Abstract
Rice is a staple food for half of the world’s population and the largest source of greenhouse gas (GHG) from the agricultural sector, responsible for approximately 48% of GHG emissions from croplands. With the rapid growth of the human population, the increasing pressure on rice systems for extensive and intensive farming is associated with an increase in GHG emissions that is impeding global efforts to mitigate climate change. The complex rice environment, with its genotypic variability among rice cultivars, as well as emerging farming practices and global climatic changes, are important challenges for research and development initiatives that aim to lower GHG emissions and increase crop productivity. A combination of approaches will likely be needed to effectively improve the resilience of modern rice farming. These will include a better understanding of the major drivers of emissions, different cropping practices to control the magnitude of emissions, and high yield performance through systems-level studies. The use of rice hybrids may give farmers an additive advantage, as hybrids may be better able to resist environmental stress than inbred varieties. Recent progress in the development and dissemination of hybrid rice has demonstrated a shift in the carbon footprint of rice production and is likely to lead the way in transforming rice systems to reduce GHG emissions. The application of innovative technologies such as high-throughput sequencing, gene editing, and AI can accelerate our understanding of the underlying mechanisms and critical drivers of GHG emissions from rice fields. We highlight advanced practical approaches to rice breeding and production that can support the increasing contribution of hybrid rice to global food and nutritional security while ensuring a sustainable and healthy planet.
Keywords: hybrid rice; climate change; machine learning; nitrous oxide, N2O; methane, CH4; greenhouse gas
Rice contributes to 48% of GHG emissions from croplands. This review discusses opportunities for lowering GHG emissions and increasing crop productivity through the use of hybrid rice. The adoption of high-yielding, shorter-duration rice hybrids that have been identified as low GHG emitters could help to significantly reduce GHG emissions. Data-driven approaches for identifying ways to reduce GHG emissions from rice paddies are highlighted.
Introduction
The most critical problem facing humanity in this century is climate change, brought about by rising atmospheric greenhouse gas (GHG) emissions. A remarkable proportion of worldwide anthropogenic methane (CH4) and nitrous oxide (N2O) emissions comes from agriculture (Esmizade et al., 2010; Mikhaylov et al., 2020; Slingo and Slingo, 2024). Agricultural operations produce the majority of non-carbon dioxide (non-CO2) emissions (84% N2O and 47% CH4), which also account for 10%–17% of all human GHG emissions (Beach et al., 2015; Bellarby et al., 2008; Frank et al., 2019; Linquist et al., 2018; Springmann and Freund, 2022). Agricultural soils alone account for 4.1% of total GHG emissions, and rice cultivation is responsible for 1.3% (Ritchie, 2020), as shown in Figure 1A. Rice (Oryza sativa L.) is an essential food crop and the second most widely cultivated cereal crop worldwide (Bodie et al., 2019). In Asia, rice is a critical and nutrient-dense staple food. Although China and India account for the majority of rice consumption, overall consumption has grown significantly, rising from 157 million tons in 1960 to 520 million tons in 2022 (USDA, 2023). By 2030, consumption is predicted to increase by an additional ∼6% (Bin Rahman and Zhang, 2023). To meet the rising demand from the accelerated increase in the human population, rice output must be raised by 40% by 2030, which may cause significant environmental problems (IMF and UNCTAD, 2011). As a result, rice crop systems will need to be balanced by producing higher grain output with potentially lower GHG emissions.
Figure 1.
Contributions of the agricultural sector and leading rice-producing countries to global GHG emissions.
(A) Right: GHG emissions (CO2, CH4, and N2O converted to CO2 equiv using a 100-year time horizon) from different segments of the agricultural sector as of 2020. This is the latest breakdown of global emissions by sector published by Climate Watch and the World Resources Institute (Pachauri et al., 2014).
(B and C) Percentages of CH4 and N2O emissions contributed by the top 10 rice-producing countries (Ritchie, 2020; Ritchie and Roser, 2023).
Rice fields cover about 1.7 million km2 (Liu et al., 2021b) in 114 nations, accounting for 11% of all arable land worldwide (Gupta et al., 2021). It is estimated that approximately 11% and 30% of global agricultural N2O and CH4 are emitted from rice fields, respectively (Hussain et al., 2015; Ritchie and Roser, 2024). Figure 1B and 1C present the contributions of CH4 and N2O emissions from leading rice-producing countries to global GHG emissions. The rice-crop global warming potential (GWP) is 467% and 169% higher than those of wheat and maize (Linquist et al., 2012). Anaerobic soil conditions are conducive to CH4 formation, whereas N2O is produced mainly under aerobic conditions. The soil microbial processes of nitrification and denitrification create this N2O gas (Islam et al., 2020). Maximum amounts of CH4 are released when rice fields are continuously flooded, whereas significant amounts of N2O are generated under a dry cycle when rice is intermittently flooded and crops are rotated (Zhao et al., 2011). According to predictions, emissions of both GHGs could rise by 35%–60% by 2030 (Netz et al., 2007).
To establish mitigation methods and reduce the harmful effects of future climate crises, we must improve our understanding of the mechanisms of GHG emissions from rice fields and re-conceptualize the complex field environment of current and future rice production systems. In this review, we clarify the processes of GHG emissions, the primary drivers of emissions, and the potential for rice-based farming to reduce GHG emissions through climate-smart crop management systems. The wide-scale adoption of rice hybrids with a low CH4 footprint could be a game changer, together with a number of cutting-edge approaches to decrease future GHG emissions from rice fields.
Mechanisms of CH4 emission from rice fields
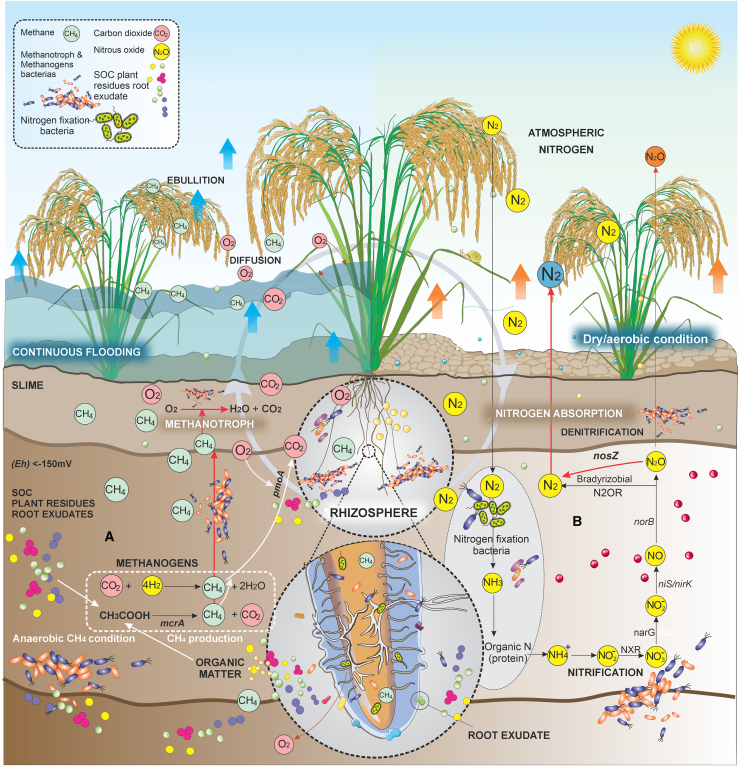
CH4 emission is one of the main components of anthropogenic GHG emissions from rice fields (Ciais et al., 2014; Tian et al., 2016). CH4 is produced through the microbial process of methanogenesis, which requires anoxic conditions and a low redox potential (Eh; < −150 mV). In rice soils, members of the domain Archaea facilitate CH4 production using fermentation products, i.e., alcohols, acetate, CO2, and H2 generated by other microorganisms during decomposition of plant matter and root exudates. Methanogenesis occurs by three biochemical pathways catalyzed by the enzyme methyl reductase. The hydrogenotrophic pathway involves the reduction of H2 to CO2 and produces CH4. The acetoclastic pathway entails splitting acetate, oxidizing the carbonyl portion of the organic molecule to CO2, and reducing the methyl portion to CH4. Methylotrophic pathways involve the production of CH4 from the methyl portion of organic compounds like methanol, methylamines, and dimethyl sulfide (Conrad, 2007; Conrad et al., 2007). Only one-third of methanogenesis in rice fields is derived from hydrogen with CO2 reduction; the rest is derived mainly from acetate (Conrad, 2007). Rice plants influence CH4 emissions by supplying root C substrates to methanogens, with the resulting CH4 carried to the atmosphere through root aerenchyma (Win et al., 2012; Kim et al., 2018). It has been reported that 90%–95% of total seasonal CH4 emissions exit the soil through rice plants and that 5%–10% of total seasonal CH4 emissions come from ebullition (Aulakh et al., 2000; Adviento-Borbe et al., 2015; Komiya et al., 2015).
Aerobic methanotrophs in the upper soil layer and rhizosphere, where O2 and CH4 gradients coincide, can convert the CH4 generated in the anoxic soil layer of rice fields to CO2 through a process known as methanotrophy or CH4 oxidation. Methanotrophs regulate the amount of CH4 gas that reaches the atmosphere. Previous studies have estimated that CH4 emissions from paddy rice could be 10%–60% higher without aerobic methanotrophs. Studies have shown that an increase in tiller number (Dubey and Singh, 2000) and plant biomass (Eller and Frenzel, 2001) can enrich the activity of CH4-oxidizing microbes by enhancing O2 transport and enlarging the volume of aerenchyma cells. Once CH4 has been produced, it is released into the atmosphere by several pathways: (i) diffusion loss of dissolved CH4 across the water–air and soil–water interfaces, (ii) ebullition loss by the release of gas bubbles, and (iii) plant transport into the roots by diffusion of CH4 gas in the aerenchyma and cortex and simultaneous release into the atmosphere via stomata (Davamani et al., 2020), as shown in Figure 2A. Co-existence of CH4-producing and CH4-oxidizing microbes in rice soils and control of the dynamic interplay between microbes and the environment by rice plants could provide opportunities to develop plant traits that lower net CH4 emissions from rice fields.
Figure 2.
CH4 and N2O production in the rice field.
The illustration depicts the complex interactions among rice roots, soil environments, and microbial communities, highlighting their roles in the production and oxidation of (A) methane (CH4) and (B) nitrous oxide (N2O). Rice root exudates influence microbial activity in the rhizosphere, promoting both the generation of N2O through nitrification and denitrification processes and the oxidation of CH4 by methanotrophic bacteria. The dynamic relationship between rice roots and microbial communities is influenced by soil properties and water management practices, which regulate the balance of GHG emissions.
Mechanisms of N2O emissions from rice fields
The microbial conversion of nitrogen (N) results in the production of N2O in soil. Nitrification and denitrification are two microbial N reactions mediated by nitrifiers (e.g., Nitrosomonas and Nitrobacter spp.) and denitrifiers (e.g., facultative anaerobic bacteria like Pseudomonas, Paracoccus, and Bacillus) (Kuypers et al., 2018). These microbes are responsible for NH3-to-N2 transformations, with N2O being a by-product of these reactions and the primary cause of net N2O emissions, as shown in Figure 2B. Nitrifiers oxidize NH3 to NO2− and then to NO3−, indirectly contributing to N2O production, especially under soil conditions where oxygen is limited, causing partial conversion of N to N2O instead of NO3−. On the other hand, during the denitrification process, the reduction of NO3− to N2 and the nitrification of NH4+ under aerobic conditions result in the loss of N as N2O. In other words, denitrification is the progressive reduction of N oxides to gaseous products like N2O or N2 in the presence of restricted O2, as seen in Figure 2B. This process, which is irreversible once NO is generated, results from bacteria using N oxide as a terminal electron acceptor rather than molecular O2 (McLain and Martens, 2005). Therefore, in an environment with low O2, a source of organic C is necessary for bacterial metabolism, and there must be enough NO3− available to act as an electron acceptor.
For denitrification to occur, all three conditions must be satisfied: a C source, low O2, and sufficient NO3 (McLain and Martens, 2006). It has been observed that greater denitrification occurs at the soil surface than in deeper subsoils, owing to the higher organic input at the soil surface caused by microbial activity. Denitrifying bacteria belong to a variety of genera. Approximately 23 genera of bacteria are capable of denitrification, including Azospirillum (Jang et al., 2019), Bacillus (Yang et al., 2020), Halobacterium (Tomlinson et al., 1986), Paracoccus (Chakravarthy et al., 2011), and Rhodopseudomonas (Kundu and Nicholas, 1985).
Nitrification involves bacterial oxidation of NH4+ or NH3 through NO2− to NO3−(Norton, 2015). Two kinds of autotrophic bacteria carry out this function. NH3 oxidizers accelerate the first step, which is the conversion of NH3 to NO2−. Nitrosomonas is the primary genus associated with this step, with other genera, like Nitrosococcus, Nitrosospira, and the subgenera Nitrosolobus and Nitrosovibrio, also capable of autotrophic NH3 oxidation. The second step is the conversion of NO2− to NO3−, which is mediated by the genus Nitrobacter. Other genera are also associated with this step, including Nitrospina, Nitrococcus, and Nitrospira (Watson et al., 1981).
The key factors that control N2O emissions from rice soils are N fertilizer application rates and water management practices (Ali et al., 2021). In addition, several field studies reported that N2O emissions varied among rice cultivars, and the differences were unaffected by genetic variations but were instead largely influenced by N input (Wang et al., 2021). A distinct soil layer is formed in rice fields after flooding, and throughout the rice-growing season, oxidizing and reducing zones form in the cultivated layer. When N fertilizer is added to rice fields, ammonium N is nitrified, and NO3− is formed at the water–soil interface in the oxidized layer. The NO3− generated in the oxidized layer travels to the reduced layer and is denitrified, creating N2O as an intermediate product (Xing et al., 2009). The denitrification process also occurs in the soil’s subsurface saturated and above-flooded cultivated layer (Xing et al., 2002). N2O is produced in rice soils after intermittent flooding during the transition from wet to dry soil conditions. Moreover, winter upland crops and the rice cycle could increase with water evaporation and add to atmospheric N2O. Rice plants serve as a route for dissolved soil gases to move from the root zone to the atmosphere, a process that results in considerable N2O emissions under flooding conditions (Yan et al., 2000). Because N2O is a water-soluble molecule, plant roots can absorb and transmit it through leaves via the transpiration pathway. Diffusion is the primary means for N2O to move to the soil surface, as shown in Figure 2B. Unlike those involved in CH4 emissions, microbial processes involved in N2O production are typically related to the amount of N available in the soil, highlighting N fertilizer rate as the major driving force for N2O emissions.
Impact of global warming on GHG emissions from rice fields
The average global surface air temperature is expected to increase by 1.4°C–4.4°C and atmospheric CO2 concentrations to reach close to 1000 ppm by the end of the 21st century (Izrael et al., 2007). Increases in atmospheric CO2 concentrations, global mean air temperature, and other factors related to climate change will significantly affect GHG emissions from rice fields. Rice paddies are one of the main anthropogenic sources of CH4, a powerful GHG, and their emissions are predicted to be affected by global warming (Qian et al., 2022). CH4 emissions from rice paddies are significantly influenced by agricultural practices (Qian et al., 2020). A report has shown that a 1°C increase in air temperature caused China’s rice fields to release 12.6% more CH4 (Qian et al., 2023). This increase probably resulted from improved C substrate availability for methanogens as well as the methanogenic activity ratio of CH4 to CO2 (Wang et al., 2018a). Furthermore, lowering the Eh of the soil induces the formation of CH4 by decreasing the solubility of O2 in water or soil solution, which speeds up the rate at which microorganisms consume O2 and other electron acceptors. In addition, air warming could increase N2O emissions from rice fields by 26% (Gao et al., 2022). The increased availability of inorganic N for N2O generation as influenced by the acceleration of soil organic matter decomposition is probably the cause of these higher N2O emissions (Bai et al., 2013; Liu et al., 2020). Furthermore, heat could alter the abundance of N2O reductase, ammonia-oxidizing, and nitrite reductase genes in bacteria and Archaea, which might increase N2O emissions through effects on the soil microbial population (Wang et al., 2022). Important soil parameters that influence the output and emissions of N2O and CH4 from rice fields are mentioned in Supplemental Table 1.
One of the critical components of global warming is the rising CO2 concentration in the atmosphere, which has increased to a new high of 415 μmol mol−1, about 149% of pre-industrial (before the year 1750) CO2 levels (Legg, 2021). This elevated CO2 has a direct feedback effect on CH4 and N2O emissions by regulating the production, oxidation, and transport of these non-CO2 gases in rice fields (Inubushi et al., 2003; Bhattacharyya et al., 2013; Wang et al., 2018b). For example, elevated CO2 increased the number and activity of methanogenic bacteria, as well as the number of tillers and aerenchyma cells, leading to enhanced gas transport and high C availability from higher root biomass (Ziska, 1998; Cheng et al., 2006; Okubo et al., 2015). Whereas elevated CO2 promotes grain yield through higher photosynthesis and root growth (Lou et al., 2008; Lv et al., 2020), variable results have been reported regarding CH4 and N2O emissions from paddy fields. Liu et al. (2019) found that CH4 and N2O emissions from global rice fields increased by 34% and 10%, respectively (Liu et al., 2019). However, other studies indicated that greater tiller counts or larger plant biomass resulted in faster O2 transport into the soil, enhancing CH4 oxidation (Ma et al., 2010; Jiang et al., 2019c). Because of higher root growth, soil denitrification potential was improved, resulting in higher N2O emissions under conditions of high organic C availability (Das et al., 2013). By contrast, Sun et al. (2018) reported a reduction in N2O emissions under elevated CO2 and attributed this decline to a decrease in soil mineral N caused mainly by high plant uptake (Sun et al., 2018). In general, most studies of elevated CO2-induced GHG emissions in rice have been performed under short-term exposure (<5 years), and their results do not represent future CO2 conditions (long-term response, >10 years). The meta-analysis performed by Yu et al. (2022) demonstrated that long-term elevated CO2 conditions significantly decreased CH4 and N2O emissions by 18% and 43%, respectively, and that emission dynamics were associated with declines in yield and biomass over time (Yu et al., 2022). A smaller increase in total plant biomass would lead to minimal C substrate accumulation.
Given the reduction in grain yields under the scenario of rising CO2, rice yield is also significantly affected by higher air temperatures. According to research performed by the International Rice Research Institute (IRRI) in the Philippines, an increase of 1°C in nighttime air temperature results in a 10% decrease in rice grain yield. An increase in atmospheric CO2 and a 1°C rise in temperature were shown to increase yield-scaled GHG emissions by 31.4% and decrease rice output by 11.8% (Van Groenigen et al., 2013). In previous studies, the enhancement of GHG emissions was attributed to additive effects of elevated CO2 and air temperature (Ziska, 1998; Tokida et al., 2010). Importantly, when air temperature rises by 1°C above historical levels, the global mean crop yields of the main staple foods (including rice) are expected to drop by 3%–10% (Wang et al., 2018a), as shown by different climate patterns and elevated temperatures in Figure 3. Although global climate changes have variable effects on GHG emissions, these changes increasingly challenge modern rice production.
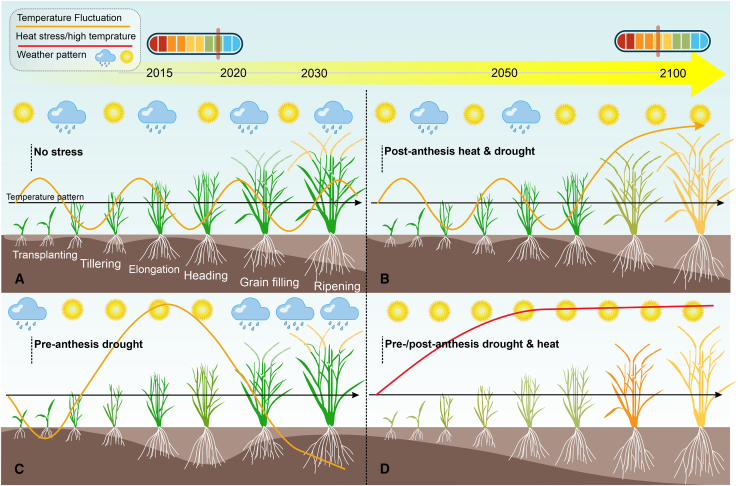
Figure 3.
Air temperature patterns during various normal, heat, and water-stress scenarios.
(A) No stress; yield benefit from optimized yield components and harvest index in rice.
(B) Post-anthesis heat and drought; possible yield losses from early maturation or possible yield benefit from stay-green characteristics.
(C) Pre-anthesis drought; severe impact on plant growth and development, leading to reduced yield. Deeper roots and/or early anthesis could help to reduce the impact of this stress.
(D) Pre-/post-anthesis drought and heat; significant impact on plant morphology, physiology, and development, causing dramatic yield reduction.
Mechanisms and processes driving the effects of mitigation practices on GHG emissions
Three primary crop-management parameters (i.e., irrigation water, soil organic matter, and fertilizer) and the use of low-GHG-emitting rice varieties can effectively decrease CH4 and N2O in rice fields. These interventions directly affect soil microbial activity by changing the availability and dynamics of microbial growth substrates, namely, carbon and N. Critical strategies for decreasing GHG emissions from rice fields are described below.
Irrigation water management
A primary strategy for decreasing CH4 emissions from paddy fields is irrigation water management. Water management changes soil moisture and soil Eh. These two factors have a substantial impact on how quickly GHGs are released and consumed (Wang et al., 2017). Non-continuous flooding (NCF) techniques, such as midseason drainage, intermittent irrigation, and alternate wetting and drying (AWD) (Minamikawa et al., 2019), usually decrease the presence and activity of methanogens (Qian et al., 2023). Increases and decreases in CH4 emissions during rice growth depend largely on the duration of flooding and rice phenology. Figure 4 shows a typical CH4 emission profile for drill or dry seeding under continuously flooded irrigation. Here, CH4 emissions are low and close to zero during the early growth stage because soils are not saturated and aerenchyma cells are not yet fully developed. As rice plants grow and reach the vegetative stage, CH4 emissions increase, peak around heading, and then decline toward physiological maturity. A sharp decline in CH4 emissions occurs when flooding is disrupted around the reproductive stages, such as during the dry-down event in AWD irrigation. Low CH4 emissions may extend during this stage if fields undergo frequent dry and wet cycles.
Figure 4.
Dynamics of GHG emissions from drill-seeded rice fields.
The trade-off between CH4 and N2O emissions in flooding and non-flooding (AWD) practices. Shaded portions indicate flooded conditions. Orange and red curves represent the patterns of CH4 and N2O, respectively, under different water-regime practices.
As soil O2 concentrations and Eh increase during a dry-down event, methanotroph activity and abundance increase, stimulating CH4 oxidation while inhibiting methanogens. Different irrigation techniques in NCF practice, such as scheduled, midseason, intermittent, AWD, and furrow irrigation, have the potential to minimize N2O and CH4 emissions without compromising grain yield. The frequency, timing, and intensity of soil drying duration could be optimized to maximize reductions in GHG emissions. For example, the total number of days without flooding is correlated with the effect of NCF on CH4 emissions; on average, single and several drying episodes decrease CH4 emissions by 33% and 64%, respectively (Jiang et al., 2019b). The Guidelines for National Greenhouse Gas Inventories, published by the Intergovernmental Panel on Climate Change in 2006, estimated a median 48% reduction in CH4 emissions compared with the baseline of transplanted puddled fields. CH4 emissions from rice were found to be 43% lower using AWD irrigation rather than conventional, continuously flooded irrigation when combined with water conservation (Sander et al., 2016). On the other hand, N2O emissions from continuously flooded rice systems are often negative or low throughout the growing season (Perry et al., 2022), because nitrification and denitrification activities predominantly occur during re-flooding and drying of soil (Bouwman, 1998). N2O peaks generally occur after the addition of N fertilizer during the early stage of rice growth or the midseason stage, when another dose of N fertilizer is applied (Figure 4). Under NCF, soil O2 concentrations increase, and N-converting microorganisms become more active, increasing N2O emissions (Hou et al., 2000; Islam et al., 2018).
Soil organic matter management
The second main management parameter is organic matter. Farmyard manure, green manure, and crop residue are conventional products used by farmers to manage soil fertility. The community composition of methanogens and methanotrophs changes when organic matter is added over an extended period of time. These changes are indicated by the relative abundance of hydrogenotrophic and acetoclastic methanogens (Zhang et al., 2018; Raheem et al., 2022) and by the abundance of methanotrophs that may prefer high CH4 concentrations (Yang et al., 2022a). Different straw management practices cause significant changes in soil organic carbon composition and dynamics (Jiang et al., 2019a; Yang et al., 2021). However, because straw incorporation increases soil organic carbon sequestration in the long term, the addition of organic matter to rice fields may have a net climatic impact (Liu et al., 2014). Crop yields have frequently been reported to increase with soil organic carbon (SOC) content (Oldfield et al., 2022). Farm strategies based on soil organic matter management can have conflicting effects on yield and emissions. However, by considering the net carbon balance of the system, techniques such as straw removal, the use of varieties with high root growth, and fields with high SOC may result in decreased emissions. Potential tradeoffs may be considered for environments with low SOC, as straw incorporation is desirable in combination with other practices. Some reports have suggested that a high-yielding variety with more extensive root growth can increase emissions. This interactions may lead to a net positive carbon gain at the system level by limiting the increase in emissions while enabling more significant gains in SOC sequestration.
Fertilizer management
CH4 and N2O emissions can be indirectly and directly affected by fertilizer N management, leading to variations in emissions. Previous studies have reported that fertilizer N can increase, decrease, or have no effect on CH4 emissions (Cai et al., 2007; Shang et al., 2011; Yao et al., 2012). However, a recent meta-analysis showed that the influence of N fertilizer depends largely on input rate, with low to moderate N rates increasing CH4 emissions and excessive N rates decreasing CH4 emissions (Linquist et al., 2012). In particular, N fertilization increases the activity of methanogens and speeds up the breakdown of organic matter, which most strongly increase CH4 emissions in acidic soils (Tang et al., 2024).
The application of mineral N has also been reported to produce higher CH4 emissions than those in low input systems without N application (Schroeder et al., 2013). A decrease in the amount of CH4 oxidation occurred as a result of CH4 monooxygenase binding and reacting with NH4+ (Gulledge and Schimel, 1998). By contrast, N2O emissions are related to the time of N fertilization and water management practices. High N2O emissions have been reported in fields with intermittent or midseason dry events or with N application rates above optimal levels (Cai et al., 1997; Zou et al., 2005). Furthermore, increased crop growth in response to N fertilizer increases shoot and root development, which in turn increases substrate availability for methanogens (Schimel, 2000). Rice yield and the type and rate of N fertilizer are also related to emissions from rice fields. Subsurface N application, enhanced-efficiency N fertilizers, and optimal N rate have been reported to reduce GHG emissions. Better land use planning, effective field management techniques, less land disturbance, direct planting, and climate-smart water management practices might also minimize CH4 and N2O emissions.
Effects of rice varieties on GHG emissions
Emissions vary significantly among high-yielding rice cultivars, likely because of differences in CH4 production, CH4 oxidation, anatomical characteristics, and gas transport capacities (Watanabe et al., 1995; Wang et al., 1997, 2000; Yang et al., 2009; Qin et al., 2015). Rice plants have two main strategies to control their CH4 emissions. The first of these processes involves the rhizodeposition of rice plants, which supplies 40%–60% of the organic C as CH4 substrate to methanogens starting at the booting stage (Watanabe et al., 1999; Yuan et al., 2012). The second mechanism is the diffusion of atmospheric O2 into the rhizosphere of rice plants through root aerenchyma, which stimulates CH4 oxidation (Conrad, 2007). Varietal effects have been demonstrated through field screening for CH4 emissions among different rice types and varieties. These effects were reported as non-significant with the limited sets of varieties tested. However, the varietal effects were observed consistently when integrated with management and environment.
A meta-analysis to quantify the effects of rice varieties on the GWP of GHG emissions at the yield scale in China revealed that indica rice varieties had a significantly higher yield-scaled GWP (1101.72 kg CO2 equiv Mg−1) than japonica rice varieties (711.38 kg CO2 equiv Mg−1) (Zheng et al., 2014). This difference may be attributed to varietal differences in root exudation, organic matter decomposition in flooded soils, and interactions with soil microbiota, which result in various levels of CH4 emissions. In addition, rice varieties can affect N2O emissions through differences in N use efficiency and N cycling in the soil. Also, a higher harvest index and productivity per unit day could reduce GHG emission relative to that of longer-duration varieties (Smith et al., 2013). Jiang et al. (2017) screened 33 rice cultivars and found that those with lower emissions were high-yielding cultivars with higher biomass and enhanced root porosity (Jiang et al., 2017). Aerobic rice varieties were reported to release 80%–85% less CH4 into the environment and to have a reduced carbon footprint, as they are grown under NCF conditions while at the same time contributing to increased carbon assimilation through greater crop growth and yield potential (Parthasarathi et al., 2012; Sandhu et al., 2013; Sritharan et al., 2015). Furthermore, studies have shown that lines that are robust to drought and show minor yield loss under different water regimes have low CH4 emissions (Sander et al., 2015). Growth duration also has a significant effect on seasonal emissions; varieties with a shorter growth duration have 25%–30% lower emissions overall compared with medium- and late-maturing varieties with similar daily emission rates.
Over the last decade, significant efforts have been made to breed climate-resilient varieties. This is a critical strategy for addressing the effect of climate change on rice production (Haefele et al., 2016; Atlin et al., 2017). No genetic engineering program is currently focused on breeding low-GHG-emitting varieties, although hypotheses about the features that contribute to differences in CH4 emissions across genotypes have been proposed. For instance, cultivars that demonstrate ozone tolerance, improved N use efficiency, and higher water use efficiency have been shown to generate less CH4. Among the traits of interest are a strong root-oxidizing capacity, a high harvest index, and fewer unproductive tillers (Wang et al., 1999). Varieties with lower respiratory losses will potentially have lower GHG emissions (Chauhan and Mahajan, 2016). Similarly, varieties with lower root exudates and less aerenchyma, may also have reduced emissions (Weller et al., 2018). As a result, breeding programs that leverage these findings, e.g., by creating genotypes with a larger rhizosphere and less carbon release from the root zone, can contribute to transforming rice systems for lower carbon emissions. It is worth noting, however, that advances in the creation of cultivars tolerant to water-scarce environments have enabled the development of high-yielding varieties adapted for Direct seeded rice. These varieties facilitate the scaling of rice systems under aerobic conditions and NCF water management, leading to lower CH4 emissions. In addition, efforts to develop highly productive genotypes with shorter growth durations continue in current breeding programs.
Mitigation through wide-scale adoption of improved low-GHG-emitting rice hybrids
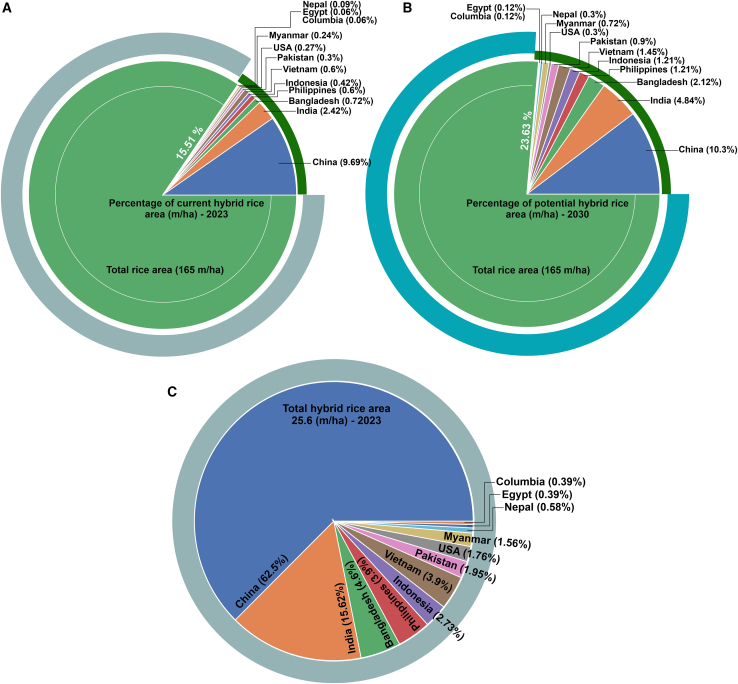
According to the latest report in 2022, approximately 165 million hectares (m/ha) of land are under rice cultivation across the world, and 15.51 m/ha of these were under hybrid rice cultivation in 2024. According to the IRRI’s hybrid rice program, the estimated area under hybrid rice cultivation by 2030 will be approximately 23.63 m/ha, as shown in Figure 5A and 5B, respectively. Figure 5C also presents the percentage of hybrid rice cultivation area that is accounted for by each of the top hybrid-rice-growing countries.
Figure 5.
Current and predicted areas under hybrid rice cultivation.
(A) Percentage of current hybrid rice area in m/ha (2023).
(B) Potential hybrid rice area in m/ha (2030).
(C) Percentages of the current hybrid rice area accounted for by top hybrid-rice-growing countries.
The amount of CH4 emitted per hectare is approximately 300 kg CH4 ha−1 season−1 (Wassmann et al., 2000a), equivalent to approximately 49.5 Mt CH4 per season worldwide. Studies have shown that paddies with high-yielding hybrid rice varieties produce approximately 19% lower CO2 emissions than those with inbred varieties under the same growth conditions (Taghavi et al., 2017). By extrapolating this value to the total area under hybrid rice cultivation in 2024, the total CO2 equiv emissions will be approximately 102.5 Mt per season for the total area under hybrid rice cultivation.
Hybrid rice technology is also a viable approach for obtaining grain yields 30% higher than those of the best inbred varieties (Wang et al., 2024). Many of the high-yielding hybrid rice varieties have a shorter growth duration than inbred varieties. Rice hybrids have higher productivity per day than inbred varieties and need less time in the field with reduced irrigation levels. This makes hybrid rice highly effective in reducing GHG emissions from rice fields. Interestingly, several climate-resilient, shorter-duration rice hybrids have recently been developed at the IRRI, with a duration of <110 days and a grain yield exceeding 10 t/ha. This directly effects the significant reduction in GHG emission compared with that of inbred varieties. It is important to know that certain hybrids produce lower CH4 emissions, and researchers need to identify them. Smartt et al. (2018) clearly demonstrated that CH4 emissions were significantly lower from three hybrid rice varieties (CLXL729, XL753, and CLXL745) than from the inbred variety RoyJoy. These hybrids have the potential to mitigate CH4 emissions from rice production on silt loam soils in the mid-southern United States. In another study, a hybrid rice (CLXP4534) produced significantly lower CH4 emissions than inbred varieties under continuous flooding conditions (Simmonds et al., 2015). From Table 1, it is very clear that there are genotypic differences among the hybrids. Certain hybrids, like RT7521 FP, CLXL745, and CLXP4534, were found to emit less CH4 than other hybrids and inbreds. Therefore, it is essential to breed and identify parental lines that emit less CH4 in order to develop low-CH4-emitting hybrids.
Table 1.
Relative performance and GHG emissions of rice inbreds and hybrids under different irrigation practices in the United States and Asian countries
| Irrigation practice | Country | Cultivar | Type | Methane (CH4) |
Nitrous oxide (N2O) |
Global warming potential (GWP)a |
Grain yield |
Reference | |
|---|---|---|---|---|---|---|---|---|---|
| kg CH4-C ha−1 season−1 |
kg N2O-N ha−1 season−1 |
kg CO2 equiv ha−1 season−1 |
kg CO2 equiv t−1 season−1 |
t ha−1 |
|||||
| – | – | Area-scaled | Yield-scaled | – | |||||
| Continuously flooded | United States | Francisb | inbred | 60 | 0.10 | 2102 | 332 | 6.2 | Simmonds et al., 2015 |
| Jupiterb | inbred | 72 | 0.04 | 2397 | 345 | 6.9 | |||
| Sabineb | inbred | 65 | 0.11 | 2286 | 391 | 5.8 | |||
| CLXP4534 | hybrid | 25 | 0.17 | 981 | 140 | 7.0 | |||
| CLXL745 | hybrid | 56 | 0.02 | 1899 | 232 | 8.2 | |||
| Continuously flooded | United States | RoyJoyb | inbred | 75 | nd | nd | nd | 9.8 | Smartt et al., 2018 |
| CLXL729 | hybrid | 55 | nd | nd | nd | 9.8 | |||
| CLXL745 | hybrid | 49 | nd | nd | nd | 9.8 | |||
| XL753 | hybrid | 53 | nd | nd | nd | 12.6 | |||
| Continuously flooded | United States | CL151b | inbred | 120 | 0.17 | 4562 | 536 | 8.0 | 2019 technical reports to RiceTec and USDA |
| XP753 | hybrid | 115 | 0.10 | 4333 | 436 | 10 | |||
| XP760 | hybrid | 129 | 0.08 | 4848 | 555 | 10 | |||
| CLXL745 | hybrid | 106 | 0.08 | 4003 | 439 | 9.0 | |||
| Continuously flooded | United States | CLL15b | inbred | 74 | 0.70 | 2984 | 352 | 10 | 2022 technical reports to RiceTec and USDA |
| RT7321 | hybrid | 82 | 0.65 | 3239 | 265 | 12 | |||
| RT7521 FP | hybrid | 56 | 0.71 | 2332 | 198 | 12 | |||
| Alternate wetting and drying (AWD) | United States | CL151b | inbred | 39 | 0.50 | 1682 | 191 | 8.0 | 2019 technical reports to RiceTec and USDA |
| XP753 | hybrid | 37 | 0.15 | 1434 | 156 | 10 | |||
| XP760 | hybrid | 38 | 0.10 | 1454 | 156 | 11 | |||
| CLXL745 | hybrid | 21 | 0.17 | 872 | 101 | 9.0 | |||
| Furrow irrigation | United States | CLL15b | inbred | 14 | 1.11 | 979 | 117 | 8.5 | 2022 technical reports to RiceTec and USDA |
| RT7321 | hybrid | 12 | 0.88 | 791 | 69 | 12 | |||
| RT7521 FP | hybrid | 5.7 | 0.72 | 513 | 44 | 12 | |||
| Continuously flooded | India | Monohar Salib | inbred | 140 | nd | nd | nd | 3.5–6 | Gogoi et al., 2008 |
| Betguti Salib | inbred | 119 | nd | nd | nd | 3–3.5 | |||
| Peolib | inbred | 107 | nd | nd | nd | 3.5–4 | |||
| IR-36b | inbred | 66 | nd | nd | nd | 3–5 | |||
| Continuously flooded | China | Zhongzhuob | inbred | 17 | nd | nd | nd | 7.7 | Wang et al., 2000 |
| Jingyoub | inbred | 37 | nd | nd | nd | 6.8 | |||
| Zhongzhuab | inbred | 33 | nd | nd | nd | 6.9 | |||
| IR72b | inbred | 24 | nd | nd | nd | 4.5 | |||
| Continuously flooded | China | Zhongfu 906b | inbred | 71 | nd | nd | nd | 5.2 | Lu et al., 2000 |
| Xiusui 11b | inbred | 75 | nd | nd | nd | 5.1 | |||
| Chungjiang 06b | inbred | 137 | nd | nd | nd | 5.2 | |||
| II-you 1568 | hybrid | 84 | nd | nd | nd | 4.9 | |||
| Jin23a/71 | hybrid | 67 | nd | nd | nd | 4.9 | |||
| Shanyou 10 | hybrid | 125 | nd | nd | nd | 5.5 | |||
| Continuously flooded | Philippines | IR65597b | inbred | 4.5 | nd | nd | nd | 1.5 | Wassmann et al., 2000b |
| PSBRc14b | inbred | 4.5 | nd | nd | nd | 3.1 | |||
| Magat | hybrid | 3 | nd | nd | nd | 5.1 | |||
| IR72 | inbred | 6 | nd | nd | nd | 3.1 | |||
| Continuously flooded | China | Huanghuazhanb | inbred | 240 | nd | nd | nd | 25c | Liao et al., 2019 |
| Rongyouhuazhan | hybrid | 188 | nd | nd | nd | 27c | |||
| Continuously flooded | Indonesia | Ciherangb | inbred | 278 | nd | nd | nd | 5.5 | Kartikawati et al., 2019 |
| Sembada 989 | hybrid | 239 | nd | nd | nd | 4.8 | |||
| Sembada 168 | hybrid | 304 | nd | nd | nd | 5.3 | |||
| Mapan 05 | hybrid | 442 | nd | nd | nd | 6.0 | |||
| Arize Gold | hybrid | 495 | nd | nd | nd | 6.2 | |||
| Intani | hybrid | 398 | nd | nd | nd | 5.7 | |||
| Hipa 8 | hybrid | 399 | nd | nd | nd | 5.2 | |||
| Hipa 18 | hybrid | 335 | nd | nd | nd | 5.2 | |||
| Hipa 19 | hybrid | 343 | nd | nd | nd | 5.0 | |||
nd, no data.
GWP (area-scaled) was computed using IPCC 2021 conversion factors of 273 and 28 over a 100-year time horizon for N2O and CH4, respectively, whereas GWP (yield-scaled) was calculated as the ratio of GWP to grain yield.
Inbred cultivars. §Pot experiment in which grain yields were extrapolated from g plant−1.
Hybrid varieties have greater root porosity than inbred cultivars and can therefore transport more O2 into the soil for methanotrophs, promoting greater CH4 oxidation (Ma et al., 2010; Kim et al., 2018). Previous studies have demonstrated that CH4 emissions vary significantly among high-yielding rice varieties, likely owing to differences in CH4 production, CH4 oxidation, anatomical characteristics, and gas transport capacities (Watanabe et al., 1995; Wang et al., 1997, 2000; Yang et al., 2009; Qin et al., 2015). With more evidence supporting reduced CH4 emissions from high-yielding rice varieties, there is increasing interest in the use of hybrid varieties to develop strategies for reducing CH4 emissions while simultaneously producing higher grain yields. In addition, because rice hybrids have higher root biomass and a deeper root system, they help with sequestration of carbon deeper in the soil. It is also possible that the robust root systems of hybrids closer to the soil surface may be able to attract methanotroph bacteria to break down CH4 into CO2 and water (Figure 3). However, the molecular and physiological mechanisms that underlie reduced GHG emission by hybrids under flooded and non-flooded conditions have yet to be studied intensively.
Several studies have shown that certain low-GHG-emitting hybrid varieties grown under continuous flooding and/or NCF consistently had lower CH4 emissions than inbred cultivars (Ma et al., 2010; Smartt et al., 2016; Brye et al., 2017; Liao et al., 2019). Field studies performed across the United States and Asia demonstrated that there was, on average, a 25% decrease in total CH4 emissions in some high-yielding hybrids compared with those in inbred varieties under continuous flooding irrigation (Table 1). CH4 emissions, especially in low-GHG-emitting, high-yielding hybrid varieties, declined by 52% relative to inbred cultivars under AWD/furrow irrigation (Table 1). Also, according to these United States studies (Table 1), there was no difference in average total N2O emissions between inbred and hybrid varieties and irrigation practices at recommended fertilizer N rates, which averaged 0.35 kg N2O-N ha−1 season−1. Expressing the GWP of CH4 and N2O emissions relative to grain yield, as opposed to total area, provides a better assessment of the agronomic and environmental benefits of GHG mitigation strategies. The results in Table 1 show that the yield-scaled GWP of some low-GHG-emitting hybrids decreased by 34% and 55% for continuous flooding and NCF irrigation, respectively. Here, the optimal cultivar response when considering a win–win strategy to mitigate GHG emissions through varietal selection is to have the lowest yield-scaled GWP with the highest grain yield and the lowest area-scaled GWP.
Carbon sequestration capacity of hybrid rice
On a global scale, rice soils (0–100 cm) contain an average of 108 Mg SOC ha−1, corresponding to 1.2% of the worldwide SOC pool (Smith et al., 2007; Liu et al., 2021a, 2021b). Although rice soils cover less than 9% of the total global cropland area, these soils retain more than 14% of their SOC stocks (Woodwell, 1984; FAO, 2017; Liu et al., 2021b), suggesting that rice soils have more SOC than upland agricultural soils (Pan et al., 2004; Wu, 2011). Unlike cropland soils, rice soils have high SOC because they are under anaerobic conditions due to periodic flooding and long-term puddling (Qiu et al., 2018). Anaerobic conditions slow the rate of organic matter decomposition, which, in turn, increases soil C accumulation compared with upland soils (Wang et al., 2015; Wei et al., 2021). Agronomic practices that increase biomass production, increase crop residue input, and slow the production of respiratory CO2 can increase SOC reserves, thereby sequestering carbon (Lal, 2004b; Wang et al., 2015). However, although rice fields store more SOC than the global average, an increase in soil C stocks does not always lead to C sequestration if there is no net removal of CO2 from the atmosphere. There are limits to the amount of C that soils can sequester, and this soil C saturation is driven by soil texture, aggregation, and, to some extent, the biophysical composition of the organic input (Six et al., 2002). According to Lal (2004a), the attainable soil C capacity is only 50%–66% of the potential soil capacity. Soils with a large C saturation deficit sequester more C than soils close to saturation.
Many rice fields, particularly in Asia, are under long-term puddled rice cultivation and have various amounts of soil C stocks, leading to various degrees of C sequestration potential (Ma et al., 2010, 2021; Kalbitz et al., 2013). Changes in C pool size could strongly affect atmospheric CO2 concentrations. The amount of soil organic C in rice paddies is directly related to the decomposition of soil organic matter, which mainly produces CO2. CO2 emissions from agriculture contribute <1% to the total global C budget because CO2 emissions are largely offset by high rates of net primary productivity and CO2 uptake by crops (Friedlingstein et al., 2020). Researchers have agreed that changes in SOC over time reflect the net balance between soil respiration and C fixation in cropland (Stewart et al., 2007; Deng et al., 2024). Hybrid rice, through its higher yield performance, may accelerate the fixation of CO2 through a high photosynthetic rate and biomass production, thereby facilitating and improving the C sequestration capacity of rice paddies. Although hybrid rice affects C sequestration, no studies have demonstrated this effect for SOC.
Future hybrid rice breeding strategies for low carbon emissions
Despite the enormous amount of research on linking various high-yielding rice cultivars to lower CH4 emissions (Table 1), the net effects of hybrid varieties on CH4 and N2O emissions and the underlying mechanisms involved in decreasing GHG emissions are largely unknown. Many studies have speculated that hybrid rice varieties have lower CH4 emissions because of different genotypic traits associated with CH4 oxidation, such as photosynthetic C partitioning, a more robust root system, greater gas transport capacity, efficient metabolic activity, and plant architecture (Table 1). Recently, several studies have tried to explain the fundamental processes that control CH4 emissions under emerging irrigation technologies such as intermittent flooding. These studies have focused on complex interactions between high-yielding hybrid varieties and microbes under drained conditions to better understand the feedback of the aerobic cycle on CH4 emissions and rice productivity (Edwards et al., 2015; Fernández-Baca et al., 2021). For example, Santos-Medellín et al. (2021) reported a compositional shift characterized by an increase in Actinobacteria (e.g., Streptomyces) in the endospheric communities of the rice root microbiota, which affected root microbial recovery during a prolonged dry cycle. Liechty et al. (2020) reported that the root microbiome of the high-CH4-emitting inbred rice variety Sabine, compared with that of the low-CH4-emitting hybrid rice variety CLXL745, was characterized by both methanogens and other bacterial groups associated with fermentation, iron, and sulfate reduction and acetogenesis, processes that support methanogenesis. This alteration of microbial communities driven by the aerobic cycle requires further research because microbial communities in rice fields exhibit considerable variation, and our current understanding of CH4 cycling is based on cultured strains and known groups of soil microbiota that may not be viable across diverse rice environments (Conrad, 2007; Zhang et al., 2017). Under current and predicted climatic conditions, the development of climate-resilient hybrid cultivars relies on acclimating these new plant types to changes in management practices and growth environments. One primary trait needed to improve hybrid response to climate-driven abiotic stresses and, at the same time, reduce GHG emissions is the efficient use of water and N fertilizer. As discussed previously, the implementation of dry-down conditions during rice growth has been recognized to reduce >50% of total CH4 emissions. However, the field performance of major rice hybrid germplasms under non-flooding irrigation practices showed that the recurring water stress commonly observed in intermittent flooding may reduce grain yield by 7%–89%, depending on the severity of the dry cycle and the duration of drought stress (Villa et al., 2012; Monkham et al., 2015; Torres and Henry, 2018; Johnson et al., 2023). Although biochemical, plant architectural, and physiological traits have been linked to grain production under water stress, there are still no consistent correlations between component traits (root biomass, stomatal conductance) and grain yields (Dixit et al., 2014; Vikram et al., 2015). This observation is not uncommon, because the response of rice plants to abiotic stress is complex and involves hundreds of genes. Recent studies have suggested that rice crops may sustain high grain yield production under water stress if certain biochemical properties (accumulation of more carbohydrates in leaves, maintenance of stomatal conductance and photosynthetic rates, and better regulation of canopy temperature) are achieved (Fukuda et al., 2018; Barnaby et al., 2019; McClung et al., 2019). Here, it appears that a detailed understanding of the response of rice plants to restrictive drought conditions, such as reoccurring dry events and the extent of dryness, is a prerequisite for developing hybrids tolerant to water stress. Breeding of new rice hybrids should also consider the interaction between plant productivity and efficient use of N in soil. N is a critical constituent of plant cells and chlorophyll and promotes the rapid growth of panicles and grains. Because N is the primary nutrient for growth and yield performance, slight N deficiencies can reduce rice growth and productivity. When fields undergo a dry cycle, soil N can be lost through denitrification, immobilization, and fixation, becoming unavailable for plant uptake (Buresh et al., 2008). In addition, drying a flooded rice field can increase N2O emissions through denitrification and nitrification, creating a trade-off between CH4 and N2O emissions. Field studies have reported a significant increase in N2O emissions with excessive N fertilizer application and inefficient irrigation water management (Adviento-Borbe et al., 2013; Lahue et al., 2016). Therefore, efforts to identify new genes responsible for the low-CH4-emission trait should include genetic traits that can produce more grains under suboptimal soil N content. This could involve the expression of genes responsible for the high-affinity transport system, which functions under low N concentrations (<250 μM) and uses Nitrate Transporter 2 (NRT2) and Ammonium Transporter 1 (AMT1) for the uptake of NO3− and NH4+, respectively (Li and Zhang, 2009; Dechorgnat et al., 2019). Another strategy that might enable hybrid rice to resist the effects of climate change is to introduce new genes that reduce net CH4 emission by enhancing CH4 oxidation through low amounts of root C exudate production. Scientists have long acknowledged that the primary factor controlling CH4 emissions in lowland rice is the presence of the plant itself. Recent studies reported that the contribution of root organic C to CH4 production was 41% at tillering and about 60% from booting to the maturity stage, demonstrating that rice roots produce organic acids and carbohydrates that are significant substrates for the formation of CH4 in rice paddies (Lu and Conrad, 2005; Yuan et al., 2012; Leichty et al., 2021). Several studies have reported that acetate, ethanoic acid, malic acid, citric acid, and succinic acid from root exudation or substrate fermentation are the main precursors for CH4 production (Wassmann et al., 1998; Chidthaisong et al., 1999; Moscôso et al., 2019; Qi et al., 2024). However, the primary source for methanogenesis among all major root-released C substrates is still unidentified. One primary focus for the development of new plant types is a better understanding of the underlying mechanisms and the target C substrates that directly control CH4 oxidation and production in rice soils and how these substrates are altered under a changing climate. As described above, the successful inclusion of hybrid rice varietal selections in multiple mitigation management practices requires identification of the genes responsible for the low-CH4-emitting, high-yielding trait and the expression of this trait in new and improved hybrid genotypes. An essential consideration for breeding of a climate-resilient hybrid is the viability of the new plant type under agricultural field conditions and its acclimation to stressful growing conditions associated with climate change (i.e., salinity, drought, high CO2 content, and high nighttime air temperature).
Smart plant breeding programs for GHG mitigation
Rice breeding programs must identify rice germplasms with lower GHG emissions, and such materials must be used for the entire rice breeding pipeline, especially targeting transplant conditions. Pre-breeding efforts must identify potential donors and place target genes relevant to different market segments into elite parental backgrounds. Varieties must be bred for short duration with lower CH4 emissions under both transplanted and direct-seeded conditions. Target traits should include higher general combination ability, higher outcrossing features, earliness, anaerobic germination tolerance, seedling vigor, nutrient use efficiency, water use efficiency, abiotic stress tolerance to drought and heat, and resistance to nematodes, blast, bacterial blight, and brown plant hopper. Breeding within the heterotic pools should follow a genomic selection approach, keeping the crosses limited to target traits related to low GHG emissions. Parental lines with lower GHG emissions will be helpful for developing a series of high-yielding, short-duration, low-carbon-footprint rice hybrids. Rice breeding must also go hand in hand with crop management practices that influence the production of GHGs in the rice environment. Identification of low-GHG-emitting hybrids is essential to maximize the gains for a given set of management practices. Furthermore, modification of the management system can also provide robust opportunities for mitigation options. The correct choice of low-GHG-emitting hybrids that are high yielding and have a short duration is of primary importance. It is vital to augment this choice with proper agricultural and management practices, such as the timing of irrigation schedules, management of organic additives, appropriate amounts and rates of N fertilizers, tillage procedures, cropping regimes, etc., to mitigate N2O and CH4 emissions from rice fields. The emergence of new technologies such as AI, genome editing, and genomic selection could bring about a revamped workflow for obtaining new types of rice varieties with minimal changes to cultural practices and could promote cost-effective farm operations while limiting the production and emission of N2O and CH4 from rice fields and maintaining high yield performance.
Genome editing strategies to target genes that control low GHG emissions
Where large-scale phenotyping facilities exist, the identification of candidate genes and quantitative trait loci could enable the investigation of plant features that contribute to mitigating CH4 emissions. Association mapping is another method for identifying genomic regions underlying a specific region linked to low CH4 emissions or associated qualities in available germplasm resources. The genetic and allelic variations between rice lines with high and low CH4 emissions can be examined, despite the lack of genomic data on regions associated with low CH4 emissions.
Researchers are now looking into new methods for precise and rapid genomic modifications to increase crop production and protect crops from various challenges (Taranto et al., 2018). The most effective method for modifying the plant genome with sequence-specific nucleases is genome editing (GE). The tremendous capacity of GE for crop development to combat food insecurity and create a worldwide climate-smart agricultural system is unmatched (Liu et al., 2013). GE technologies have had a significant impact on plant breeding techniques. The site-specific endonucleases used in GE technologies include CRISPR-Cas9, transcription activator-like effector nucleases, and zinc-finger nucleases (Zhu et al., 2017). The CRISPR-Cas9 system is emerging as the most effective GE technique because it is affordable, quick, and accurate. It enables site-specific editing inside the genome, in contrast to GE tools based on zinc-finger nucleases and transcription activator-like effector nucleases (Raza et al., 2019). GE could be used as a rapid breeding approach for editing critical genes that reduce GHG emissions in rice and the surrounding microbiome. The CRISPR-Cas9 system can precisely alter plant DNA to achieve desired features, which can be used to produce rice with lower GHG emissions. In the quest to remove carbon, three steps can be defined for gene editing in rice crops. The first step is to strive to increase photosynthesis so that plants can absorb as much CO2 as possible, for example, by improving OsHXK1 through the CRISPR-Cas9 system (Zheng et al., 2021). Second, efforts should focus on developing rice with longer roots and compatibility with lower GHG emissions. Rice plants transport carbon to the soil through their roots (as well as from crop residues upon harvest). Longer roots can bury carbon deeper in the ground, thus reducing the likelihood that it is released into the atmosphere (Kirschbaum et al., 2021). Third, the ability of the soil to retain GHGs must be increased rather than removing or converting GHGs to less destructive forms such as CH4 to CO2, N2O to N2, and CO2 to bicarbonates. Carbon is often not retained in the soil for long. One potential outcome of CRISPR-Cas9 research is a product that could be added to the soil to nurture a soil microbiome that holds on to carbon for a longer period of time (Jansson et al., 2023).
Rhizosphere engineering to reduce GHG emissions from rice paddies
Rhizosphere engineering, which focuses on strategically manipulating plant–microbe interactions in the root zone to achieve desired outcomes (Khatibi et al., 2024), offers a promising strategy for reducing GHG emissions from rice paddies. There are four key strategies for mitigating CH4 emissions through rhizosphere engineering: (1) optimizing carbon allocation, directing more carbon toward rice grains; (2) regulating root exudate composition to lessen the preference for rhizospheric methanogens; (3) increasing the abundance of rhizospheric and endospheric methanotrophs; and (4) modifying root architecture to enhance oxygen transport (Kwon et al., 2024). Evidence indicates that redirecting photosynthates to favor seeds over roots can reduce CH4 emissions and increase rice yields (Kwon et al., 2023). In addition, modulating the composition of root exudates to decrease glucose levels could reduce CH4 emissions by up to 50% (Luo et al., 2022). Rice genes that induce the growth and activity of beneficial microbes, such as CH4 consumers or methanotrophs, can also significantly reduce CH4 emissions. Furthermore, limiting the formation of aerenchyma potentially results in lower CH4 emissions and reduces oxygen release from roots. Systematic breeding of hybrid rice varieties with optimized carbon allocation, regulated root exudates, minimal aerenchyma formation, intense oxidation activity, and effective induction of methanotroph activity could collectively help to reduce CH4 emissions in rice fields (Supplemental Figure 1). Nevertheless, the complexity of plant–microbe interactions in the rhizosphere makes it difficult to predict the outcomes of rhizosphere engineering. At the same time, environmental factors such as soil type, water management, and climate can have a considerable effect on its success. Future directions in rhizosphere engineering should focus on better understanding the intricacies of plant–microbe interactions in the rhizosphere and their influence on CH4 emissions. Advances in microbial ecology, genomics, and biotechnology offer opportunities to fine-tune rhizosphere engineering strategies, specifically the use of gene-editing tools to precisely manipulate rice root traits to optimize microbial interactions and reduce CH4 emissions. In addition, the integration of rice rhizosphere engineering with sustainable agricultural practices such as AWD irrigation could enhance its effectiveness.
Using machine learning to develop low-carbon practices for hybrid rice systems
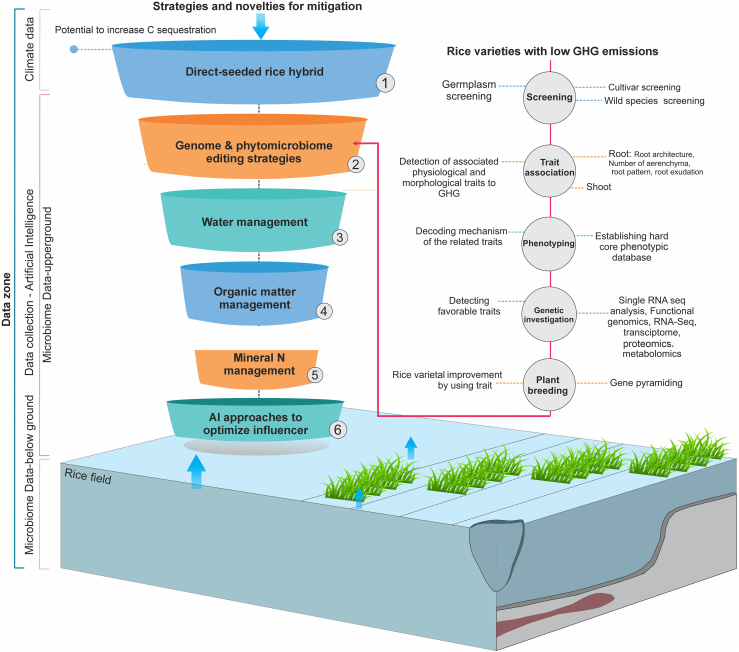
To decipher the multi-layer complexity of GHG emissions and develop appropriate management practices for paddy fields, it will be necessary to consider all the drivers of GHG emissions in this complex environment. Machine learning (ML) and deep learning (DL) approaches can handle the complicated relationships between predictors and target variables and can therefore offer the advantages of rapid computation, good heterogeneity, and high prediction performance (Kamir et al., 2020; Khatibi and Ali, 2024). Recently, ML has been used to estimate GHG emissions from soil, primarily in drylands. For instance, a prior study used a random forest approach to estimate N2O emissions from no-tillage canola under various N application rates and found that moisture and soil N availability were the most crucial factors (Glenn et al., 2021). Another study evaluated the effectiveness of many ML models for forecasting soil CO2 and N2O emissions from oat, maize, and soybean rotation systems (Jiang et al., 2023). The results showed that the long short-term memory network model was more accurate than the root zone water quality model performance (Hamrani et al., 2020). ML has also been used to close the gaps in CH4 fluxes detected by eddy covariance (Irvin et al., 2021). Supplemental Table 2 presents the updated applications of ML and DL to CO2 and N2O emission research in the crop sector. However, plenty of opportunities remain to decipher the multi-layer complexity of GHG emissions from rice paddies using ML and DL approaches. The primary advantage of ML is its ability to enable classification, simplification, and forecasting with highly complex datasets, including diverse types of datasets with different types of data. The power of AI enables comprehensive monitoring and mobilization by analyzing data across larger geographic and temporal scales, enabling detailed observations of intricate processes. ML and DL can help to predict and select the best practices for management and comprehensive strategies applicable to all aspects of GHG challenges. Also, AI approaches have robust capacity and potential for decoding microbiome relations in rice plants with underlying agro/and molecular mechanisms with GHG emission from rice paddies. Since low-cost, high-throughput sequencing technologies have become available, the collection of microbiome data has become increasingly common. Because AI has massive potential to analyze highly complex data with multi-layer interactions, it may be the best option for automated decision-making when optimizing management systems and genomic selection to reduce CH4 and N2O emissions from rice fields. Figure 6 illustrates all the possible main drivers and parameters, together with accessible datasets, that should be considered for reducing GHG emissions from rice paddies. These multi-layer datasets could be used as model feeders for robust AI and ML models.
Figure 6.
Strategic workflow and potential datasets for use in reducing the carbon footprint of rice paddies.
The diagram explains the different types of datasets that could be used to extract comprehensive patterns for all major and minor drivers of GHG emissions from rice paddies. This uniform pattern can help develop the most feasible strategy to significantly reduce the carbon footprint of rice fields through robust approaches like AI and ML. AI and ML algorithms have a strong ability to understand complicated patterns that belong to different layers of GHG emissions from rice fields, such as omics, genetics, management practices, and environmental data.
Summary and future perspectives
Paddy rice is one of the most significant sources of CH4 and N2O emissions. The breakdown of organic matter produces CH4 under anaerobic conditions, whereas N2O is produced through nitrification and denitrification processes during the transition from flooded to dry conditions in rice fields. On the basis of in situ data, global CH4 emissions, N2O emissions, and yield-scaled GHG emissions from rice fields are estimated to average 283 kg CH4 ha−1, 1.7 kg N2O ha−1, and 0.9 kg CO2 kg−1, respectively. Under climate-change scenarios, these emissions are expected to rise and to reduce crop productivity. Field trials indicate that warming will result in a 15%–23% increase in worldwide CH4 emissions and a 10%–13% reduction in rice yield. Differences in CH4 and N2O emissions are affected mainly by irrigation, organic matter management, N fertilization, and rice selection. Understanding the major drivers of emissions and the processes that control their magnitude through system-level studies will likely improve the development of strategies for GHG mitigation. A key consideration for mitigation practices is that they must involve little to no modification of current crop management and farm equipment in order to increase grower adoption. Sustained high-yield performance with lower GHG emissions is a valuable metric and resource for rice breeders trying to address the negative effects of the climate crisis. Hybrid selection is an effective strategy that offers multiple benefits to farmers, such as sustained high grain yields, minimal yield-scaled GHG emissions, and tremendous potential for C storage in fields. Furthermore, the adoption of nutrient-use-efficient and direct-seeded rice hybrids will accelerate reductions in the C footprint of paddy fields.
Despite enormous amounts of research on GHG emissions and reduction approaches, the complex interactions among major drivers and how their effects are altered by different agronomic management practices remain unclear. Emerging techniques, such as ML and DL, have tremendous potential to bring about an understanding of the most sophisticated patterns and extract the best decisions using whole datasets collected on management practices, plant genotypes, phytomicrobiomes, and environmental and climate patterns. Advanced breeding techniques will also aid in the development of rice cultivars with lower GHG emissions under future climate-change scenarios. Genomic selection with high-throughput phenotyping, genome-wide association studies, ML/AI approaches, and genotyping strategies are important for the identification of genes for rice improvement to reduce GHG emissions. We must produce environmentally friendly genome-modified rice to combat these emissions using the CRISPR-Cas9 system or other new approaches. However, there are still several leading players that should be gaining attention to reduce GHG emissions; these include the phytomicrobiome, as one of the generators and regulators of GHG emissions in rice fields. Our current ability to disentangle the functional relationships between GHG emissions and soil microorganisms is made possible by advanced developments in molecular techniques for soil microbiology. This information could be instrumental in establishing novel and comprehensive mitigation strategies. In other words, to comprehensively understand the mechanisms and regulators involved in GHG emissions from rice fields, the best approach is to integrate all possible data from all main actors to extract a unique and realistic pattern, leading to the best and most optimized strategy for permanent reduction of the GHG emissions associated with rice production.
Funding
The authors declare that financial support was received for the authorship and publication of this article. This publication was funded by the International Rice Research Institute (IRRI)–Hybrid Rice Development Consortium (HRDC) and the AGGRi Alliance project “Accelerated Genetic Gains in Rice Alliance” by the Bill and Melinda Gates Foundation through grant OPP1194925-INV 008226.
Acknowledgments
No conflict of interest is declared.
Author contributions
S.M.H.K., M.A.A.-B., N.G.D., A.M.R., and J.A. contributed to the conceptualization, design, and manuscript writing; all authors contributed to revising the manuscript. All authors read and approved the manuscript.
Published: December 28, 2024
Footnotes
Supplemental information is available at Plant Communications Online.
Supplemental information
References
- Adviento-Borbe M.A., Necita Padilla G., Pittelkow C.M., Simmonds M., Van Kessel C., Linquist B. Methane and nitrous oxide emissions from flooded rice systems following the end-of-season drain. J. Environ. Qual. 2015;44:1071–1079. doi: 10.2134/jeq2014.11.0497. [DOI] [PubMed] [Google Scholar]
- Adviento-Borbe M.A., Pittelkow C.M., Anders M., van Kessel C., Hill J.E., McClung A.M., Six J., Linquist B.A. Optimal fertilizer nitrogen rates and yield-scaled global warming potential in drill seeded rice. J. Environ. Qual. 2013;42:1623–1634. doi: 10.2134/jeq2013.05.0167. [DOI] [PubMed] [Google Scholar]
- Ali I., Zhao Q., Wu K., Ullah S., Iqbal A., Liang H., Zhang J., Muhammad I., Khan A., Khan A., et al. Biochar in combination with nitrogen fertilizer is a technique: to enhance physiological and morphological traits of Rice (Oryza sativa L.) by improving soil physio-biochemical properties. J. Plant Growth Regul. 2021;41:2406–2420. doi: 10.1007/s00344-021-10454-8. [DOI] [Google Scholar]
- Atlin G.N., Cairns J.E., Das B. Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Global Food Secur. 2017;12:31–37. doi: 10.1016/j.gfs.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulakh M., Bodenbender J., Wassmann R., Rennenberg H. Methane transport capacity of rice plants. I. Influence of methane concentration and growth stage analyzed with an automated measuring system. Nutrient Cycl. Agroecosyst. 2000;58:357–366. doi: 10.1023/A:1009831712602. [DOI] [Google Scholar]
- Bai E., Li S., Xu W., Li W., Dai W., Jiang P. A meta-analysis of experimental warming effects on terrestrial nitrogen pools and dynamics. New Phytol. 2013;199:441–451. doi: 10.1111/nph.12252. [DOI] [PubMed] [Google Scholar]
- Barnaby J.Y., Rohila J.S., Henry C.G., Sicher R.C., Reddy V.R., McClung A.M. Physiological and metabolic responses of rice to reduced soil moisture: Relationship of water stress tolerance and grain production. Int. J. Mol. Sci. 2019;20:1846. doi: 10.3390/ijms20081846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach R.H., Creason J., Ohrel S.B., Ragnauth S., Ogle S., Li C., Ingraham P., Salas W. Global mitigation potential and costs of reducing agricultural non-CO2 greenhouse gas emissions through 2030. J. Integr. Environ. Sci. 2015;12:87–105. doi: 10.1080/1943815X.2015.1110183. [DOI] [Google Scholar]
- Bellarby, J., Foereid, B., Hastings, A.F.S.J., and Smith, P. (2008). Cool Farming: Climate impacts of agriculture and mitigation potential.
- Bhattacharyya P., Roy K.S., Neogi S., Dash P.K., Nayak A.K., Mohanty S., Baig M.J., Sarkar R.K., Rao K.S. Impact of elevated CO2 and temperature on soil C and N dynamics in relation to CH4 and N2O emissions from tropical flooded rice (Oryza sativa L.) Sci. Total Environ. 2013;461–462:601–611. doi: 10.1016/j.scitotenv.2013.05.035. [DOI] [PubMed] [Google Scholar]
- Bin Rahman A.N.M.R., Zhang J. Trends in rice research: 2030 and beyond. Food Energy Secur. 2023;12:e390. doi: 10.1002/fes3.390. [DOI] [Google Scholar]
- Bodie A.R., Micciche A.C., Atungulu G.G., Rothrock M.J., Jr., Ricke S.C. Current trends of rice milling byproducts for agricultural applications and alternative food production systems. Front. Sustain. Food Syst. 2019;3:47. doi: 10.3389/fsufs.2019.00047. [DOI] [Google Scholar]
- Bouwman A.F. Nitrogen oxides and tropical agriculture. Nature. 1998;392:866–867. doi: 10.1038/31809. [DOI] [Google Scholar]
- Brye K.R., Rogers C.W., Smartt A.D., Norman R.J., Hardke J.T., Gbur E.E. Methane emissions as affected by crop rotation and rice cultivar in the Lower Mississippi River Valley, USA. Geoderma regional. 2017;11:8–17. doi: 10.1016/j.geodrs.2017.08.004. [DOI] [Google Scholar]
- Buresh R.J., Ramesh Reddy K., Van Kessel C. Nitrogen transformations in submerged soils. Nitrogen in agricultural systems. 2008;49:401–436. [Google Scholar]
- Cai Z., Shan Y., Xu H. Effects of nitrogen fertilization on CH4 emissions from rice fields. Soil Sci. Plant Nutr. 2007;53:353–361. doi: 10.1111/j.1747-0765.2007.00153.x. [DOI] [Google Scholar]
- Cai Z., Xing G., Yan X., Xu H., Tsuruta H., Yagi K., Minami K. Methane and nitrous oxide emissions from rice paddy fields as affected by nitrogen fertilisers and water management. Plant Soil. 1997;196:7–14. [Google Scholar]
- Chakravarthy S.S., Pande S., Kapoor A., Nerurkar A.S. Comparison of denitrification between Paracoccus sp. and Diaphorobacter sp. Appl. Biochem. Biotechnol. 2011;165:260–269. doi: 10.1007/s12010-011-9248-5. [DOI] [PubMed] [Google Scholar]
- Chauhan B.S., Mahajan G. Strategies for Boosting Rice Yield in the Face of Climate Change in India. J. Rice Res. 2016;4:105. doi: 10.4172/jrr.1000105. Page 2 of 5 Volume 1⋅ Issue 1⋅ 1000105 J Rice Res ISSN: JRR, an open access journal Center for Atmospheric Research, US) scenario. South Asia and world production of rice in 2050. [DOI] [Google Scholar]
- Cheng Y., Han Y., Wang Q., Wang Z. Seasonal dynamics of fine root biomass, root length density, specific root length, and soil resource availability in a Larix gmelinii plantation. Front. Biol. China. 2006;1:310–317. doi: 10.1007/s11515-006-0039-2. [DOI] [Google Scholar]
- Chidthaisong A., Rosenstock B., Conrad R. Measurement of monosaccharides and conversion of glucose to acetate in anoxic rice field soil. Appl. Environ. Microbiol. 1999;65:2350–2355. doi: 10.1128/aem.65.6.2350-2355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciais P., Sabine C., Bala G., Bopp L., Brovkin V., House J.I. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Change. Cambridge University Press; 2014. Carbon and other biogeochemical cycles; pp. 465–570. [DOI] [Google Scholar]
- Conrad R. Microbial ecology of methanogens and methanotrophs. Adv. Agron. 2007;96:1–63. doi: 10.1016/S0065-2113(07)96005-8. [DOI] [Google Scholar]
- Conrad R., Chan O.-C., Claus P., Casper P. Characterization of methanogenic Archaea and stable isotope fractionation during methane production in the profundal sediment of an oligotrophic lake (Lake Stechlin, Germany) Limnol. Oceanogr. 2007;52:1393–1406. doi: 10.4319/lo.2007.52.4.1393. [DOI] [Google Scholar]
- Das T., Bhattacharyya R., Sharma A., Das S., Saad A., Pathak H. Impacts of conservation agriculture on total soil organic carbon retention potential under an irrigated agro-ecosystem of the western Indo-Gangetic Plains. Eur. J. Agron. 2013;51:34–42. doi: 10.1016/j.eja.2013.07.003. [DOI] [Google Scholar]
- Davamani V., Parameswari E., Arulmani S. Mitigation of methane gas emissions in flooded paddy soil through the utilization of methanotrophs. Sci. Total Environ. 2020;726 doi: 10.1016/j.scitotenv.2020.138570. [DOI] [PubMed] [Google Scholar]
- Dechorgnat J., Francis K.L., Dhugga K.S., Rafalski J.A., Tyerman S.D., Kaiser B.N. Tissue and nitrogen-linked expression profiles of ammonium and nitrate transporters in maize. BMC Plant Biol. 2019;19:206–213. doi: 10.1186/s12870-019-1768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L., Cheung S., Liu J., Chen J., Chen F., Zhang X., Liu H. Nanoplastics impair growth and nitrogen fixation of marine nitrogen-fixing cyanobacteria. Environ. Pollut. 2024;350 doi: 10.1016/j.envpol.2024.123960. [DOI] [PubMed] [Google Scholar]
- Dixit S., Singh A., Kumar A. Rice breeding for high grain yield under drought: a strategic solution to a complex problem. International Journal of Agronomy. 2014;2014:1–15. doi: 10.1155/2014/863683. [DOI] [Google Scholar]
- Dubey S., Singh J. Spatio-temporal variation and effect of urea fertilization on methanotrophs in a tropical dryland rice field. Soil Biol. Biochem. 2000;32:521–526. doi: 10.1016/S0038-0717(99)00181-9. [DOI] [Google Scholar]
- Edwards J., Johnson C., Santos-Medellín C., Lurie E., Podishetty N.K., Bhatnagar S., Eisen J.A., Sundaresan V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA. 2015;112:E911–E920. doi: 10.1073/pnas.1414592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller G., Frenzel P. Changes in activity and community structure of methane-oxidizing bacteria over the growth period of rice. Appl. Environ. Microbiol. 2001;67:2395–2403. doi: 10.1128/AEM.67.6.2395-2403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmizade, S., Landi, A., and Gilani, A. (2010). Evaluating the amount of carbonic greenhouse gasses (GHGes) emission from rice paddies.
- FAO (2017). FAOSTAT: FAO Statistical Databases. Food and Agriculture Organization of the United Nations.
- Fernández-Baca C.P., Rivers A.R., Maul J.E., Kim W., Poudel R., McClung A.M., Roberts D.P., Reddy V.R., Barnaby J.Y. Rice plant–soil microbiome interactions driven by root and shoot biomass. Diversity. 2021;13:125. doi: 10.3390/d13030125. [DOI] [Google Scholar]
- Frank H., Schmid H., Hülsbergen K.-J. Modelling greenhouse gas emissions from organic and conventional dairy farms. J. Sustain. Org. Agric. Syst. 2019;69:37–46. doi: 10.3168/jds.2017-13272. [DOI] [Google Scholar]
- Friedlingstein P., O'sullivan M., Jones M.W., Andrew R.M., Hauck J., Olsen A., Peters G.P., Peters W., Pongratz J., Sitch S. Global carbon budget 2020. Earth Syst. Sci. Data Discuss. 2020;2020:1–3. doi: 10.5194/essd-12-3269-2020. [DOI] [Google Scholar]
- Fukuda Y., Hirao T., Mishima K., Ohira M., Hiraoka Y., Takahashi M., Watanabe A. Transcriptome dynamics of rooting zone and aboveground parts of cuttings during adventitious root formation in Cryptomeria japonica D. Don. BMC Plant Biol. 2018;18:201–214. doi: 10.1186/s12870-018-1401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Tian H., Zhang Z., Xia X. Warming-induced greenhouse gas fluxes from global croplands modified by agricultural practices: A meta-analysis. Sci. Total Environ. 2022;820 doi: 10.1016/j.scitotenv.2022.153288. [DOI] [PubMed] [Google Scholar]
- Glenn A.J., Moulin A.P., Roy A.K., Wilson H.F. Soil nitrous oxide emissions from no-till canola production under variable rate nitrogen fertilizer management. Geoderma. 2021;385 doi: 10.1016/j.geoderma.2020.114857. [DOI] [Google Scholar]
- Gogoi N., Baruah K.K., Gupta P.K. Selection of rice genotypes for lower methane emission. Agron. Sustain. Dev. 2008;28:181–186. doi: 10.1051/agro:2008005. [DOI] [Google Scholar]
- Gulledge J., Schimel J.P. Low-concentration kinetics of atmospheric CH4 oxidation in soil and mechanism of NH4+ inhibition. Appl. Environ. Microbiol. 1998;64:4291–4298. doi: 10.1128/aem.64.11.4291-4298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K., Kumar R., Baruah K.K., Hazarika S., Karmakar S., Bordoloi N. Greenhouse gas emission from rice fields: a review from Indian context. Environ. Sci. Pollut. Res. Int. 2021;28:30551–30572. doi: 10.1007/s11356-021-13935-1. [DOI] [PubMed] [Google Scholar]
- Haefele S.M., Kato Y., Singh S. Climate ready rice: augmenting drought tolerance with best management practices. Field Crops Res. 2016;190:60–69. doi: 10.1016/j.fcr.2016.02.001. [DOI] [Google Scholar]
- Hamrani A., Akbarzadeh A., Madramootoo C.A. Machine learning for predicting greenhouse gas emissions from agricultural soils. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140338. [DOI] [PubMed] [Google Scholar]
- Hou A.X., Chen G.X., Wang Z.P., Van Cleemput O., Patrick W.H., Jr. Methane and nitrous oxide emissions from a rice field in relation to soil redox and microbiological processes. Soil Sci. Soc. Am. J. 2000;64:2180–2186. doi: 10.2136/sssaj2000.6462180x. [DOI] [Google Scholar]
- Hussain S., Peng S., Fahad S., Khaliq A., Huang J., Cui K., Nie L. Rice management interventions to mitigate greenhouse gas emissions: a review. Environ. Sci. Pollut. Res. Int. 2015;22:3342–3360. doi: 10.1007/s11356-014-3760-4. [DOI] [PubMed] [Google Scholar]
- IMF O., UNCTAD W. FAO; Roma, Italy: 2011. Price Volatility in Food and Agricultural Markets: Policy Responses. [Google Scholar]
- Inubushi K., Cheng W., Aonuma S., Hoque M., Kobayashi K., Miura S., Kim H.Y., Okada M. Effects of free-air CO2 enrichment (FACE) on CH4 emission from a rice paddy field. Global Change Biol. 2003;9:1458–1464. doi: 10.1046/j.1365-2486.2003.00665.x. [DOI] [Google Scholar]
- Irvin J., Zhou S., McNicol G., Lu F., Liu V., Fluet-Chouinard E., Ouyang Z., Knox S.H., Lucas-Moffat A., Trotta C., et al. Gap-filling eddy covariance methane fluxes: Comparison of machine learning model predictions and uncertainties at FLUXNET-CH4 wetlands. Agric. For. Meteorol. 2021;308–309 doi: 10.1016/j.agrformet.2021.108528. [DOI] [Google Scholar]
- Islam S.F.-u., van Groenigen J.W., Jensen L.S., Sander B.O., de Neergaard A. The effective mitigation of greenhouse gas emissions from rice paddies without compromising yield by early-season drainage. Sci. Total Environ. 2018;612:1329–1339. doi: 10.48550/arXiv.2006.02917. [DOI] [PubMed] [Google Scholar]
- Islam S.F.-u., Sander B.O., Quilty J.R., De Neergaard A., Van Groenigen J.W., Jensen L.S. Mitigation of greenhouse gas emissions and reduced irrigation water use in rice production through water-saving irrigation scheduling, reduced tillage and fertiliser application strategies. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.140215. [DOI] [PubMed] [Google Scholar]
- Izrael Y.A., Semenov S.M., Anisimov O.A., Anokhin Y.A., Velichko A.A., Revich B.A., Shiklomanov I.A. The fourth assessment report of the intergovernmental panel on climate change: Working group II contribution. Russ. Meteorol. Hydrol. 2007;32:551–556. [Google Scholar]
- Jang J., Sakai Y., Senoo K., Ishii S. Potentially mobile denitrification genes identified in Azospirillum sp. strain TSH58. Appl. Environ. Microbiol. 2019;85:e02474-18–e02418. doi: 10.1128/AEM.02474-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson J.K., McClure R., Egbert R.G. Soil microbiome engineering for sustainability in a changing environment. Nat. Biotechnol. 2023;41:1716–1728. doi: 10.1038/s41587-023-01932-3. [DOI] [PubMed] [Google Scholar]
- Jiang X., Tan X., Cheng J., Haddix M.L., Cotrufo M.F. Interactions between aged biochar, fresh low molecular weight carbon and soil organic carbon after 3.5 years soil-biochar incubations. Geoderma. 2019;333:99–107. doi: 10.1016/j.fcr.2019.02.010. [DOI] [Google Scholar]
- Jiang Y., Carrijo D., Huang S., Chen J., Balaine N., Zhang W., van Groenigen K.J., Linquist B. Water management to mitigate the global warming potential of rice systems: A global meta-analysis. Field Crops Res. 2019;234:47–54. doi: 10.1126/sciadv.aau9038. [DOI] [Google Scholar]
- Jiang Y., van Groenigen K.J., Huang S., Hungate B.A., van Kessel C., Hu S., Zhang J., Wu L., Yan X., Wang L., et al. Higher yields and lower methane emissions with new rice cultivars. Global Change Biol. 2017;23:4728–4738. doi: 10.1111/gcb.13737. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Qian H., Huang S., Zhang X., Wang L., Zhang L., Shen M., Xiao X., Chen F., Zhang H., et al. Acclimation of methane emissions from rice paddy fields to straw addition. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aau9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Yang S., Smith P., Pang Q. Ensemble machine learning for modeling greenhouse gas emissions at different time scales from irrigated paddy fields. Field Crops Res. 2023;292 doi: 10.1016/j.fcr.2023.108821. [DOI] [Google Scholar]
- Johnson K., Vo T.T.B., Van Nha D., Asch F. Genotypic responses of rice to alternate wetting and drying irrigation in the Mekong Delta. J. Agron. Crop Sci. 2023;209:593–612. doi: 10.1111/jac.12649. [DOI] [Google Scholar]
- Kalbitz K., Kaiser K., Fiedler S., Kölbl A., Amelung W., Bräuer T., Cao Z., Don A., Grootes P., Jahn R., et al. The carbon count of 2000 years of rice cultivation. Global Change Biol. 2013;19:1107–1113. doi: 10.1111/gcb.12080. [DOI] [PubMed] [Google Scholar]
- Kamir E., Waldner F., Hochman Z. Estimating wheat yields in Australia using climate records, satellite image time series and machine learning methods. ISPRS J. Photogrammetry Remote Sens. 2020;160:124–135. doi: 10.1016/j.isprsjprs.2019.11.008. [DOI] [Google Scholar]
- Kartikawati R., Yulianingsih E., Yuniati I., Wihardjaka A. IOP Conference Series: Earth and Environmental Science. IOP Publishing; 2019. A strategy for reducing methane emission using hybrid rice variety. [DOI] [Google Scholar]
- Khatibi S.M.H., Ali J. Harnessing the power of machine learning for crop improvement and sustainable production. Front. Plant Sci. 2024;15 doi: 10.3389/fpls.2024.1417912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatibi S.M.H., Dimaano N.G., Veliz E., Sundaresan V., Ali J. Exploring and exploiting the rice phytobiome to tackle climate change challenges. Plant Communications. 2024;5 doi: 10.1016/j.xplc.2024.101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.-J., Bui L.T., Chun J.-B., McClung A.M., Barnaby J.Y. Correlation between methane (CH4) emissions and root aerenchyma of rice varieties. Plant breeding and biotechnology. 2018;6:381–390. doi: 10.9787/PBB.2018.6.4.381. [DOI] [Google Scholar]
- Kirschbaum M.U., Don A., Beare M.H., Hedley M.J., Pereira R.C., Curtin D., McNally S.R., Lawrence-Smith E.J. Sequestration of soil carbon by burying it deeper within the profile: a theoretical exploration of three possible mechanisms. Soil Biol. Biochem. 2021;163 doi: 10.1016/j.soilbio.2021.108432. [DOI] [Google Scholar]
- Komiya S., Noborio K., Katano K., Pakoktom T., Siangliw M., Toojinda T. Contribution of ebullition to methane and carbon dioxide emission from water between plant rows in a tropical rice paddy field. Int. Sch. Res. Notices. 2015;2015 doi: 10.1155/2015/623901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu B., Nicholas D.J.D. Denitrification in Rhodopseudomonas sphaeroides f. denitrificans. Arch. Microbiol. 1985;141:57–62. doi: 10.1007/BF00446740. [DOI] [Google Scholar]
- Kuypers M.M.M., Marchant H.K., Kartal B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018;16:263–276. doi: 10.1038/nrmicro.2018.9. [DOI] [PubMed] [Google Scholar]
- Kwon Y., Jin Y., Lee J.-H., Sun C., Ryu C.-M. Rice rhizobiome engineering for climate change mitigation. Trends Plant Sci. 2024;29:1299–1309. doi: 10.1016/j.tplants.2024.06.006. [DOI] [PubMed] [Google Scholar]
- Kwon Y., Lee J.-Y., Choi J., Lee S.-M., Kim D., Cha J.-K., Park H., Kang J.-W., Kim T.H., Chae H.G., et al. Loss-of-function gs3 allele decreases methane emissions and increases grain yield in rice. Nat. Clim. Change. 2023;13:1329–1333. doi: 10.1038/s41558-023-01872-5. [DOI] [Google Scholar]
- Lahue G.T., van Kessel C., Linquist B.A., Adviento-Borbe M.A., Fonte S.J. Residual Effects of Fertilization History Increase Nitrous Oxide Emissions from Zero-N Controls: Implications for Estimating Fertilizer-Induced Emission Factors. J. Environ. Qual. 2016;45:1501–1508. doi: 10.2134/jeq2015.07.0409. [DOI] [PubMed] [Google Scholar]
- Lal R. Soil carbon sequestration in India. Climatic Change. 2004;65:277–296. doi: 10.1023/B:CLIM.0000038202.46720.37. [DOI] [Google Scholar]
- Lal R. Soil carbon sequestration to mitigate climate change. Geoderma. 2004;123:1–22. doi: 10.1016/j.geoderma.2004.01.032. [DOI] [Google Scholar]
- Legg S. IPCC, 2021: Climate change 2021-the physical science basis. Interaction. 2021;49:44–45. [Google Scholar]
- Leichty S.I., Kasanke C.P., Bell S.L., Hofmockel K.S. Site and Bioenergy Cropping System Similarly Affect Distinct Live and Total Soil Microbial Communities. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.725756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI X., ZHANG J.-l. Uptake of ammonium and nitrate by external hyphae of arbuscular mycorrhizal fungi. Journal of Plant Nutrition and Fertilizers. 2009;15:683–689. [Google Scholar]
- Liao P., Sun Y., Jiang Y., Zeng Y., Wu Z., Huang S. Hybrid rice produces a higher yield and emits less methane. Plant Soil Environ. 2019;65:549–555. doi: 10.17221/330/2019-PSE. [DOI] [Google Scholar]
- Liechty Z., Santos-Medellín C., Edwards J., et al. Comparative analysis of root microbiomes of rice cultivars with high and low methane emissions reveals differences in abundance of methanogenic archaea and putative upstream fermenters. mSystems. 2020;5:10–1128. doi: 10.1128/msystems.00897-00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linquist B.A., Adviento-Borbe M.A., Pittelkow C.M., van Kessel C., van Groenigen K.J. Fertilizer management practices and greenhouse gas emissions from rice systems: a quantitative review and analysis. Field Crops Res. 2012;135:10–21. doi: 10.1016/j.fcr.2012.06.007. [DOI] [Google Scholar]
- Linquist B.A., Marcos M., Adviento-Borbe M.A., Anders M., Harrell D., Linscombe S., Reba M.L., Runkle B.R.K., Tarpley L., Thomson A. Greenhouse gas emissions and management practices that affect emissions in US rice systems. J. Environ. Qual. 2018;47:395–409. doi: 10.2134/jeq2017.11.0445. [DOI] [PubMed] [Google Scholar]
- Liu S., Zheng Y., Ma R., Yu K., Han Z., Xiao S., Li Z., Wu S., Li S., Wang J., et al. Increased soil release of greenhouse gases shrinks terrestrial carbon uptake enhancement under warming. Global Change Biol. 2020;26:4601–4613. doi: 10.1111/gcb.15156. [DOI] [PubMed] [Google Scholar]
- Liu W., Yuan J.S., Stewart C.N., Jr. Advanced genetic tools for plant biotechnology. Nat. Rev. Genet. 2013;14:781–793. doi: 10.1038/nrg3583. [DOI] [PubMed] [Google Scholar]
- Liu X., Wang P., Song H., Zeng X. Determinants of net primary productivity: Low-carbon development from the perspective of carbon sequestration. Technol. Forecast. Soc. Change. 2021;172 doi: 10.1016/j.techfore.2021.121006. [DOI] [Google Scholar]
- Liu X., Zhou T., Liu Y., Zhang X., Li L., Pan G. Effect of mid-season drainage on CH4 and N2O emission and grain yield in rice ecosystem: A meta-analysis. Agric. Water Manag. 2019;213:1028–1035. doi: 10.1016/j.agwat.2018.12.025. [DOI] [Google Scholar]
- Liu Y.B., Zhou Y.L., Ju W.M., Wang S.Q., Wu X.C., He M.Z., Zhu G. Impacts of droughts on carbon sequestration by China's terrestrial ecosystems from 2000 to 2011. Biogeosciences. 2014;11:2583–2599. doi: 10.5194/bgd-10-17469-2013. [DOI] [Google Scholar]
- Liu Y., Ge T., van Groenigen K.J., Yang Y., Wang P., Cheng K., Zhu Z., Wang J., Li Y., Guggenberger G., et al. Rice paddy soils are a quantitatively important carbon store according to a global synthesis. Commun. Earth Environ. 2021;2:154. doi: 10.1016/10.1038/s43247-021-00229-0. [DOI] [Google Scholar]
- Lou Y., Inubushi K., Mizuno T., Hasegawa T., Lin Y., Sakai H., Cheng W., Kobayashi K. CH4 emission with differences in atmospheric CO2 enrichment and rice cultivars in a Japanese paddy soil. Global Change Biol. 2008;14:2678–2687. doi: 10.1111/j.1365-2486.2008.01665.x. [DOI] [Google Scholar]
- Lu W., Chen W., Duan B., Guo W., Lu Y., Lantin R., Wassmann R., Neue H. Methane emissions and mitigation options in irrigated rice fields in southeast China. Nutrient Cycl. Agroecosyst. 2000;58:65–73. doi: 10.1023/A:1009830232650. [DOI] [Google Scholar]
- Lu Y., Conrad R. In situ stable isotope probing of methanogenic archaea in the rice rhizosphere. Science. 2005;309:1088–1090. doi: 10.1126/science.1113435. [DOI] [PubMed] [Google Scholar]
- Luo D., Li Y., Yao H., Chapman S.J. Effects of different carbon sources on methane production and the methanogenic communities in iron rich flooded paddy soil. Sci. Total Environ. 2022;823 doi: 10.1016/j.scitotenv.2022.153636. [DOI] [PubMed] [Google Scholar]
- Lv Y., Li Y., Liu X., Xu K. Photochemistry and proteomics of ginger (Zingiber officinale Roscoe) under drought and shading. Plant Physiol. Biochem. 2020;151:188–196. doi: 10.1016/j.plaphy.2020.03.021. [DOI] [PubMed] [Google Scholar]
- Ma J., Li X., Baoquan J., Liu X., Li T., Zhang W., Liu W. Spatial variation analysis of urban forest vegetation carbon storage and sequestration in built-up areas of Beijing based on i-Tree Eco and Kriging. Urban For. Urban Green. 2021;66 doi: 10.1016/j.ufug.2021.127413. [DOI] [Google Scholar]
- Ma K., Qiu Q., Lu Y. Microbial mechanism for rice variety control on methane emission from rice field soil. Global Change Biol. 2010;16:3085–3095. doi: 10.1111/j.1365-2486.2009.02145.x. [DOI] [Google Scholar]
- McClung A.M., Rohila J.S., Henry C.G., Lorence A. Response of US rice cultivars grown under non-flooded irrigation management. Agronomy. 2019;10:55. doi: 10.3390/agronomy10010055. [DOI] [Google Scholar]
- McLain J.E., Martens D.A. Nitrous oxide flux from soil amino acid mineralization. Soil Biol. Biochem. 2005;37:289–299. doi: 10.1016/j.soilbio.2004.03.013. [DOI] [Google Scholar]
- McLain J.E., Martens D.A. N2O production by heterotrophic N transformations in a semiarid soil. Appl. Soil Ecol. 2006;32:253–263. doi: 10.1016/j.apsoil.2005.06.005. [DOI] [Google Scholar]
- Mikhaylov A., Moiseev N., Aleshin K., Burkhardt T. Global climate change and greenhouse effect. Entrepreneurship and Sustainability Issues. 2020;7:2897–2913. doi: 10.9770/jesi.2020.7.4. [DOI] [Google Scholar]
- Minamikawa K., Yamaguchi T., Tokida T. Climate Smart Agriculture for the Small-Scale Farmers in the Asian and Pacific Region. 2019. Dissemination of water management in rice paddies in Asia; pp. 19–36. [DOI] [Google Scholar]
- Monkham T., Jongdee B., Pantuwan G., Sanitchon J., Mitchell J., Fukai S. Genotypic variation in grain yield and flowering pattern in terminal and intermittent drought screening methods in rainfed lowland rice. Field Crops Res. 2015;175:26–36. doi: 10.1016/j.fcr.2015.02.003. [DOI] [Google Scholar]
- Moscôso J.S.C., Silva L.S.d., Pujol S.B., Giacomini S.J., Severo F.F., Marzari L.B., Molin G.D. Methane emission induced by short-chain organic acids in lowland soil. Rev. Bras. Ciência do Solo. 2019;43 doi: 10.1590/18069657rbcs20180252. [DOI] [Google Scholar]
- Netz, B., Davidson, O., Bosch, P., Dave, R., and Meyer, L. (2007). Climate change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Summary for Policymakers.
- Norton J.M. Nitrification in agricultural soils. Nitrogen in agricultural systems. 2015;49:173–199. doi: 10.2134/agronmonogr49.c6. [DOI] [Google Scholar]
- Okubo T., Liu D., Tsurumaru H., Ikeda S., Asakawa S., Tokida T., Tago K., Hayatsu M., Aoki N., Ishimaru K., et al. Elevated atmospheric CO2 levels affect community structure of rice root-associated bacteria. Front. Microbiol. 2015;6:136. doi: 10.3389/fmicb.2015.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield E.E., Bradford M.A., Augarten A.J., Cooley E.T., Radatz A.M., Radatz T., Ruark M.D. Positive associations of soil organic matter and crop yields across a regional network of working farms. Soil Sci. Soc. Am. J. 2022;86:384–397. doi: 10.1002/saj2.20349. [DOI] [Google Scholar]
- Pachauri R.K., Allen M.R., Barros V.R., Broome J., Cramer W., Christ R., Church J.A., Clarke L., Dahe Q., Dasgupta P. Intergovernmental Panel on Climate Change (Ipcc); 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the. [Google Scholar]
- Pan G., Li L., Wu L., Zhang X. Storage and sequestration potential of topsoil organic carbon in China's paddy soils. Global Change Biol. 2004;10:79–92. doi: 10.1111/j.1365-2486.2003.00717.x. [DOI] [Google Scholar]
- Parthasarathi, T., Vanitha, K., Lakshamanakumar, P., and Kalaiyarasi, D. (2012). Aerobic rice-mitigating water stress for the future climate change.
- Perry H., Carrijo D., Linquist B. Single midseason drainage events decrease global warming potential without sacrificing grain yield in flooded rice systems. Field Crops Res. 2022;276 doi: 10.1016/j.fcr.2021.108312. [DOI] [Google Scholar]
- Qi X., Jia X., Li M., Chen W., Hou J., Wei Y., Fu S., Xi B. Enhancing CH4 production in microbial electrolysis cells: Optimizing electric field via carbon cathode resistivity. Sci. Total Environ. 2024;920 doi: 10.1016/j.scitotenv.2024.170992. [DOI] [PubMed] [Google Scholar]
- Qian H., Huang S., Chen J., Wang L., Hungate B.A., van Kessel C., Zhang J., Deng A., Jiang Y., van Groenigen K.J., et al. Lower-than-expected CH4 emissions from rice paddies with rising CO2 concentrations. Global Change Biol. 2020;26:2368–2376. doi: 10.1111/gcb.14984. [DOI] [PubMed] [Google Scholar]
- Qian H., Zhang N., Chen J., Chen C., Hungate B.A., Ruan J., Huang S., Cheng K., Song Z., Hou P., et al. Unexpected parabolic temperature dependency of CH4 emissions from rice paddies. Environ. Sci. Technol. 2022;56:4871–4881. doi: 10.1021/acs.est.2c00738. [DOI] [PubMed] [Google Scholar]
- Qian H., Zhu X., Huang S., Linquist B., Kuzyakov Y., Wassmann R., Minamikawa K., Martinez-Eixarch M., Yan X., Zhou F., et al. Greenhouse gas emissions and mitigation in rice agriculture. Nat. Rev. Earth Environ. 2023;4:716–732. doi: 10.1038/s43017-023-00482-1. [DOI] [Google Scholar]
- Qin X., Li Y., Wang H., Li J., Wan Y., Gao Q., Liao Y., Fan M. Effect of rice cultivars on yield-scaled methane emissions in a double rice field in South China. J. Integr. Environ. Sci. 2015;12:47–66. doi: 10.1080/1943815X.2015.1118388. [DOI] [Google Scholar]
- Qiu H., Ge T., Liu J., Chen X., Hu Y., Wu J., Su Y., Kuzyakov Y. Effects of biotic and abiotic factors on soil organic matter mineralization: Experiments and structural modeling analysis. Eur. J. Soil Biol. 2018;84:27–34. doi: 10.1016/j.ejsobi.2017.12.003. [DOI] [Google Scholar]
- Raheem A., Wang T., Huang J., Danso F., Bankole O.O., Deng A., Gao J., Zhang J., Zhang W. Leguminous green manure mitigates methane emissions in paddy field by regulating acetoclastic and hydrogenotrophic methanogens. Eur. J. Soil Biol. 2022;108 doi: 10.1016/j.ejsobi.2021.103380. [DOI] [Google Scholar]
- Raza A., Razzaq A., Mehmood S.S., Zou X., Zhang X., Lv Y., Xu J. Impact of climate change on crops adaptation and strategies to tackle its outcome. Plants. 2019;8:34. doi: 10.3390/plants8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, H. (2020). Sector by sector: where do global greenhouse gas emissions come from? Our World in Data.
- Ritchie, H., and Roser, M. (2023). Sector by sector: where do global greenhouse gas emissions come from? Our World in data.
- Ritchie, H., and Roser, M. (2024). Sector by sector: where do global greenhouse gas emissions come from? Our World in data.
- Sander B.O., Wassmann R., Siopongco J., Hoanh C., Johnston R., Smakhtin V. Mitigating greenhouse gas emissions from rice production through water-saving techniques: potential, adoption and empirical evidence. Climate change and agricultural water management. in developing countries. 2015;8:193. doi: 10.1079/9781780643663.0193. [DOI] [Google Scholar]
- Sander B.O., Wassmann R., Siopongco J.D.L.C., Hoanh C., Johnston R., Smakhtin V. Mitigating greenhouse gas emissions from rice production through water-saving techniques: potential, adoption and empirical evidence. Climate change and agricultural water management in developing countries. 2016;8:193–207. doi: 10.1079/9781780643663.0193. [DOI] [Google Scholar]
- Sandhu N., Jain S., Kumar A., Mehla B.S., Jain R. Genetic variation, linkage mapping of QTL and correlation studies for yield, root, and agronomic traits for aerobic adaptation. BMC Genet. 2013;14:104–116. doi: 10.1186/1471-2156-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Medellín C., Liechty Z., Edwards J., Nguyen B., Huang B., Weimer B.C., Sundaresan V. Prolonged drought imparts lasting compositional changes to the rice root microbiome. Nat. Plants. 2021;7:1065–1077. doi: 10.1038/s41477-021-00967-1. [DOI] [PubMed] [Google Scholar]
- Schimel J. Rice, microbes and methane. Nature. 2000;403:375–377. doi: 10.1038/35000325. [DOI] [PubMed] [Google Scholar]
- Schroeder J.I., Delhaize E., Frommer W.B., Guerinot M.L., Harrison M.J., Herrera-Estrella L., Horie T., Kochian L.V., Munns R., Nishizawa N.K., et al. Using membrane transporters to improve crops for sustainable food production. Nature. 2013;497:60–66. doi: 10.1038/nature11909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Q., Yang X., Gao C., Wu P., Liu J., Xu Y., Shen Q., Zou J., Guo S. Net annual global warming potential and greenhouse gas intensity in Chinese double rice-cropping systems: a 3-year field measurement in long-term fertilizer experiments. Global Change Biol. 2011;17:2196–2210. doi: 10.1111/j.1365-2486.2010.02374.x. [DOI] [Google Scholar]
- Simmonds M.B., Anders M., Adviento-Borbe M.A., van Kessel C., McClung A., Linquist B.A. Seasonal methane and nitrous oxide emissions of several rice cultivars in direct-seeded systems. J. Environ. Qual. 2015;44:103–114. doi: 10.2134/jeq2014.07.0286. [DOI] [PubMed] [Google Scholar]
- Six J., Callewaert P., Lenders S., De Gryze S., Morris S.J., Gregorich E.G., Paul E.A., Paustian K. Measuring and understanding carbon storage in afforested soils by physical fractionation. Soil Sci. Soc. Am. J. 2002;66:1981–1987. doi: 10.2136/sssaj2002.1981. [DOI] [Google Scholar]
- Slingo J.M., Slingo M.E. The science of climate change and the effect of anaesthetic gas emissions. Anaesthesia. 2024;79:252–260. doi: 10.1111/anae.16189. [DOI] [PubMed] [Google Scholar]
- Smartt A., Brye K., Norman R. Methane emissions among hybrid rice cultivars in the mid-southern United States. Ann. Adv. Agric. Sci. 2018;2:1–13. doi: 10.22606/as.2018.21001. [DOI] [Google Scholar]
- Smartt A.D., Brye K.R., Rogers C.W., Norman R.J., Gbur E.E., Hardke J.T., Roberts T.L. Characterization of methane emissions from rice production on a clay soil in Arkansas. Soil Sci. 2016;181:57–67. doi: 10.1097/SS.0000000000000139. [DOI] [Google Scholar]
- Smith, P., Martino, Z., and Cai, D. (2007). 'Agriculture', in Climate change 2007: mitigation.
- Smith W., Grant B., Desjardins R., Kroebel R., Li C., Qian B., Worth D., McConkey B., Drury C. Assessing the effects of climate change on crop production and GHG emissions in Canada. Agric. Ecosyst. Environ. 2013;179:139–150. doi: 10.1016/j.agee.2013.08.015. [DOI] [Google Scholar]
- Springmann M., Freund F. Options for reforming agricultural subsidies from health, climate, and economic perspectives. Nat. Commun. 2022;13:82. doi: 10.1038/s41467-021-27645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sritharan N., Vijayalakshmi C., Subramanian E., Boomiraj K. Supremacy of rice genotypes under aerobic condition for mitigating water scarcity and future climate change. Afr. J. Agric. Res. 2015;10:235–243. doi: 10.5897/AJAR2013.8064. [DOI] [Google Scholar]
- Stewart J.C., Villasmil M.L., Frampton M.W. Changes in fluorescence intensity of selected leukocyte surface markers following fixation. Cytometry A. 2007;71:379–385. doi: 10.1002/cyto.a.20392. [DOI] [PubMed] [Google Scholar]
- Sun H., Feng Y., Ji Y., Shi W., Yang L., Xing B. N2O and CH4 emissions from N-fertilized rice paddy soil can be mitigated by wood vinegar application at an appropriate rate. Atmos. Environ. 2018;185:153–158. doi: 10.1016/j.atmosenv.2018.05.015. [DOI] [Google Scholar]
- Taghavi S.M., Mendoza T.C., Acero Jr B., Li T., Siddiq S.A., Yorobe Jr J., Li Z., Ali J. Carbon dioxide equivalent emissions of newly developed rice varieties. J. Agric. Sci. 2017;9:107–123. doi: 10.5539/jas.v9n5p107. [DOI] [Google Scholar]
- Tang J., Qian H., Zhu X., Liu Z., Kuzyakov Y., Zou J., Wang J., Xu Q., Li G., Liu Z., et al. Soil pH Determines Nitrogen Effects on Methane Emissions From Rice Paddies. Global Change Biol. 2024;30 doi: 10.1111/gcb.17577. [DOI] [PubMed] [Google Scholar]
- Taranto F., Nicolia A., Pavan S., De Vita P., D’Agostino N. Biotechnological and digital revolution for climate-smart plant breeding. Agronomy. 2018;8:277. doi: 10.3390/agronomy8120277. [DOI] [Google Scholar]
- Tian H., Lu C., Ciais P., Michalak A.M., Canadell J.G., Saikawa E., Huntzinger D.N., Gurney K.R., Sitch S., Zhang B., et al. The terrestrial biosphere as a net source of greenhouse gases to the atmosphere. Nature. 2016;531:225–228. doi: 10.1038/nature16946. [DOI] [PubMed] [Google Scholar]
- Tokida T., Fumoto T., Cheng W., Matsunami T., Adachi M., Katayanagi N., Matsushima M., Okawara Y., Nakamura H., Okada M., et al. Effects of free-air CO 2 enrichment (FACE) and soil warming on CH 4 emission from a rice paddy field: impact assessment and stoichiometric evaluation. Biogeosciences. 2010;7:2639–2653. doi: 10.5194/bg-7-2639-2010. [DOI] [Google Scholar]
- Tomlinson G.A., Jahnke L.L., Hochstein L.I. Halobacterium denitrificans sp. nov., an extremely halophilic denitrifying bacterium. Int. J. Syst. Bacteriol. 1986;36:66–70. doi: 10.1099/00207713-36-1-66. [DOI] [PubMed] [Google Scholar]
- Torres R.O., Henry A. Yield stability of selected rice breeding lines and donors across conditions of mild to moderately severe drought stress. Field Crops Res. 2018;220:37–45. doi: 10.1016/j.fcr.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA (2023). Rice Yearbook. Economic Research Service (U.S. Department of Agriculture).
- Van Groenigen K.J., Van Kessel C., Hungate B.A. Increased greenhouse-gas intensity of rice production under future atmospheric conditions. Nat. Clim. Change. 2013;3:288–291. doi: 10.1038/nclimate1712. [DOI] [Google Scholar]
- Vikram P., Swamy B.P.M., Dixit S., Singh R., Singh B.P., Miro B., Kohli A., Henry A., Singh N.K., Kumar A. Drought susceptibility of modern rice varieties: an effect of linkage of drought tolerance with undesirable traits. Sci. Rep. 2015;5 doi: 10.1038/srep14799.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa J.E., Henry A., Xie F., Serraj R. Hybrid rice performance in environments of increasing drought severity. Field Crops Res. 2012;125:14–24. doi: 10.1016/j.fcr.2011.08.009. [DOI] [Google Scholar]
- Wang B., Neue H., Samonte H. Effect of cultivar difference (‘IR72’,‘IR65598’and ‘Dular’) on methane emission. Agric. Ecosyst. Environ. 1997;62:31–40. [Google Scholar]
- Wang B., Xu Y., Wang Z., Li Z., Guo Y., Shao K., Chen Z. Methane emissions from ricefields as affected by organic amendment, water regime, crop establishment, and rice cultivar. Environ. Monit. Assess. 1999;57:213–228. [Google Scholar]
- Wang C., Amon B., Schulz K., Mehdi B. Factors that influence nitrous oxide emissions from agricultural soils as well as their representation in simulation models: a review. Agronomy. 2021;11:770. [Google Scholar]
- Wang C., Lai D.Y.F., Sardans J., Wang W., Zeng C., Peñuelas J. Factors related with CH4 and N2O emissions from a paddy field: clues for management implications. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Jin Y., Ji C., Zhang N., Song M., Kong D., Liu S., Zhang X., Liu X., Zou J., et al. An additive effect of elevated atmospheric CO2 and rising temperature on methane emissions related to methanogenic community in rice paddies. Agric. Ecosyst. Environ. 2018;257:165–174. doi: 10.1016/j.agee.2018.02.003. [DOI] [Google Scholar]
- Wang C., Wang Z., Cai Y., Zhu Z., Yu D., Hong L., Wang Y., Lv W., Zhao Q., Si L., et al. A higher-yield hybrid rice is achieved by assimilating a dominant heterotic gene in inbred parental lines. Plant Biotechnol. J. 2024;22:1669–1680. doi: 10.1111/pbi.14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yang T., Chen J., Bell S.M., Wu S., Jiang Y., Sun Y., Zeng Y., Zeng Y., Pan X., et al. Effects of free-air temperature increase on grain yield and greenhouse gas emissions in a double rice cropping system. Field Crops Res. 2022;281 doi: 10.1016/j.fcr.2022.108489. [DOI] [Google Scholar]
- Wang P., Liu Y., Li L., Cheng K., Zheng J., Zhang X., Zheng J., Joseph S., Pan G. Long-term rice cultivation stabilizes soil organic carbon and promotes soil microbial activity in a salt marsh derived soil chronosequence. Sci. Rep. 2015;5 doi: 10.1038/srep15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Han P., Lv X., Zhang L., Zheng G. Defect and interface engineering for aqueous electrocatalytic CO2 reduction. Joule. 2018;2:2551–2582. doi: 10.1016/j.joule.2018.09.021. [DOI] [Google Scholar]
- Wang Z., Xu Y., Li Z., Guo Y., Wassmann R., Neue H., Lantin R., Buendia L., Ding Y., Wang Z. A four-year record of methane emissions from irrigated rice fields in the Beijing region of China. Nutrient Cycl. Agroecosyst. 2000;58:55–63. doi: 10.1023/A:1009878115811. [DOI] [Google Scholar]
- Wassmann R., Neue H.-U., Lantin R., Buendia L., Rennenberg H. Characterization of methane emissions from rice fields in Asia. I. Comparison among field sites in five countries. Nutrient Cycl. Agroecosyst. 2000;58:1–12. doi: 10.1023/A:1009848813994. [DOI] [Google Scholar]
- Wassmann R., Neue H., Bueno C., Lantin R., Alberto M., Buendia L., Bronson K., Papen H., Rennenberg H. Methane production capacities of different rice soils derived from inherent and exogenous substrates. Plant Soil. 1998;203:227–237. doi: 10.1023/A:1004357411814. [DOI] [Google Scholar]
- Wassmann R., Buendia L., Lantin R., Bueno C., Lubigan L., Umali A., Nocon N., Javellana A., Neue H. Mechanisms of crop management impact on methane emissions from rice fields in Los Baños, Philippines. Nutrient Cycl. Agroecosyst. 2000;58:107–119. doi: 10.1023/1009838401699. [DOI] [Google Scholar]
- Watanabe A., Takeda T., Kimura M. Evaluation of origins of CH4 carbon emitted from rice paddies. J. Geophys. Res. 1999;104:23623–23629. doi: 10.1029/1999JD900467. [DOI] [Google Scholar]
- Watanabe A., Kajiwara M., Tashiro T., Kimura M. Influence of rice cultivar on methane emission from paddy fields. Plant Soil. 1995;176:51–56. [Google Scholar]
- Watson S.W., Valois F.W., Waterbury J.B. The Prokaryotes: A Handbook on Habitats, Isolation, and Identification of Bacteria. Springer; 1981. The family nitrobacteraceae; pp. 1005–1022. [DOI] [Google Scholar]
- Wei L., Ge T., Zhu Z., Luo Y., Yang Y., Xiao M., Yan Z., Li Y., Wu J., Kuzyakov Y. Comparing carbon and nitrogen stocks in paddy and upland soils: Accumulation, stabilization mechanisms, and environmental drivers. Geoderma. 2021;398 doi: 10.1016/j.geoderma.2021.115121. [DOI] [Google Scholar]
- Weller, S., Wassmann, R., Romasanta, R.R., Van Phu, N., and Sander, B.O. (2018). Plant traits influencing greenhouse gas emission potential and assessment of technical options for emission screening with large number of rice varieties.
- Win K.T., Nonaka R., Win A.T., Sasada Y., Toyota K., Motobayashi T., Hosomi M. Comparison of methanotrophic bacteria, methane oxidation activity, and methane emission in rice fields fertilized with anaerobically digested slurry between a fodder rice and a normal rice variety. Paddy Water Environ. 2012;10:281–289. doi: 10.1007/s10333-011-0279-x. [DOI] [Google Scholar]
- Woodwell G. The carbon dioxide problem. The role of terrestrial vegetation in the global carbon cycle: Measurement by remote sensing. 1984;23:3–17. [Google Scholar]
- Wu J. Carbon accumulation in paddy ecosystems in subtropical China: evidence from landscape studies. Eur. J. Soil Sci. 2011;62:29–34. doi: 10.1111/j.1365-2389.2010.01325.x. [DOI] [Google Scholar]
- Xing G., Shi S., Shen G., Du L., Xiong Z. Nitrous oxide emissions from paddy soil in three rice-based cropping systems in China. Nutrient Cycl. Agroecosyst. 2002;64:135–143. doi: 10.1023/A:1021131722165. [DOI] [Google Scholar]
- Xing G., Zhao X., Xiong Z., Yan X., Xu H., Xie Y., Shi S. Nitrous oxide emission from paddy fields in China. Acta Ecol. Sin. 2009;29:45–50. doi: 10.1016/j.chnaes.2009.04.006. [DOI] [Google Scholar]
- Yan X., Du L., Shi S., Xing G. Nitrous oxide emission from wetland rice soil as affected by the application of controlled-availability fertilizers and mid-season aeration. Biol. Fertil. Soils. 2000;32:60–66. doi: 10.1007/s003740000215. [DOI] [Google Scholar]
- Yang S.-S., Lai C.-M., Chang H.-L., Chang E.-H., Wei C.-B. Estimation of methane and nitrous oxide emissions from paddy fields in Taiwan. Renew. Energy. 2009;34:1916–1922. doi: 10.1016/j.renene.2008.12.016. [DOI] [Google Scholar]
- Yang T., Xin Y., Zhang L., Gu Z., Li Y., Ding Z., Shi G. Characterization on the aerobic denitrification process of Bacillus strains. Biomass Bioenergy. 2020;140 doi: 10.1016/j.biombioe.2020.105677. [DOI] [Google Scholar]
- Yang Y.-l., Shen L.-d., Zhao X., Shan J., Wang S.-w., Zhou W., Liu J.-q., Liu X., Tian M.-h., Yang W.-t., et al. Long-term incorporation of wheat straw changes the methane oxidation potential, abundance and community composition of methanotrophs in a paddy ecosystem. Appl. Soil Ecol. 2022;173 doi: 10.1016/j.apsoil.2021.104384. [DOI] [Google Scholar]
- Yang Y., Li M., Wu J., Pan X., Gao C., Tang D.W.S. Impact of combining long-term subsoiling and organic fertilizer on soil microbial biomass carbon and nitrogen, soil enzyme activity, and water use of winter wheat. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.788651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Miao Y., Huang S., Gao L., Ma X., Zhao G., Jiang R., Chen X., Zhang F., Yu K., et al. Active canopy sensor-based precision N management strategy for rice. Agron. Sustain. Dev. 2012;32:925–933. doi: 10.1007/s13593-012-0094-9. [DOI] [Google Scholar]
- Yu H., Wang T., Huang Q., Song K., Zhang G., Ma J., Xu H. Effects of elevated CO 2 concentration on CH 4 and N 2 O emissions from paddy fields: A meta-analysis. Sci. China Earth Sci. 2022;65:96–106. doi: 10.1016/j.agee.2024.109055. [DOI] [Google Scholar]
- Yuan Q., Pump J., Conrad R. Partitioning of CH4 and CO2 production originating from rice straw, soil and root organic carbon in rice microcosms. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Sheng R., Zhang M., Xiong G., Hou H., Li S., Wei W. Effects of continuous manure application on methanogenic and methanotrophic communities and methane production potentials in rice paddy soil. Agric. Ecosyst. Environ. 2018;258:121–128. doi: 10.1016/j.agee.2018.02.018. [DOI] [Google Scholar]
- Zhang X., Zhang R., Gao J., Wang X., Fan F., Ma X., Yin H., Zhang C., Feng K., Deng Y. Thirty-one years of rice-rice-green manure rotations shape the rhizosphere microbial community and enrich beneficial bacteria. Soil Biol. Biochem. 2017;104:208–217. doi: 10.1016/j.soilbio.2016.10.023. [DOI] [Google Scholar]
- Zhao X., Min J., Wang S., Shi W., Xing G. Further understanding of nitrous oxide emission from paddy fields under rice/wheat rotation in south China. J. Geophys. Res. 2011;116 doi: 10.1029/2010JG001528. [DOI] [Google Scholar]
- Zheng H., Huang H., Yao L., Liu J., He H., Tang J. Impacts of rice varieties and management on yield-scaled greenhouse gas emissions from rice fields in China: A meta-analysis. Biogeosciences. 2014;11:3685–3693. doi: 10.5194/bg-11-3685-2014. [DOI] [Google Scholar]
- Zheng S., Ye C., Lu J., Liufu J., Lin L., Dong Z., Li J., Zhuang C. Improving the rice photosynthetic efficiency and yield by editing OsHXK1 via CRISPR/Cas9 system. Int. J. Mol. Sci. 2021;22:9554. doi: 10.3390/ijms22179554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Bortesi L., Baysal C., Twyman R.M., Fischer R., Capell T., Schillberg S., Christou P. Characteristics of genome editing mutations in cereal crops. Trends Plant Sci. 2017;22:38–52. doi: 10.1016/j.tplants.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Ziska L. The influence of root zone temperature on photosynthetic acclimation to elevated carbon dioxide concentrations. Ann. Bot. 1998;81:717–721. doi: 10.1016/j.tplants.2016.08.009. [DOI] [Google Scholar]
- Zou J., Huang Y., Jiang J., Zheng X., Sass R.L. A 3-year field measurement of methane and nitrous oxide emissions from rice paddies in China: Effects of water regime, crop residue, and fertilizer application. Global Biogeochem. Cycles. 2005;19 doi: 10.1029/2004GB002401. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.