theor. Appl. Genet. 2017;130:2165–2189. doi: 10.1007/s00122-017-2949-6. [DOI] [PubMed] [Google Scholar]
- Gudi S., Kumar P., Singh S., Tanin M.J., Sharma A. Strategies for accelerating genetic gains in crop plants: Special focus on speed breeding. Physiol. Mol. Biol. Plants. 2022;28:1921–1938. doi: 10.1007/s12298-022-01247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay K.W., Wilkinson G.N. The analysis of adaptation in a plant-breeding programme. Aust. J. Agric. Res. 1963;14:742–754. [Google Scholar]
- Fitzpatrick M.C., Dunn R.R. Contemporary climatic analogs for 540 North American urban areas in the late 21st century. Nat. Commun. 2019;10:1–7. doi: 10.1038/s41467-019-08540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin-McCormick A., Campbell Q., Negrao S., Morrell P.L., Hubner S., Neyhart J., Kantar M.B. Back to the Future: Environmental genomic selection to take advantage of polygenic local adaptation. bioRxiv. 2024:2024–2110. doi: 10.1101/2024.10.09.617488. Preprint at. [DOI] [Google Scholar]
- Hancock A.M., Brachi B., Faure N., Horton M.W., Jarymowycz L.B., Sperone F.G., Toomajian C., Roux F., Bergelson J., Bergelson J. Adaptation to climate across the Arabidopsis thaliana genome. Science. 2011;334:83–86. doi: 10.1126/science.1209244. [DOI] [PubMed] [Google Scholar]
- Hajjar R., Hodgkin T. The use of wild relatives in crop improvement: a survey of developments over the last 20 years. Euphytica. 2007;156:1–13. [Google Scholar]
- Harlan J.R. Genetic resources in wild relatives of crops 1. Crop Sci. 1976;16:329–333. doi: 10.2135/cropsci1976.0011183X001600030004x. [DOI] [Google Scholar]
- Harlan J.R., de Wet J.M.J. Toward a rational classification of cultivated plants. Taxon. 1971;20:509–517. doi: 10.2307/1218252. [DOI] [Google Scholar]
- Henry R.J. Crop Breeding. Apple Academic Press; 2016. Genomics strategies for germplasm characterization and the development of climate resilient crops; pp. 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K., Rieseberg L.H. Frequency, Origins, and Evolutionary Role of Chromosomal Inversions in Plants. Front. Plant Sci. 2020;11:296. doi: 10.3389/fpls.2020.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftekharuddaula K.M., Newaz M.A., Salam M.A., Ahmed H.U., Mahbub M.A.A., Septiningsih E.M., Collard B.C.Y., Sanchez D.L., Pamplona A.M., Mackill D.J. Rapid and high-precision marker assisted backcrossing to introgress the SUB1 QTL into BR11, the rainfed lowland rice mega variety of Bangladesh. Euphytica. 2011;178:83–97. [Google Scholar]
- Isabel N., Holliday J.A., Aitken S.N. Forest genomics: Advancing climate adaptation, forest health, productivity, and conservation. Evol. Appl. 2020;13:3–10. doi: 10.1111/eva.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson L., Knight K.S., Prasad A., Herms D.A., Matthews S., Peters M., Smith A., Hartzler D.M., Long R., Almendinger J. Potential species replacements for black ash (Fraxinus nigra) at the confluence of two threats: emerald ash borer and a changing climate. Ecosystems. 2016;19:248–270. [Google Scholar]
- Joost S., Bonin A., Bruford M.W., Després L., Conord C., Erhardt G., Taberlet P. A spatial analysis method (SAM) to detect candidate loci for selection: towards a landscape genomics approach to adaptation. Mol. Ecol. 2007;16:3955–3969. doi: 10.1111/j.1365-294X.2007.03442.x. [DOI] [PubMed] [Google Scholar]
- Kantar M.B., Sosa C.C., Khoury C.K., Castañeda-Álvarez N.P., Achicanoy H.A., Bernau V., Kane N.C., Marek L., Seiler G., Rieseberg L.H. Ecogeography and utility to plant breeding of the crop wild relatives of sunflower (Helianthus annuus L.) Front. Plant Sci. 2015;6:841. doi: 10.3389/fpls.2015.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawecki T.J., Ebert D. Conceptual issues in local adaptation. Ecol. Lett. 2004;7:1225–1241. [Google Scholar]
- Khoury C.K., Brush S., Costich D.E., Curry H.A., De Haan S., Engels J.M.M., Guarino L., Hoban S., Mercer K.L., Miller A.J., et al. Crop genetic erosion: understanding and responding to loss of crop diversity. New Phytol. 2022;233:84–118. doi: 10.1111/nph.17733. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M., Barton N.H. Evolution of a species' range. Am. Nat. 1997;150:1–23. doi: 10.1086/286054. [DOI] [PubMed] [Google Scholar]
- Koo B., Pardey P.G., Wright B.D. The economic costs of conserving genetic resources at the CGIAR centers. Agric. Econ. 2003;29:287–297. [Google Scholar]
- Koo B., Pardey P., Wright B. The price of conserving agricultural biodiversity. Nat. Biotechnol. 2003;21:126–128. doi: 10.1038/nbt0203-126. [DOI] [PubMed] [Google Scholar]
- Krieg C.P., Smith D.D., Adams M.A., Berger J., Layegh Nikravesh N., von Wettberg E.J. Greater ecophysiological stress tolerance in the core environment than in extreme environments of wild chickpea (Cicer reticulatum) Sci. Rep. 2024;14:5744. doi: 10.1038/s41598-024-56457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Verma R.P.S., Singh A., Kumar Sharma H., Devi G. Barley landraces: Ecological heritage for edaphic stress adaptations and sustainable production. Environmental and Sustainability Indicators. 2020;6 [Google Scholar]
- Lasky J.R., Upadhyaya H.D., Ramu P., Deshpande S., Hash C.T., Bonnette J., Juenger T.E., Hyma K., Acharya C., Mitchell S.E., et al. Genome-environment associations in sorghum landraces predict adaptive traits. Sci. Adv. 2015;1 doi: 10.1126/sciadv.1400218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky J.R., Josephs E.B., Morris G.P. Genotype–environment associations to reveal the molecular basis of environmental adaptation. Plant Cell. 2023;35:125–138. doi: 10.1093/plcell/koac267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levins R. Theory of fitness in a heterogeneous environment. I. The fitness set and adaptive function. Am. Nat. 1962;96:361–373. [Google Scholar]
- Leimu R., Fischer M. A meta-analysis of local adaptation in plants. PLoS One. 2008;3 doi: 10.1371/journal.pone.0004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipper L., Thornton P., Campbell B.M., Baedeker T., Braimoh A., Bwalya M., Caron P., Cattaneo A., Garrity D., Henry K., et al. Climate-smart agriculture for food security. Nat. Clim. Change. 2014;4:1068–1072. doi: 10.1038/nclimate2437. [DOI] [Google Scholar]
- Langridge P., Waugh R. Harnessing the potential of germplasm collections. Nat. Genet. 2019;51:200–201. doi: 10.1038/s41588-018-0340-4. [DOI] [PubMed] [Google Scholar]
- Langstroff A., Heuermann M.C., Stahl A., Junker A. Opportunities and limits of controlled-environment plant phenotyping for climate response traits. Theor. Appl. Genet. 2022;135:1–16. doi: 10.1007/s00122-021-03892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry D.B. Local adaptation in the model plant. New Phytol. 2012;194:888–890. doi: 10.1111/j.1469-8137.2012.04146.x. [DOI] [PubMed] [Google Scholar]
- Lotterhos K.E., Whitlock M.C. The relative power of genome scans to detect local adaptation depends on sampling design and statistical method. Mol. Ecol. 2015;24:1031–1046. doi: 10.1111/mec.13100. [DOI] [PubMed] [Google Scholar]
- Lotterhos K.E. The paradox of adaptive trait clines with nonclinal patterns in the underlying genes. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2220313120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K., Wei L., Li X., Wang Y., Wu J., Liu M., Zhang C., Chen Z., Xiao Z., Jian H., et al. Whole-genome resequencing reveals Brassica napus origin and genetic loci involved in its improvement. Nat. Commun. 2019;10:1154. doi: 10.1038/s41467-019-09134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucier G. US Department of Agriculture, Economic Research Service; 1991. US Potato Statistics, 1949-89. [Google Scholar]
- Mastretta-Yanes A., Tobin D., Bellon M.R., von Wettberg E., Cibrián-Jaramillo A., Wegier A. Human management of ongoing evolutionary processes in agroecosystems. Plants, People, Planet. 2024 doi: 10.1002/ppp3.10521. [DOI] [Google Scholar]
- Martin P., Russell J., Wishart J., Brown L.K., Wallace M., Iannetta P.P.M., George T.S. Back to the future: Using ancient Bere barley landraces for a sustainable future. Plants, People, Planet. 2023;2023:1–16. [Google Scholar]
- Mercer K., Martínez-Vásquez Á., Perales H.R. Asymmetrical local adaptation of maize landraces along an altitudinal gradient. Evol. Appl. 2008;1:489–500. doi: 10.1111/j.1752-4571.2008.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignolo W.D. Duke University Press; 2011. The Darker Side of Western Modernity: Global Futures, Decolonial Options. [Google Scholar]
- Milner S.G., Jost M., Taketa S., Mazón E.R., Himmelbach A., Oppermann M., Weise S., Knüpffer H., Basterrechea M., König P., et al. Genebank genomics highlights the diversity of a global barley collection. Nat. Genet. 2019;51:319–326. doi: 10.1038/s41588-018-0266-x. [DOI] [PubMed] [Google Scholar]
- Monteagudo A., Casas A.M., Cantalapiedra C.P., Contreras-Moreira B., Gracia M.P., Igartua E. Harnessing novel diversity from landraces to improve an elite barley variety. Front. Plant Sci. 2019;10 doi: 10.3389/fpls.2019.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.W. Verso; 2015. Capitalism in the Web of Life: Ecology and the Accumulation of Capital. [Google Scholar]
- Morales N., Ogbonna A.C., Ellerbrock B.J., Bauchet G.J., Tantikanjana T., Tecle I.Y., Powell A.F., Lyon D., Menda N., Simoes C.C., et al. Breedbase: a digital ecosystem for modern plant breeding. G3. 2022;12 doi: 10.1093/g3journal/jkac078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R., Williamson S., Kim Y., Hubisz M.J., Clark A.G., Bustamante C. Genomic scans for selective sweeps using SNP data. Gene. 2005;15:1566–1575. doi: 10.1101/gr.4252305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyhart J.L., Kantar M.B., Zalapa J., Vorsa N. Genomic-environmental associations in wild cranberry (Vaccinium macrocarpon Ait.) G3 (Bethesda). 2022;12 doi: 10.1093/g3journal/jkac203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell M.A., Jannick J.L. Genomic selection in plant breeding. Crop breeding: Methods and protocols. 2014:117–130. doi: 10.1007/978-1-4939-0446-4_10. [DOI] [PubMed] [Google Scholar]
- Oakey H., Cullis B., Thompson R., Comadran J., Halpin C., Waugh R. Genomic selection in multi-environment crop trials. G3 (Bethesda). 2016;6:1313–1326. doi: 10.1534/g3.116.027524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L., Pathak T., Ostoja S. Climate change reduces frost exposure for high-value California orchard crops. Sci. Total Environ. 2021;762 doi: 10.1016/j.scitotenv.2020.143971. [DOI] [PubMed] [Google Scholar]
- Pais A.L., Whetten R.W., Xiang Q.J. Population structure, landscape genomics, and genetic signatures of adaptation to exotic disease pressure in Cornus florida L.—Insights from GWAS and GBS data. J. Systemat. Evol. 2020;58:546–570. doi: 10.1111/jse.12592. [DOI] [Google Scholar]
- Patnaik P. Globalization and the Peasantry in the South. Agrarian South: J. Pol. Econ. 2018;7:234–248. [Google Scholar]
- Pauli D., Chapman S.C., Bart R., Topp C.N., lawrence-Dill C.J., Poland J., Gore M.A. The quest for understanding phenotypic variation via integrated approaches in the field environment. Plant. Physiol. 2016;172:622–634. doi: 10.1104/pp.16.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz K. The great divergence: China, Europe, and the making of the modern world economy. Princeton University Press; 2000. [Google Scholar]
- Qin X., Chiang C.W.K., Gaggiotti O.E. Deciphering signatures of natural selection via deep learning. Brief. Bioinformatics. 2022;23 doi: 10.1093/bib/bbac354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalski J.A. Association genetics in crop improvement. Curr. Opin. Plant Biol. 2010;13:174–180. doi: 10.1016/j.pbi.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Rana M.M., Takamatsu T., Baslam M., Kaneko K., Itoh K., Harada N., Sugiyama T., Ohnishi T., Kinoshita T., Takagi H., et al. Salt tolerance improvement in rice through efficient SNP marker-assisted selection coupled with speed-breeding. Int. J. Mol. Sci. 2019;20:2585. doi: 10.3390/ijms20102585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson D.C., Phillips R.L. Plant breeding progress and genetic diversity from de novo variation and elevated epistasis. Crop. Sci. 1997;37:303–310. [Google Scholar]
- Raven P.H. Research in botanical gardens. Bot. Jahrb. Syst. 1981;102:53–72. [Google Scholar]
- Rahaman M.M., Chen D., Gillani Z., Klukas C., Chen M. Advanced phenotyping and phenotype data analysis for the study of plant growth and development. Front. Plant Sci. 2015;6:619. doi: 10.3389/fpls.2015.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellstab C., Gugerli F., Eckert A.J., Hancock A.M., Holderegger R. A practical guide to environmental association analysis in landscape genomics. Mol. Ecol. 2015;24:4348–4370. doi: 10.1111/mec.13322. [DOI] [PubMed] [Google Scholar]
- Ren Z.H., Gao J.P., Li L.G., Cai X.L., Huang W., Chao D.Y., Zhu M.Z., Wang Z.Y., Luan S., Lin H.X. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005;37:1141–1146. doi: 10.1038/ng1643. [DOI] [PubMed] [Google Scholar]
- Rising J., Devineni N. Crop switching reduces agricultural losses from climate change in the United States by half under RCP 8.5. Nat. Commun. 2020;11:4991. doi: 10.1038/s41467-020-18725-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Ibarra J., Morrell P.L., Gaut B.S. Plant domestication, a unique opportunity to identify the genetic basis of adaptation. Proc. Natl. Acad. Sci. USA. 2007;104:8641–8648. doi: 10.1073/pnas.0700643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J., Mascher M., Dawson I.K., Kyriakidis S., Calixto C., Freund F., Bayer M., Milne I., Marshall-Griffiths T., Heinen S., et al. Exome sequencing of geographically diverse barley landraces and wild relatives gives insights into environmental adaptation. Nat. Genet. 2016;48:1024–1030. doi: 10.1038/ng.3612. [DOI] [PubMed] [Google Scholar]
- Runck B.C., Joglekar A., Silverstein K.A.T., Chan-Kang C., Pardey P.G., Wilgenbusch J.C. Digital agriculture platforms: Driving data-enabled agricultural innovation in a world fraught with privacy and security concerns. Agron. J. 2022;114:2635–2643. [Google Scholar]
- Sanchez D., Sadoun S.B., Mary-Huard T., Allier A., Moreau L., Charcosset A. Improving the use of plant genetic resources to sustain breeding programs’ efficiency. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2205780119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandercock A.M., Westbrook J.W., Zhang Q., Holliday J.A. A genome-guided strategy for climate resilience in American chestnut restoration populations. Proc. Natl. Acad. Sci. USA. 2024;121 doi: 10.1073/pnas.2403505121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen O., Pyhäjärvi T., Knürr T. Gene flow and local adaptation in trees. Annu. Rev. Ecol. Evol. Syst. 2007;38:595–619. [Google Scholar]
- Savolainen O., Lascoux M., Merilä J. Ecological genomics of local adaptation. Nat. Rev. Genet. 2013;14:807–820. doi: 10.1038/nrg3522. [DOI] [PubMed] [Google Scholar]
- Schlötterer C., Tobler R., Kofler R., Nolte V. Sequencing pools of individuals—mining genome-wide polymorphism data without big funding. Nat. Rev. Genet. 2014;15:749–763. doi: 10.1038/nrg3803. [DOI] [PubMed] [Google Scholar]
- Schreiber M., Himmelbach A., Börner A., Mascher M. Genetic diversity and relationship between domesticated rye and its wild relatives as revealed through genotyping-by-sequencing. Evol. Appl. 2019;12:66–77. doi: 10.1111/eva.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S.B., Brown L.K., Booth A., Wishart J., Hedley P.E., Martin P. Heritage genetics for adaptation to marginal soils in barley. Trends Plant Sci. 2023;28:544–551. doi: 10.1016/j.tplants.2023.01.008. [DOI] [PubMed] [Google Scholar]
- Shan D., Ali M., Shahid M., Arif A., Waheed M.Q., Xia X., Trethowan R., Tester M., Poland J., Ogbonnaya F.C., et al. Genetic networks underlying salinity tolerance in wheat uncovered with genome-wide analyses and selective sweeps. Theor. Appl. Genet. 2022;135:2925–2941. doi: 10.1007/s00122-022-04153-5. [DOI] [PubMed] [Google Scholar]
- Sharma R., Cockram J., Gardner K.A., Russell J., Ramsay L., Thomas W.T.B., O'Sullivan D.M., Powell W., Mackay I.J. Trends of genetic changes uncovered by Env- and Eigen-GWAS in wheat and barley. Theor. Appl. Genet. 2022;135:667–678. doi: 10.1007/s00122-021-03991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds N.W. Selection for local adaptation in a plant breeding programme. Theor. Appl. Genet. 1991;82:363–367. doi: 10.1007/BF02190624. [DOI] [PubMed] [Google Scholar]
- Singh R.P., Chintagunta A.D., Agarwal D.K., Kureel R.S., Kumar S.J. Varietal replacement rate: Prospects and challenges for global food security. Global Food Secur. 2020;25 [Google Scholar]
- Smale M., Jamora N. Valuing genebanks. Food Secur. 2020;12:905–918. [Google Scholar]
- Song Q., Hyten D.L., Jia G., Quigley C.V., Fickus E.W., Nelson R.L., Cregan P.B. Development and evaluation of SoySNP50K, a high-density genotyping array for soybean. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear M.M., Levi S.J., Etterson J.R., Gross B.L. Resurrecting urban sunflowers: Phenotypic and molecular changes between antecedent and modern populations separated by 36 years. Mol. Ecol. 2023;32:5241–5259. doi: 10.1111/mec.17112. [DOI] [PubMed] [Google Scholar]
- Stafleu F.A. Botanical garden before 1818. BOISSIERA. 1969;14:31–46. [Google Scholar]
- Sun Y., Shang L., Zhu Q.H., Fan L., Guo L. Twenty years of plant genome sequencing: achievements and challenges. Trends Plant Sci. 2022;27:391–401. doi: 10.1016/j.tplants.2021.10.006. [DOI] [PubMed] [Google Scholar]
- Todesco M., Owens G.L., Bercovich N., Légaré J.S., Soudi S., Burge D.O., Huang K., Ostevik K.L., Drummond E.B.M., Imerovski I., et al. Massive haplotypes underlie ecotypic differentiation in sunflowers. Nature. 2020;584:602–607. doi: 10.1038/s41586-020-2467-6. [DOI] [PubMed] [Google Scholar]
- Tuberosa R. Phenotyping for drought tolerance of crops in the genomics era. Front. Physiol. 2012;3 doi: 10.3389/fphys.2012.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajana E., Barbato M., Colli L., Milanesi M., Rochat E., Fabrizi E., Mukasa C., Del Corvo M., Masembe C., Muwanika V.B., et al. Combining Landscape Genomics and Ecological Modelling to Investigate Local Adaptation of Indigenous Ugandan Cattle to East Coast Fever. Front. Genet. 2018;9:385. doi: 10.3389/fgene.2018.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaminck L., Sang-Aram C., Botterman D., Uy C.J.C., Harper M.K., Inzé D., Gheysen G., Depuydt S., Depuydt S. Development of a novel and rapid phenotype-based screening method to assess rice seedling growth. Plant Methods. 2020;16:1–19. doi: 10.1186/s13007-020-00682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorsa N., Zalapa J. Domestication, genetics, and genomics of the American cranberry. Plant. Breed. Rev. 2019;43:279–315. [Google Scholar]
- Wang C., Hu S., Gardner C., Lübberstedt T. Emerging avenues for utilization of exotic germplasm. Trends Plant Sci. 2017;22:624–637. doi: 10.1016/j.tplants.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Wang D.R., Kantar M.B., Murugaiyan V., Neyhart J. Where the wild things are: genetic associations of environmental adaptation in the Oryza rufipogon species complex. G3 (Bethesda). 2023;13 doi: 10.1093/g3journal/jkad128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A., Ghosh S., Williams M.J., Cuddy W.S., Simmonds J., Rey M.D., Asyraf Md Hatta M., Hinchliffe A., Steed A., Reynolds D., et al. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants. 2018;4:23–29. doi: 10.1038/s41477-017-0083-8. [DOI] [PubMed] [Google Scholar]
- Wambugu P.W., Ndjiondjop M.N., Henry R.J. Role of genomics in promoting the utilization of plant genetic resources in genebanks. Brief. Funct. Genomics. 2018;17:198–206. doi: 10.1093/bfgp/ely014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting J.R., Booker T.R., Rougeux C., Lind B.M., Singh P., Lu M., Huang K., Whitlock M.C., Aitken S.N., Andrew R.L., et al. The genetic architecture of repeated local adaptation to climate in distantly related plants. Nat. Ecol. Evol. 2024;8:1933–1947. doi: 10.1038/s41559-024-02514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.L., Sherman J.D., Lamb P., Cook J., Lachowiec J.A., Bourgault M. Relationships between roots, the stay-green phenotype, and agronomic performance in barley and wheat grown in semi-arid conditions. The Plant Phenome Journal. 2022;5 [Google Scholar]
- Wu D., Shen E., Jiang B., Feng Y., Tang W., Lao S., Jia L., Lin H.Y., Xie L., Weng X., et al. Genomic insights into the evolution of Echinochloa species as weed and orphan crop. Nat. Commun. 2022;13:689. doi: 10.1038/s41467-022-28359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Xu X., Fukao T., Canlas P., Maghirang-Rodriguez R., Heuer S., Ismail A.M., Bailey-Serres J., Ronald P.C., Mackill D.J. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- Yeaman S., Hodgins K.A., Lotterhos K.E., Suren H., Nadeau S., Degner J.C., Nurkowski K.A., Smets P., Wang T., Gray L.K., et al. Convergent local adaptation to climate in distantly related conifers. Science. 2016;353:1431–1433. doi: 10.1126/science.aaf7812. [DOI] [PubMed] [Google Scholar]
- Yan L., Loukoianov A., Tranquilli G., Helguera M., Fahima T., Dubcovsky J. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA. 2003;100:6263–6268. doi: 10.1073/pnas.0937399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Loukoianov A., Blechl A., Tranquilli G., Ramakrishna W., SanMiguel P., Bennetzen J.L., Echenique V., Dubcovsky J. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science (New York, N.Y.) 2004;303:1640–1644. doi: 10.1126/science.1094305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C.Y., Fan L. Orphan Crops and their Wild Relatives in the Genomic Era. Mol. Plant. 2021;14:27–39. doi: 10.1016/j.molp.2020.12.013. ISSN 1674-2052. [DOI] [PubMed] [Google Scholar]
- Yeaman S. Evolution of polygenic traits under global vs local adaptation. Gene. 2022;220 doi: 10.1093/genetics/iyab134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Li X., Guo T., Zhu C., Wu Y., Mitchell S.E., Roozeboom K.L., Wang D., Wang M.L., Pederson G.A., et al. Genomic prediction contributing to a promising global strategy to turbocharge gene banks. Nat. Plants. 2016;2:1–7. doi: 10.1038/nplants.2016.150. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Jiang Y., Wang Z., Gou Z., Lyu J., Li W., Yu Y., Shu L., Zhao Y., Ma Y., et al. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat. Biotechnol. 2015;33:408–414. doi: 10.1038/nbt.3096. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Song R., Nevo E., Fu X., Wang X., Wang Y., Wang C., Chen J., Sun G., Sun D., et al. Genomic evidence for climate-linked diversity loss and increased vulnerability of wild barley spanning 28 years of climate warming. Sci. Total. Environ. 2024;913 doi: 10.1016/j.scitotenv.2023.169679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.