Abstract
Epitranscriptomic chemical modifications of RNAs have emerged as potent regulatory mechanisms in the process of plant stress adaptation. Currently, over 170 distinct chemical modifications have been identified in mRNAs, tRNAs, rRNAs, microRNAs (miRNAs), and long noncoding RNAs (lncRNAs). Genetic and molecular studies have identified the genes responsible for addition and removal of chemical modifications from RNA molecules, which are known as “writers” and “erasers,” respectively. N6-methyladenosine (m6A) is the most prevalent chemical modification identified in eukaryotic mRNAs. Recent studies have identified m6A writers and erasers across different plant species, including Arabidopsis (Arabidopsis thaliana), rice (Oryza sativa), cotton (Gossypium hirsutum), and tomato (Solanum lycopersicum). Accumulating discoveries have improved our understanding of the functions of RNA modifications in plant stress responses. This review highlights the latest research on RNA modification, emphasizing the biological and cellular roles of diverse chemical modifications of mRNAs, tRNAs, rRNAs, miRNAs, and lncRNAs in plant responses to environmental and hormonal signals. We also propose and discuss critical questions and future challenges for enhancing our understanding of the cellular and mechanistic roles of RNA modifications in plant stress responses. Integrating molecular insights into the regulatory roles of RNA modifications in stress responses with novel genome- and RNA-editing technologies will facilitate the breeding of stress-tolerant crops through precise engineering of RNA modifications.
Keywords: RNA modification, RNA methylation, abiotic stress, epitranscriptomics, stress adaptation
This review highlights the latest progress in the understanding of the vital and mechanistic roles played by epitranscriptomic RNA modifications in plant responses to diverse environmental signals, and suggests that the engineering of RNA modifications is a powerful strategy for the development of stress-tolerant crops.
Introduction
Increasing abiotic stresses—including drought, salinity, extreme temperatures, high light, and heavy metals—significantly inhibit plant growth and crop productivity. Understanding the underlying mechanisms that govern plant responses to these abiotic stresses is crucial for developing strategies to improve stress tolerance and promote agricultural sustainability in challenging environmental conditions. Plants respond to harsh environmental stresses by regulating gene expression at multiple levels, including transcription, posttranscriptional RNA metabolism, translational and posttranslational regulation, and epigenetic modifications (reviewed by Zhang et al., 2022a; Zhang et al., 2023; Shilpa et al., 2024).
Recent studies have identified chemical modifications of RNAs as a significant regulatory mechanism in the process of plant stress adaptation (reviewed by Hu et al., 2022; Tang et al., 2023a). Currently, >170 distinct chemical modifications have been documented for mRNAs, transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), and other noncoding RNAs (ncRNAs), including microRNAs (miRNAs) and long noncoding RNAs (lncRNAs) (Boccaletto et al., 2022; reviewed by Sharma et al., 2023). The proteins responsible for addition and removal of chemical modifications to and from RNA molecules are known as “writers” and “erasers,” respectively. Several writers responsible for tRNA and rRNA modifications have been identified in plants (Chen et al., 2010; reviewed by Wang et al., 2017a; Sharma et al., 2023). However, the corresponding erasers for tRNA and rRNA remain largely unidentified (Sharma et al., 2023). N6-methyladenosine (m6A) is the most prevalent chemical modification identified in eukaryotic mRNAs (Boccaletto et al., 2022). Recent studies have identified m6A writers and associated complexes primarily in Arabidopsis (Arabidopsis thaliana). These include methyltransferase A (MTA), MTB, VIRILIZER (VIR), a ubiquitin E3 ligase (HAKAI), FKBP12-interacting protein 37 (FIP37), HAKAI-interacting zinc finger protein 2 (HIZ2), and FIONA1 (FIO1) (Zhong et al., 2008; Bodi et al., 2012; Růžička et al., 2017; Wang et al., 2022a; Zhang et al., 2022b; Cai et al., 2023). In addition, the α-ketoglutarate-dependent dioxygenase homolog (ALKBH) proteins, including ALKBH9B, ALKBH9C, and ALKBH10B in Arabidopsis; ALKBH9 in rice (Oryza sativa); ALKBH10 in cotton (Gossypium hirsutum); and ALKBH2 and ALKBH10B in tomato (Solanum lycopersicum), have been identified as m6A erasers in plants (Duan et al., 2017; Martínez-Pérez et al., 2017; Zhou et al., 2019; Amara et al., 2022; Shen et al., 2023; Tang et al., 2024). This m6A writer- and eraser-mediated methylation and demethylation of mRNAs is crucial for abiotic stress responses, as well as plant development, senescence, and fruit ripening (reviewed by Zhou et al., 2022; Hu et al., 2022; Tang et al., 2023a). In addition to m6A, 5-methylcytosine (m5C) and N4-acetylcytidine (ac4C) have also been identified as novel modifications of plant mRNAs (Cui et al., 2017; Li et al., 2023b). tRNA-specific methyltransferase 4B (TRM4B) has been identified as an m5C methyltransferase in Arabidopsis (Cui et al., 2017). In addition, N-acetyltransferases for cytidine in RNA (ACYR1 and ACYR2) have been identified as ac4C writers in Arabidopsis (Wang et al., 2023a), playing a crucial role in seed, leaf, and root development (Cui et al., 2017; Wang et al., 2023a).
This review aims to explore recent advances in RNA modification research, with a focus on the occurrence and biological functions of RNA modifications in mRNAs, tRNAs, rRNAs, and other ncRNAs, including miRNAs and lncRNAs, that are related to plant stress responses. The review also explores existing limitations and proposes future research directions and challenges to further understand the mechanistic roles of RNA modifications during plant stress adaptation.
Types of RNA modifications that govern abiotic stress responses in plants
Recent transcriptome-wide analyses have identified over 170 RNA modifications across different RNA species (Boccaletto et al., 2022), including N1-methyladenosine (m1A), m6A, m5C, 2-methylguanosine (m2G), 7-methylguanosine (m7G), ac4C, pseudouridine (Ψ), and 2′-O-methylation. Emerging evidence highlights the crucial role of RNA modifications as a potent regulatory mechanism in plant responses to abiotic stresses. The occurrence and roles of chemical modifications in various types of RNA (Figure 1) and the key genes involved in the RNA modifications that influence abiotic stress responses (Table 1) will be explored in the next section.
Figure 1.
RNA modifications of mRNAs, tRNAs, and rRNAs associated with plant abiotic stress responses.
Multiple RNA modifications modulate various abiotic stress responses in plants. Salt stress affects various modifications of mRNA (e.g., m6A and ac4C) and tRNA (e.g., m1A, m7G, Am, and Cm). RNA modifications associated with the drought-stress response include m6A and m5C of mRNA and various modifications of tRNA. Temperature fluctuations influence m6A, m5C, and ac4C modifications. m6A methylation not only participates in heavy metal stress responses but also plays a regulatory role in the response to photodamage. m5C modification of tRNA is involved in theoxidative stress response. rRNA modifications are mainly involved in temperature acclimation and heavy metal stress. Abbreviations: m6A, N6-methyladenosine; m5C, 5-methylcytosine; ac4C, N4-acetylcytidine; m1A, N1-methyladenosine; m2G, 2-methylguanosine; m7G, 7-methylguanosine; Am, 2ʹ-O-methyladenosine; Cm, 2ʹ-O-methylcytidine.
Table 1.
Confirmed writers, erasers, and readers associated with abiotic stress responses in plants.
| RNA modification | Abiotic stress | m6A machinery | Gene name | Plant speciesa | Reference |
|---|---|---|---|---|---|
| mRNA | |||||
| m6A | salt | writer | VIR | Arabidopsis | Hu et al. (2021) |
| FIONA1 | Arabidopsis | Cai et al. (2024a) | |||
| eraser | ALKBH8B | Arabidopsis | Huong et al. (2022) | ||
| ALKBH9B | Arabidopsis | Tang et al. (2022) | |||
| ALKBH9C | Arabidopsis | Amara et al. (2022) | |||
| ALKBH10B | Arabidopsis | Tang et al. (2021) | |||
| GhALKBH10 | cotton | Cui et al. (2022) | |||
| reader | GhECT6 | cotton | Wang et al. (2022b) | ||
| ECT8 | Arabidopsis | Cai et al. (2024b) | |||
| ECT12 | Arabidopsis | Amara et al. (2024) | |||
| drought | writer | MdMTA | apple | Hou et al. (2022) | |
| eraser | ALKBH10B | Arabidopsis | Han et al. (2023a) | ||
| GhALKBH10 | cotton | Li et al. (2023a) | |||
| cold | writer | MTA | Arabidopsis | Wang et al. (2023b) | |
| MTA | Arabidopsis | Govindan et al. (2022) | |||
| FIP37 | Arabidopsis | Wang et al. (2023b) | |||
| reader | SlYTP8 | tomato | Zhang et al. (2024a) | ||
| heat | eraser | ALKBH10B | Arabidopsis | Wang et al. (2022c) | |
| high light | writer | VIR | Arabidopsis | Zhang et al. (2022c) | |
| m5C | heat | writer | OsNSUN2 | rice | Tang et al. (2020a) |
| tRNA | |||||
| Nm | salt | writer | OsTRM13 | rice | Wang et al. (2017b) |
| nm5U | TRM9/TRM11 | Arabidopsis | Janssen et al. (2022) | ||
| mcm5U | TRM9/TRM11 | Arabidopsis | |||
| nchm5U | TRM9/TRM11 | Arabidopsis | |||
| ncm5U | AtELP1 | Arabidopsis | |||
| m5C | reactive oxygen species | writer | TRM4b | Arabidopsis | David et al. (2017) |
| rRNA | |||||
| m2G | cold | writer | RsmD | Arabidopsis | Ngoc et al. (2021) |
| Ψ | low temperature | writer | OsPUS1 | rice | Wang et al. (2022d) |
nm5U, aminomethyluridine; ncm5U, 5-aminomethylurine; nchm5U, 5-aminosamethyluridine; mcm5U, 5-methoxymethyluridine.
Arabidopsis (Arabidopsis thaliana), cotton (Gossypium hirsutum), apple (Malus pumila), tomato (Solanum lycopersicum), rice (Oryza sativa).
mRNAs
Among the diverse RNA modifications identified to date, m6A is the most prevalent internal modification found on mRNA. The mapping of mRNA modifications under different abiotic stresses is essential for understanding the role of mRNA modifications in stress responses. At present, transcriptome-wide mapping of mRNA m6A modifications has been performed in a number of plant species subjected to various abiotic stresses (reviewed by Hu et al., 2022). Notably, global and transcript-specific m6A levels are dynamically regulated in response to diverse stresses. For instance, global m6A levels increase in Arabidopsis under salt stress (Hu et al., 2021) but decrease in sea buckthorn under drought stress (Zhang et al., 2021) and in tomato under moderate low-temperature stress (Yang et al., 2021). By contrast, global m6A levels remain unchanged in apples exposed to drought stress (Hou et al., 2022), in pak-choi subjected to heat stress (Liu et al., 2020), and in rice exposed to heavy metal stress (Cheng et al., 2021). Although overall mRNA m6A levels do not change noticeably in several plant species subjected to abiotic stress, m6A levels may be altered in specific mRNAs associated with individual stresses (reviewed by Hu et al., 2022). These findings highlight the dynamic nature of mRNA m6A modification during abiotic stress responses in plants. Combined m6A-sequencing (m6A-seq) and RNA-sequencing (RNA-seq) analyses have revealed that mRNA m6A modification can either increase or decrease the transcript levels of genes involved in stress responses (reviewed by Hu et al., 2022; Tang et al., 2023a).
Studies of loss-of-function mutants for m6A writers, erasers, and readers have demonstrated the critical role of m6A in regulating plant responses to abiotic stresses, including salinity, drought, extreme temperatures, high light intensity, and heavy metal exposure. Hu et al. (2021) demonstrated, for the first time, that mutants of m6A writers, including mta, mtb, vir, and hakai, exhibit increased sensitivity to salt stress, indicating that m6A modification acts as a positive regulator of salt tolerance in Arabidopsis. Subsequently, FIONA1, a recently identified m6A writer, was shown to promote seedling growth in Arabidopsis under salt-stress conditions (Cai et al., 2024a). In addition, the m6A erasers ALKBH8B and ALKBH9C in Arabidopsis and the m6A reader GhECT6 in cotton enhance seedling growth in Arabidopsis and cotton under salt-stress conditions (Amara et al., 2022; Wang et al., 2022b; Huong et al., 2022). By contrast, Arabidopsis ALKBH6, cotton ALKBH10, and tomato ALKBH10B negatively regulate salt tolerance (Huong et al., 2020; Cui et al., 2022; Shen et al., 2023). Recent studies have demonstrated the critical role of the m6A reader ECT8 in Arabidopsis responses to salt and drought stresses (Cai et al., 2024b; Wu et al., 2024). These findings indicate that m6A writers, erasers, and readers play distinct roles in the regulation of plant responses to salt stress. Further investigation into the regulatory mechanisms underlying the m6A-mediated salt response by these writers, erasers, and readers will be crucial for advancing our understanding of m6A modifications in salt tolerance.
The role of m6A in plant responses to various abiotic stresses is also emerging as a significant area of research. The m6A writers PtrMTA in poplar (Populus trichocarpa) and MdMTA in apple (Malus domestica) enhance drought tolerance (Lu et al., 2020; Hou et al., 2022). By contrast, the m6A eraser ALKBH9C in Arabidopsis negatively affects seedling growth under drought-stress conditions (Amara et al., 2022). Loss-of-function mutants of Arabidopsis ALKBH10B exhibit sensitivity to drought stress (Han et al., 2023a), whereas ALKBH10B mutants in cotton and tomato demonstrate drought tolerance (Li et al., 2023a; Shen et al., 2023). These findings indicate that ALKBH10B-mediated m6A demethylation has opposite effects on drought tolerance in different plant species. SiYTH1, an m6A reader in foxtail millet (Setaria italica), stabilizes m6A-modified transcripts associated with stomatal closure and reactive oxygen species to regulate the drought response (Luo et al., 2023). Furthermore, m6A exhibits contrasting roles in cold and heat tolerance. For example, Arabidopsis mta and fip37 mutants exhibit cold-sensitive growth phenotypes (Govindan et al., 2022; Wang et al., 2023b), indicating that m6A serves as a positive regulator of cold tolerance. By contrast, disruption of ALKBH10B inhibits the expression of heat-activated genes, leading to heat sensitivity in Arabidopsis (Wang et al., 2022c), suggesting that m6A functions as a negative regulator of heat tolerance. The m6A reader ECT2 in Arabidopsis relocates from the cytoplasm to stress granules (SGs) upon heat exposure, suggesting its role in the heat-stress response (Scutenaire et al., 2018). Interestingly, in Arabidopsis under heat stress, ALKBH9B selectively demethylates a heat-activated retrotransposon Onsen mRNA to release it from SGs and allow for its mobilization, suggesting a crucial role for m6A in transposon-mediated heat-stress response (Fan et al., 2023). A recent study in tomato revealed that the m6A reader SlYTH8 participates in the low-temperature response (Zhang et al., 2024a).
Because light is a key environmental signal that regulates plant responses to abiotic stresses, it will be crucial to understand the link between m6A and light responses. A recent study demonstrated that photosynthetic activity and the abundance of photosystem proteins decreased significantly in Arabidopsis vir mutants under high-light conditions, indicating a crucial role for m6A in photosynthesis during photodamage in plants (Zhang et al., 2022c). Cryptochrome 2 (CRY2), a blue-light receptor in Arabidopsis, regulates m6A modification of mRNAs, especially those modulated by the circadian clock, by interacting with MTA, MTB, and FIP37, thus influencing circadian rhythm and chlorophyll homeostasis (Wang et al., 2021). In addition, a recent study demonstrated that the CRY2/SPA1 (suppressor of phytochrome A1)/FIO1 complex regulates the m6A methylation and translation of chlorophyll-biosynthesis-related mRNAs to maintain chlorophyll homeostasis in response to light (Jiang et al., 2023). On the other hand, CRY1 interacts with FIP37 to promote m6A methylation of photomorphogenesis-related genes such as phytochrome-interacting factor 3 (PIF3), PIF4, and PIF5, thereby reducing their mRNA stability and promoting photomorphogenesis (Yang et al., 2023a). These results highlight the crucial role of m6A modification in light responses. Recent studies have demonstrated that m6A is implicated in the response to heavy metal. Transcriptome-wide m6A mapping performed in the roots of barley and soybean under cadmium stress indicated that m6A serves as a crucial regulator of the expression of genes associated with cadmium tolerance (Su et al., 2022; Han et al., 2023b). The m6A reader CPSF30-L, a core alternative polyadenylation factor, regulates nitrate metabolism by affecting the alternative polyadenylation of nitrate-signaling-related genes in Arabidopsis (Hou et al., 2021).
Other modifications of mRNAs, including m5C, ac4C, m1A, and m7G, are emerging as potent regulatory mechanisms for stress tolerance alongside m6A (Figure 1). For instance, m5C has been implicated in responses to heat and drought stress in Arabidopsis (Cui et al., 2017). In rice, OsNSUN2-mediated m5C modification of mRNA enhances plant adaptation to heat stress by maintaining chloroplast function (Tang et al., 2020a). The recently identified ac4C modification of mRNA regulates genes associated with cold and salt stress responses in Arabidopsis but has not been observed to play a similar role in rice (Li et al., 2023b). Combining enzymatic digestion with liquid chromatography–tandem mass spectrometry analysis revealed that m7G levels in mRNAs decrease in rice subjected to cadmium stress (Chu et al., 2018). In addition, transcriptome-wide profiling of m1A in petunia (Petunia hybrida) mRNAs revealed a dynamic pattern of mRNA m1A modification in various tissues and at different developmental stages (Yang et al., 2020). Further investigation into the occurrence and functional roles of these RNA modifications across different plant species under various environmental signals remains essential.
tRNAs
tRNAs are the most extensively modified RNA species among diverse RNA types (Frye et al., 2018; Dannfald et al., 2021). Compared with mRNA modifications, tRNA modifications exhibit greater diversity, ranging from simple chemical modifications to the addition of complex compounds. Various types of tRNA modifications regulate key aspects of tRNA biogenesis and function, including maturation, stability, structural integrity, and interactions with ribosomes (Barraud and Tisné, 2019; Dannfald et al., 2021). One study showed that 5-methoxymethyluridine and m5C modifications near the wobble position of the tRNA anticodon region can be induced by alkylation or oxidative stress, affecting tRNA function (Gu et al., 2014). A liquid chromatography–tandem mass spectrometry analysis indicated that four specific tRNA modifications—2ʹ-O-methyladenosine, 2ʹ-O-methylcytidine, m1A, and m7G—are differentially altered in Arabidopsis and rice in response to drought, cold, and salt stress (Wang et al., 2017b). The tRNA C5-methyltransferase mutant trm4b specifically acts in oxidative stress responses in Arabidopsis (David et al., 2017), and trm13 mutants in rice exhibit salt hypersensitivity (Wang et al., 2017b). A recent study demonstrated that various RNA modifications at the highly modified wobble positions of Arabidopsis tRNAs, including 5-aminomethyluridine, 5-aminomethylurine, 5-aminosamethyluridine, and 5-methoxymethyluridine, significantly increase under salt-stress conditions (Janssen et al., 2022). The Arabidopsis TRM61/TRM6 complex is a tRNA m1A methyltransferase crucial for embryo and seed development (Tang et al., 2020b). In addition, the tRNA methyltransferases TRM9 and TRM11 increase in response to salt stress (Janssen et al., 2022), suggesting that these enzymes directly regulate specific tRNA modifications under stress conditions. Given the emerging role of tRNAs as key stress regulators in plants (Aswathi et al., 2023), identification of the specific writers and/or erasers of tRNA modifications and characterization of their mechanistic roles in plant stress adaptation are promising areas for further research.
rRNAs
rRNAs are the most conserved RNA species in eukaryotes and contain several common chemical modifications, including m5C, m6A, m2G, m7G, m1A, ac4C, 2ʹ-O-methyladenosine, and Ψ. These modifications contribute to ribosomal biogenesis and stability (Polikanov et al., 2015; Dannfald et al., 2021). Owing to the high conservation and low turnover of rRNA molecules, rRNA modifications are believed to occur primarily during the initial processing and maturation stages of rRNA synthesis (Sharma et al., 2023). However, recent research has challenged this assumption by revealing significant rRNA modifications in response to abiotic stresses. For instance, cadmium treatment significantly increases the levels of m6A in 18S rRNA, alongside N2,N2-dimethylguanosine, N6,N6-dimethyladenosine, and N7-methylguanosine (m7G) in 25S rRNA (Tang et al., 2023b), suggesting the dynamic nature of rRNA modifications. Further investigation is needed to determine whether the increased levels of these rRNA modifications are associated with changes in the expression or activity of methyltransferases. Notably, a recent study demonstrated that ATMETTL5 in Arabidopsis is a novel m6A methyltransferase responsible for m6A methylation of 18S rRNA and regulates blue-light-mediated hypocotyl growth by modulating the translation of blue-light-related mRNAs (Song et al., 2024). A loss-of-function mutant of the rRNA methyltransferase RsmD, which is responsible for m2G methylation of 16S rRNA in Arabidopsis chloroplasts, exhibited sensitivity to cold stress (Ngoc et al., 2021). rRNAs in the cytoplasm, chloroplasts, and mitochondria of Arabidopsis contain 2′-O-methylated nucleotides whose formation is catalyzed by Fibrillarin 1 (FIB1) and FIB2 and that mediate ribosome subunit joining (Wu et al., 2021). The identification of tRNA and rRNA modifications responsive to specific abiotic stresses presents a new research area for analyzing the functional roles of these dynamic modifications during plant stress adaptation.
miRNAs and lncRNAs
Most cellular transcriptomes include ncRNAs, including miRNAs and lncRNAs, that are significant for plant stress responses (reviewed by Shriram et al., 2016; Yang et al., 2023b). However, the roles of RNA modifications in the biogenesis and function of miRNAs and lncRNAs are only gradually being recognized. One study demonstrated that the m6A writer MTA interacts with RNA polymerase II and TOUGH to deposit m6A on primary miRNAs, affecting the secondary structures of miRNA precursors and regulating the levels of miRNAs (Bhat et al., 2020). Furthermore, two recent studies showed that the m6A writer MTB interacts with SERRATE (SE), a miRNA processor component, to mediate binding of the m6A writer complex to primary miRNAs, thus regulating miRNA biogenesis in Arabidopsis (Bai et al., 2024; Zhong et al., 2024). By contrast, SE influences transcriptome-wide m6A deposition (Bai et al., 2024; Zhong et al., 2024). These studies reveal the regulatory interplay between m6A modifications and miRNA biogenesis in plants. However, the role of m6A in the biogenesis and function of miRNAs during plant stress responses remains largely unexplored. Hence, further research is needed to determine whether m6A and other RNA modifications of miRNAs regulate their processing and function in response to different abiotic stresses.
The role of RNA modifications in lncRNA biogenesis and function is gradually being discovered. A recent study in mammals demonstrated that the lncRNA HSATIII, which is extensively m6A modified, is recruited to nuclear stress bodies that regulate temperature-dependent splicing (Ninomiya et al., 2021). In addition, the heat-shock-inducible lncRNA Heat is heavily m6A methylated and is associated with enhanced heat-stress tolerance (Ji et al., 2021). The crucial role of ncRNAs in plant responses to various abiotic stresses, including drought, salinity, heat, cold, heavy metal toxicity, and nutrient deficiency, has been established (Shuai et al., 2014; Song et al., 2019; Tang et al., 2019; Wang et al., 2019). However, the role of m6A in the biogenesis and function of lncRNAs during plant stress responses remains largely unexplored. m6A methylation of the lncRNA COOLAIR enhances FCA-mediated repression of FLC during the floral transition (Xu et al., 2021). Stress-induced lncRNAs trigger RNA-directed DNA methylation (Ariel et al., 2020) and chromatin modifications (Jha et al., 2020), which are vital for plant responses to environmental cues. A recent nanopore direct RNA-seq analysis revealed the transcriptome-wide m6A landscape of lncRNAs in Arabidopsis (Liang et al., 2024). Notably, the expression patterns of lncRNAs were associated with differential m6A modification during the transition from the vegetative stage to the reproductive stage (Liang et al., 2024), suggesting the dynamic regulation of m6A deposition on lncRNAs. As the role of m6A in the biogenesis and function of lncRNAs during plant stress responses remains unknown, further research is needed to determine whether m6A and other RNA modifications of lncRNAs are modulated in response to different abiotic stresses. A growing body of evidence indicates the presence of crosstalk between RNA modifications and epigenetic factors, including DNA methylation, histone modifications, chromatin remodeling, and small RNAs (Hu et al., 2024). Thus, further research is needed to determine whether RNA modifications of ncRNAs directly regulate their processing in response to stress and whether ncRNAs serve as scaffolds for recruitment of writers, erasers, or readers to regulate stress-responsive genes. Furthermore, investigating the association between RNA modifications of ncRNAs and the epigenetic regulation of stress adaptation is essential.
Integration of RNA modifications with hormone signaling for adaptation to environmental challenges
The plant response to abiotic stress is a complex and intricate process involving the regulation of multiple phytohormones and signaling molecules, including abscisic acid (ABA), auxin, gibberellin, jasmonic acid, and ethylene. In response to external environmental stimuli, plants initiate adaptive mechanisms by modulating the biosynthesis of endogenous phytohormones. RNA methylation and other epigenetic factors regulate plant responses to abiotic stress by modulating the synthesis and signaling of phytohormones (Figure 2). Recent work in strawberry (Fragaria virginiana) demonstrated that FvMTA enhances the stability of transcripts involved in ABA biosynthesis and signaling pathways via m6A modification (Zhou et al., 2021). OsFIP37-mediated m6A methylation of OsYUCCA3 enhances local auxin biosynthesis, a process critical for meiotic division and subsequent pollen development in rice (Cheng et al., 2022). In addition, overexpression of ALKBH8B reduces global m6A levels, resulting in significant decreases in the transcript levels of genes involved in ABA signaling (Huong et al., 2022). These findings suggest that m6A methylation plays a crucial role in regulating the biosynthesis and signaling of phytohormones during plant growth and development.
Figure 2.
mRNA m6A modification integrates the crosstalk between abiotic stress response and phytohormone signaling.
(A) OsFIP37-mediated mRNA m6A methylation stabilizes YUCCA3, a key enzyme for auxin biosynthesis, thus ensuring male meiosis and fertility in rice.
(B) MTA-mediated m6A modification of the transcripts of ABA biosynthesis- and signaling-related genes (e.g., NCED3, AREB1, and ABAR) regulates their stability or translation, promoting fruit ripening.
(C) Chilling stress induces the m6A methylation of transcripts of phytohormone-related genes (e.g., ACO, ARF2A, GA2ox2, PE, and SABP2), modulating their stability to enhance cold tolerance.
(D) ABA induces the demethylation of ABI1 and BES1 by ALKBH9B, which enhances their stability and subsequently suppresses the expression of ABI3 and ABI5, leading to the inhibition of seed germination and early seedling growth. In addition, ABA and auxin induce the m6A modification of genes associated with heat, cold, and salt stress to modulate the stability or translation of the m6A-modified transcripts, promoting stress tolerance.
The significance of m6A modification in the regulation of phytohormone biosynthesis and signaling during abiotic stress responses is becoming increasingly evident. Integrated m6A-seq and RNA-seq analyses in tomato fruits exposed to chilling injury revealed that m6A modification is closely associated with the expression of several key regulatory genes, including 1-aminocyclopropane-1-carboxylate oxidase, auxin response factor 2A, gibberellin 2-oxidase 2, pectin esterase, and salicylic acid-binding protein 2, which are critical for modulating responses to fruit chilling injury (Bai et al., 2021). The m6A eraser ALKBH9B demethylates ABA INSENSITIVE 1 and BRI1-EMS-SUPPRESSOR 1, affecting their stability and ABA response in Arabidopsis (Tang et al., 2022). The expression of ALKBH10B is regulated by salt or osmotic stress, along with ABA or jasmonic acid (Tang et al., 2021). Further studies have demonstrated that the m6A eraser ALKBH10B and the m6A reader CPSF30-L are critical for the response of Arabidopsis to ABA (Shoaib et al., 2021; Song et al., 2021; Tang et al., 2021). In addition, the cotton m6A demethylase GhALKBH10B facilitates the decay of mRNAs related to ABA biosynthesis and signaling, such as GhZEP, GhNCED4, and GhPP2CA, which are upregulated under drought-stress conditions (Li et al., 2023a). The m6A reader ECT8 plays a key role in ABA and drought responses in Arabidopsis (Wu et al., 2024). Recent research has demonstrated that treatment with auxin or ABA induces differential m6A modifications of genes involved in their respective signaling pathways, as well as genes associated with responses to heat, cold, and salt stress (Zhang et al., 2024b). Although the relationship of phytohormones with RNA modifications other than m6A remains largely unexplored, the transcriptome-wide profiling of m1A in petunia mRNA revealed that several m1A peaks were upregulated or downregulated in petunia corollas after ethylene treatment (Yang et al., 2020). These findings suggest that m6A and other RNA modifications directly regulate the phytohormone-signaling network while integrating the crosstalk between phytohormone signaling and abiotic stress responses. However, further research is necessary to explore how phytohormones influence global m6A and other types of RNA methylation, as well as how phytohormone-mediated RNA methylation modulates plant responses to different abiotic stresses.
Cellular roles of RNA modifications in response to abiotic stresses
The fate of RNA molecules is determined by various cellular processes, collectively known as RNA metabolism, which include stability, splicing, translation, and export (reviewed by Manavella et al., 2023). Recent studies have begun to demonstrate the cellular and molecular roles of RNA modifications in RNA metabolism during abiotic stress responses. m6A modification specifically plays a pivotal role in stress adaptation by modulating these cellular processes under stress conditions (Anderson et al., 2018; Miao et al., 2020; Cheng et al., 2022; Hu et al., 2022). Growing evidence indicates that stress-responsive m6A modification influences RNA metabolism, primarily through its effects on mRNA stability and translation control (Figure 3). In addition, liquid–liquid phase separation (LLPS) is emerging as a pivotal process governing mRNA stability and translation under abiotic stresses, as discussed below.
Figure 3.
RNA modifications regulate diverse RNA processes under abiotic-stress conditions.
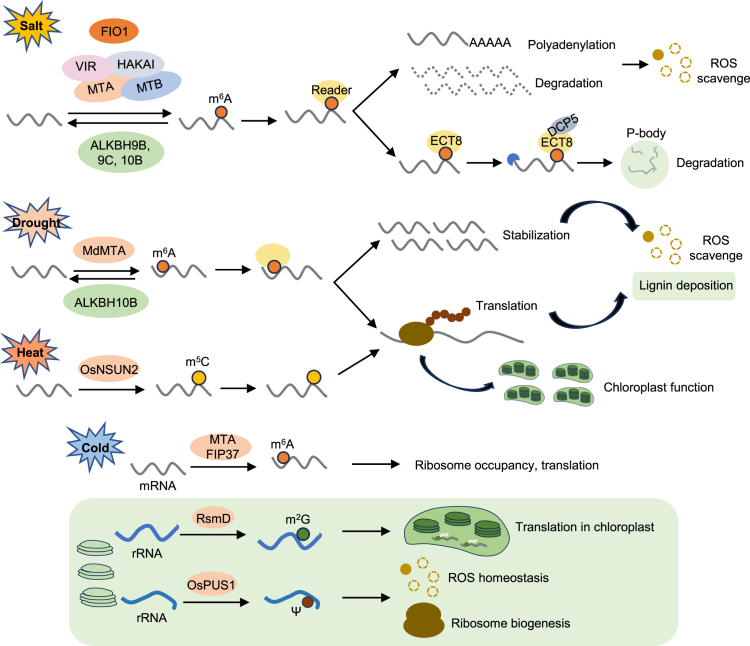
Under salt stress, m6A modifications mediated by m6A writers (MTA, MTB, VIR, HAKAI, and FIO1) or erasers (ALKBH9B, ALKBH9C, and ALKBH10B) modulate the stability of target transcripts, affecting reactive oxygen species (ROS) homeostasis. Under drought stress, the m6A writer MdMTA in apple and the eraser ALKBH10B in Arabidopsis alter m6A modification and modulate mRNA stability and translation, leading to ROS homeostasis and lignin deposition. Under heat stress, the m5C writer OsNSUN2 in rice modulates m5C levels, affecting translation and chloroplast function. Under cold or low-temperature stress, mRNA m6A modification by MTA and FIP37 in the cytoplasm regulates ribosome occupancy and translation, promoting cold tolerance. In addition, rRNA m2G modification by RsmD and Ψ modification by OsPUS1 in chloroplasts regulate ribosome biogenesis, translation, and ROS homeostasis, leading to cold or low-temperature response.
m6A in mRNA stability
Transcriptome-wide m6A-seq and RNA-seq analyses have revealed that m6A modification stabilizes salt-responsive genes by preventing ribonucleolytic cleavage (Anderson et al., 2018). A previous study demonstrated that m6A-mediated loss of RNA secondary structures enhances the stability of transcripts associated with salt-stress responses (Kramer et al., 2020). m6A modification, enriched in the 3′ untranslated regions (UTRs) of eukaryotic mRNAs, regulates mRNA degradation and stabilization. VIR-mediated m6A methylation influences the stability of salt-stress-responsive genes by affecting alternative polyadenylation at the 3′ UTRs of target mRNAs in Arabidopsis (Hu et al., 2021). In addition, VIR positively regulates the stability of transcripts associated with photoprotection and helps to maintain photosynthetic efficiency under conditions of high-light stress (Zhang et al., 2022c). In apple, the m6A writer MdMTA enhances the mRNA stability of drought-related genes involved in oxidative stress and lignin deposition (Hou et al., 2022). The m6A writer FIONA1 regulates reactive oxygen species production and the transcript levels of several salt-stress-responsive genes by modulating their mRNA stability in an m6A-dependent manner (Cai et al., 2024a). Demethylation mediated by m6A erasers regulates the stability of stress-responsive genes. For instance, ALKBH9C and ALKBH10B in Arabidopsis modulate the stability of genes responsive to salt or drought stress, thereby influencing stress tolerance (Amara et al., 2022; Han et al., 2023a). Furthermore, ALKBH10B-mediated demethylation facilitates the decay of mRNAs involved in ABA and Ca2+ signaling in cotton under drought-stress conditions (Li et al., 2023a).
A critical question is how m6A modification regulates the stability and decay of target transcripts. Recent studies have demonstrated that the m6A-dependent stabilization and destabilization of mRNAs are influenced by specific m6A reader proteins (Nguyen and Kang, 2024). For instance, the Arabidopsis m6A reader ECT2 enhances the stability of m6A-modified target mRNAs by interacting with the poly(A)-binding proteins PAB2 and PAB4 (Wei et al., 2018; Song et al., 2023). By contrast, FLK, a novel m6A reader in Arabidopsis, facilitates the decay of FLC mRNA by binding to the m6A site in the 3ʹ UTRs of FLC transcripts (Amara et al., 2023). Although these studies demonstrate that m6A readers significantly influence the stability of m6A-modified transcripts in plants under normal conditions, the role of m6A readers in regulating mRNA stability under stress conditions is still emerging. For instance, the Arabidopsis m6A reader CPSF30-L enhances the stability of mRNAs involved in ABA responses by regulating polyadenylation site selection (Song et al., 2021). In addition, ECT12 regulates the stability of mRNAs that encode positive and negative effectors associated with responses to salt and drought stress (Amara et al., 2024). A recent study demonstrated that the ECT8-decapping protein DECAPPING 5 complex facilitates the degradation of m6A-modified negative regulators of salt stress within processing bodies (PBs; Cai et al., 2024b).
m6A in mRNA translation
The role of m6A modification in translation regulation is emerging as a significant area of research. In animal models, m6A modifications in mRNAs positively or negatively influence translation, depending on the specific locations of the m6A marks (Rodriguez et al., 2022). A study in maize demonstrated that transcripts containing m6A marks near the start codon exhibit a high translational status, suggesting that these m6A marks may be associated with enhanced mRNA translation (Luo et al., 2020). Furthermore, the m6A writer MTA in apple enhances the translation efficiency of genes involved in oxidative stress and lignin deposition under drought-stress conditions (Hou et al., 2022). The m6A writer VIR in Arabidopsis positively regulates the translation of transcripts associated with photoprotection under conditions of high-light stress (Zhang et al., 2022c). In addition, a recent analysis of the m6A methylome, transcriptome, and translatome in the mta mutant revealed that m6A-containing mRNAs exhibit higher translation efficiency than non-m6A-containing mRNAs under cold-stress conditions (Wang et al., 2023b). The m6A reader MhYTP2 in apple regulates the translation efficiency of antioxidant genes (Guo et al., 2022). SlYTH2, a newly identified m6A reader in tomato, impedes polysome assembly and translation of target mRNAs (Bian et al., 2024). Furthermore, a recent study demonstrated that the m6A reader ECT8 influences the ABA response by inhibiting translation of the ABA receptor gene PYL7 in response to ABA (Wu et al., 2024). These findings raise the important questions of whether m6A readers regulate translation efficiency in plants under abiotic stress conditions and whether a specific m6A reader mediates translation control based on the positioning of m6A marks within a transcript.
m6A-mediated phase separation modulates mRNA stability and translation
The formation of membraneless biomolecular condensates, including SGs and PBs, via LLPS is a potent cellular mechanism that governs mRNA stability and translation in cells (reviewed by Field et al., 2023; Solis-Miranda et al., 2023). Recent studies have shown that m6A modification and m6A readers are key components associated with phase separation (reviewed by Kang and Xu, 2023; Nguyen and Kang, 2024). Relocation of the Arabidopsis m6A reader ECT2 from the cytoplasm to SGs upon heat exposure suggests an association of phase separation with the heat-stress response (Scutenaire et al., 2018). The Arabidopsis m6A reader CPSF30-L enhances the formation of liquid-like nuclear bodies to regulate polyadenylation site selection (Song et al., 2021). Notably, Arabidopsis ECT1 forms PBs and SGs to aid in the degradation of m6A-modified mRNAs associated with the salicylic acid response (Lee et al., 2024). The m6A reader SiYTH1 in foxtail millet promotes the formation of cytoplasmic condensates to stabilize m6A-modified transcripts involved in the drought-stress response (Luo et al., 2023). Selective demethylation of the retrotransposon Onsen mRNA by ALKBH9B under heat stress releases the Onsen mRNA from the SGs into the cytoplasm, enabling its mobilization to mediate heat-dependent transposition in Arabidopsis (Fan et al., 2023). A recent study demonstrated that the ECT8-decapping protein DECAPPING 5 complex forms PBs to facilitate the degradation of m6A-modified negative regulators in response to salt stress (Cai et al., 2024b). SlYTH2, a newly identified m6A reader in tomato, affects the sequestration of m6A-modified transcripts into liquid-like condensates, impeding polysome assembly and translation of target mRNAs (Bian et al., 2024). Furthermore, a recent study revealed that the m6A reader ECT8 acts as a driver of LLPS to sequester m6A-modified transcripts, including the ABA receptor gene PYL7, into SGs for mRNA storage, thereby inhibiting mRNA translation in response to ABA (Wu et al., 2024). These findings suggest a crucial role for m6A reader-mediated phase separation in mRNA stabilization, decay, and translation. Because many ECT family proteins contain intrinsically disordered regions or prion-like domains essential for phase separation (Lee et al., 2022; Nguyen and Kang, 2024), determining the phase-separation potential of other ECT family members and investigating the interactions of ECT proteins and other m6A readers with cellular proteins involved in decapping and endoribonucleolytic cleavage could provide insights into their roles in phase separation and mRNA cleavage under specific stress conditions.
A role for m6A-mediated phase separation in light signaling and response has recently been emerging. CRY2, together with m6A writer components such as MTA, MTB, and FIP37, undergoes light-induced phase separation to modulate methyltransferase activity, m6A abundance, and circadian rhythms in Arabidopsis (Wang et al., 2021). Moreover, CRY2 co-condenses FIO1 in the presence of SPA1 to form photobodies, inducing the m6A methylation and translation of mRNAs related to chlorophyll biosynthesis in response to light (Jiang et al., 2023). These results highlight the emerging role of m6A modification in light-induced phase separation in plants. Given that light is a key environmental signal that regulates plant responses to abiotic stresses and that photosynthesis functions as a global sensor of abiotic stress (Biswal et al., 2011), further research is needed to determine whether m6A and other modifications of mRNAs associated with light signaling and response are modulated in response to abiotic stresses. Furthermore, it will be crucial to explore whether m6A-modified mRNAs involved in light signaling and response undergo phase separation under abiotic stresses.
tRNA and rRNA modifications in translation control
tRNA modifications play diverse roles in cellular responses to environmental stress (Huber et al., 2019). Typically, complex modifications are primarily localized in the anticodon loop, whereas simpler modifications are associated with the tRNA core (Huber et al., 2019). One study demonstrated that modifications at the wobble position of the tRNA anticodon affect the translation of stress-responsive transcripts (Gu et al., 2014). Stress-induced alterations at the wobble position enhance translation, leading to increased synthesis of critical stress-response proteins (Barraud and Tisné, 2019). A recent study demonstrated that RsmD, a chloroplast rRNA m2G methyltransferase, catalyzes m2G methylation at position 915 of the chloroplast 16S rRNA, thereby influencing the translation of chloroplast proteins in Arabidopsis under cold-stress conditions (Ngoc et al., 2021). In rice, OsPUS1 is implicated in chloroplast rRNA pseudouridylation, affecting ribosome biogenesis and the translation of photosynthesis-related and stress-responsive genes under low-temperature conditions (Wang et al., 2022d).
Growing evidence underscores the critical roles of tRNA and rRNA modifications in regulating translation during plant stress responses. However, the mechanistic connections between tRNA modifications and translation have been investigated primarily in mammalian systems. To gain a comprehensive understanding of the functional roles of tRNA and rRNA modifications in regulating translation during plant stress responses, genetic manipulation of genes associated with these modifications, along with proteome analysis, will be necessary across different plant species under abiotic stresses.
Questions, outlook, and future challenges
The transcriptome-wide mapping of m6A and other RNA modifications, combined with the genetic characterization of genes associated with these modifications, has broadened our understanding of the crucial role of RNA modifications in plant stress responses. However, because most recent studies have focused on model plants such as Arabidopsis, investigating how different RNA modifications are associated with abiotic stress responses across diverse plant species is essential. Recent advances in m6A-seq tools and gene-editing technologies have led to significant progress in discovering the novel functions of RNA modifications in diverse plant species, including Arabidopsis, rice, tomato, cotton, and apple, during the process of stress adaptation. However, several critical questions and challenges remain regarding the cellular and mechanistic roles of RNA modifications in plant stress responses.
First, as an RNA modification, m6A is a dynamic and reversible process influenced by environmental and developmental signals. Therefore, it will be necessary to investigate how abiotic stresses influence the expression and activity of m6A writers and erasers and how m6A is added to or removed from mRNA transcripts in a stress-dependent manner across different plant species.
Second, growing evidence highlights the crucial role of chemical modifications in tRNAs and rRNAs during plant stress responses. Given that many genes responsible for tRNA and rRNA modifications have been identified in bacteria, yeasts, and animals (reviewed by Swinehart and Jackman, 2015; Sergiev et al., 2018), identifying the writers and erasers responsible for tRNA and rRNA modifications and characterizing their functions in plants under stress conditions are essential.
Third, considering that ncRNAs, including miRNAs and lncRNAs, play significant roles in plant stress responses and that m6A modification affects miRNA biogenesis (Bhat et al., 2020; Bai et al., 2024; Zhong et al., 2024) and lncRNA function (Xu et al., 2021), investigating how m6A and other modifications regulate the fate and functions of ncRNAs in response to abiotic stresses is needed.
Fourth, because phase separation mediated by m6A readers has emerged as a significant mechanism for gene regulation during stress responses (Luo et al., 2023; Cai et al., 2024b; Wu et al., 2024), it will be necessary to investigate whether m6A and other RNA modifications facilitate the phase separation of essential genes involved in stress responses to specific environmental cues.
Fifth, considering that most m6A mapping studies to date have relied on antibody-based methylated RNA immunoprecipitation sequencing (MeRIP-seq) data, which lacks single-base resolution for pinpointing precise m6A sites within transcripts, advanced sequencing technologies, including nanopore direct RNA-seq, MAZTER sequencing (MAZTER-seq), m6A-sensitive RNA-endoribonuclease–facilitated sequencing (m6A-REF-seq), and m6A individual-nucleotide-resolution cross-linking and immunoprecipitation sequencing (miCLIP-seq), should be improved and further developed for use in plants (reviewed by Prall et al., 2023). These methods will facilitate the precise measurement of stoichiometry and dynamic changes in specific m6A and other RNA modifications under different stress conditions.
Sixth, because the Arabidopsis m6A writer FIP37 is regulated by transmembrane kinase 4-mediated phosphorylation (Li et al., 2024) and the m6A writer METTL3 and m6A readers YTHDF1 and YTHDF2 in animals are regulated by phosphorylation, sumoylation, or glycosylation (Sun et al., 2020; Li et al., 2023c; Sugiokto et al., 2024), it will be necessary to determine whether the activity and functionality of writers, erasers, and readers are regulated by posttranslational modifications, including phosphorylation, sumoylation, and ubiquitination, in plants in response to various developmental and environmental signals.
Finally, the identification of stress-induced RNA modifications is crucial for differentiating between “constitutive” and “dynamic” modifications associated with specific stress conditions. This information will offer valuable insights to support the precise engineering of RNA modifications to enhance crop resilience. The CRISPR-Cas13-based RNA methylation/demethylation system (Wilson et al., 2020) and programmable m6A editing tools, by fusing the catalytic domain of an m6A writer or eraser to catalytically dead Cas13a (Shi et al., 2024; Yu et al., 2024), hold promise for directly modifying single bases that exhibit stress-induced modifications, thereby improving stress resistance in crops.
In summary, rapid advances in transcriptome-wide mapping of RNA modifications have significantly enhanced our understanding of their roles in plant responses to diverse abiotic stresses. However, significant challenges remain in identifying and characterizing the cellular components responsible for addition and removal of RNA modification marks. Establishing the molecular links between RNA modifications and stress adaptation remains an ongoing effort in model plants and agriculturally important crops. Integrating molecular insights into the regulatory roles of RNA modifications in stress responses with novel genome- and RNA-editing technologies will facilitate the breeding of stress-tolerant crops through precisely engineered RNA modifications. Although this research area presents significant challenges, it holds the potential for exciting discoveries in the future.
Funding
This work was supported by the Mid-Career Researcher Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning (NRF-2021R1A2C1004187), Republic of Korea; the Qing Lan Project of Jiangsu Province (2024); the Natural Science Foundation of Jiangsu Province (grant NoBK20241054); the Program of the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (23KJB550004); and the High-Level Innovation and Entrepreneurship Talents Introduction Program of Jiangsu Province of China (JSSCBS20230419).
Acknowledgments
No conflict of interest is declared.
Author contributions
J.C., L.S., H.K., and T.X. conceptualized the ideas, performed the literature review, and wrote the manuscript.
Published: December 21, 2024
Contributor Information
Hunseung Kang, Email: hskang@jnu.ac.kr.
Tao Xu, Email: xutao_yr@126.com.
References
- Amara U., Hu J., Cai J., Kang H. FLK is an mRNA m6A reader that regulates floral transition by modulating the stability and splicing of FLC in Arabidopsis. Mol. Plant. 2023;16:919–929. doi: 10.1016/j.molp.2023.04.005. [DOI] [PubMed] [Google Scholar]
- Amara U., Hu J., Park S.J., Kang H. ECT12, an YTH-domain protein, is a potential mRNA m6A reader that affects abiotic stress responses by modulating mRNA stability in Arabidopsis. Plant Physiol. Biochem. 2024;206 doi: 10.1016/j.plaphy.2023.108255. [DOI] [PubMed] [Google Scholar]
- Amara U., Shoaib Y., Kang H. ALKBH9C, a potential RNA m6A demethylase, regulates the response of Arabidopsis to abiotic stresses and abscisic acid. Plant Cell Environ. 2022;45:3566–3581. doi: 10.1111/pce.14447. [DOI] [PubMed] [Google Scholar]
- Anderson S.J., Kramer M.C., Gosai S.J., Yu X., Vandivier L.E., Nelson A.D.L., Anderson Z.D., Beilstein M.A., Fray R.G., Lyons E., et al. N6-methyladenosine inhibits local ribonucleolytic cleavage to stabilize mRNAs in Arabidopsis. Cell Rep. 2018;25:1146–1157.e3. doi: 10.1016/j.celrep.2018.10.020. [DOI] [PubMed] [Google Scholar]
- Ariel F., Lucero L., Christ A., Mammarella M.F., Jegu T., Veluchamy A., Mariappan K., Latrasse D., Blein T., Liu C., et al. R-loop mediated trans-action of the APOLO long noncoding RNA. Mol. Cell. 2020;77:1055–1065.e4. doi: 10.1016/j.molcel.2019.12.015. [DOI] [PubMed] [Google Scholar]
- Aswathi U., Viswam P., Kattupalli D., Vasudevan S.E. Elucidation of transfer RNAs as stress regulating agents and the experimental strategies to conceive the functional role of tRNA-derived fragments in plants. Crit. Rev. Biotechnol. 2023;43:275–292. doi: 10.1080/07388551.2022.2026288. [DOI] [PubMed] [Google Scholar]
- Bai C., Fang M., Zhai B., Ma L., Fu A., Gao L., Kou X., Meng D., Wang Q., Zheng S., et al. Regulations of m6A methylation on tomato fruit chilling injury. Hortic. Plant J. 2021;7:434–442. [Google Scholar]
- Bai H., Dai Y., Fan P., Zhou Y., Wang X., Chen J., Jiao Y., Du C., Huang Z., Xie Y., et al. The METHYLTRANSFERASE B–SERRATE interaction mediates the reciprocal regulation of microRNA biogenesis and RNA m6A modification. J. Integr. Plant Biol. 2024;66:2613–2631. doi: 10.1111/jipb.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud P., Tisné C. To be or not to be modified: miscellaneous aspects influencing nucleotide modifications in tRNAs. IUBMB Life. 2019;71:1126–1140. doi: 10.1002/iub.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat S.S., Bielewicz D., Gulanicz T., Bodi Z., Yu X., Anderson S.J., Szewc L., Bajczyk M., Dolata J., Grzelak N., et al. mRNA adenosine methylase (MTA) deposits m6A on pri-miRNAs to modulate miRNA biogenesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2020;117:21785–21795. doi: 10.1073/pnas.2003733117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian H., Song P., Gao Y., Deng Z., Huang C., Yu L., Wang H., Ye B., Cai Z., Pan Y., et al. The m6A reader SlYTH2 negatively regulates tomato fruit aroma by impeding the translation process. Proc. Natl. Acad. Sci. USA. 2024;121 doi: 10.1073/pnas.2405100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal, Joshi P.N., Raval M.K., Biswal U.C. Photosynthesis, a global sensor of environmental stress in green plants: stress signaling and adaptation. Curr. Sci. 2011;101:47–56. [Google Scholar]
- Boccaletto P., Stefaniak F., Ray A., Cappannini A., Mukherjee S., Purta E., Kurkowska M., Shirvanizadeh N., Destefanis E., Groza P., et al. MODOMICS: a database of RNA modification pathways. 2021 update. Nucleic Acids Res. 2022;50:D231–D235. doi: 10.1093/nar/gkab1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodi Z., Zhong S., Mehra S., Song J., Graham N., Li H., May S., Fray R.G. Adenosine methylation in Arabidopsis mRNA is associated with the 3' end and reduced levels cause developmental defects. Front. Plant Sci. 2012;3:48. doi: 10.3389/fpls.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Hu J., Amara U., Park S.J., Li Y., Jeong D., Lee I., Xu T., Kang H. Arabidopsis N6-methyladenosine methyltransferase FIONA1 regulates floral transition by affecting the splicing of FLC and the stability of floral activators SPL3 and SEP3. J. Exp. Bot. 2023;74:864–877. doi: 10.1093/jxb/erac461. [DOI] [PubMed] [Google Scholar]
- Cai J., Hu J., Xu T., Kang H. FIONA1-mediated mRNA m6A methylation regulates the response of Arabidopsis to salt stress. Plant Cell Environ. 2024;47:900–912. doi: 10.1111/pce.14807. [DOI] [PubMed] [Google Scholar]
- Cai Z., Tang Q., Song P., Tian E., Yang J., Jia G. The m6A reader ECT8 is an abiotic stress sensor that accelerates mRNA decay in Arabidopsis. Plant Cell. 2024;36:2908–2926. doi: 10.1093/plcell/koae149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Jäger G., Zheng B. Transfer RNA modifications and genes for modifying enzymes in Arabidopsis thaliana. BMC Plant Biol. 2010;10:201. doi: 10.1186/1471-2229-10-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P., Bao S., Li C., Tong J., Shen L., Yu H. RNA N6-methyladenosine modification promotes auxin biosynthesis required for male meiosis in rice. Dev. Cell. 2022;57:246–259.e4. doi: 10.1016/j.devcel.2021.12.014. [DOI] [PubMed] [Google Scholar]
- Cheng Q., Wang P., Wu G., Wang Y., Tan J., Li C., Zhang X., Liu S., Huang S., Huang T., et al. Coordination of m6A mRNA methylation and gene transcriptome in rice response to cadmium stress. Rice. 2021;14:62. doi: 10.1186/s12284-021-00502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J.M., Ye T.T., Ma C.J., Lan M.D., Liu T., Yuan B.F., Feng Y.Q. Existence of internal N7-methylguanosine modification in mRNA determined by differential enzyme treatment coupled with mass spectrometry analysis. ACS Chem. Biol. 2018;13:3243–3250. doi: 10.1021/acschembio.7b00906. [DOI] [PubMed] [Google Scholar]
- Cui C., Ma Z., Wan H., Gao J., Zhou B. GhALKBH10 negatively regulates salt tolerance in cotton. Plant Physiol. Biochem. 2022;192:87–100. doi: 10.1016/j.plaphy.2022.09.029. [DOI] [PubMed] [Google Scholar]
- Cui X., Liang Z., Shen L., Zhang Q., Bao S., Geng Y., Zhang B., Leo V., Vardy L.A., Lu T., et al. 5-methylcytosine RNA methylation in Arabidopsis thaliana. Mol. Plant. 2017;10:1387–1399. doi: 10.1016/j.molp.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Dannfald A., Favory J.J., Deragon J.M. Variations in transfer and ribosomal RNA epitranscriptomic status can adapt eukaryote translation to changing physiological and environmental conditions. RNA Biol. 2021;18:4–18. doi: 10.1080/15476286.2021.1931756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R., Burgess A., Parker B., Li J., Pulsford K., Sibbritt T., Preiss T., Searle I.R. Transcriptome-wide mapping of RNA 5-methylcytosine in Arabidopsis mRNAs and noncoding RNAs. Plant Cell. 2017;29:445–460. doi: 10.1105/tpc.16.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H.C., Wei L.H., Zhang C., Wang Y., Chen L., Lu Z., Chen P.R., He C., Jia G. ALKBH10B is an RNA N6-methyladenosine demethylase affecting Arabidopsis floral transition. Plant Cell. 2017;29:2995–3011. doi: 10.1105/tpc.16.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W., Wang L., Lei Z., Li H., Chu J., Yan M., Wang Y., Wang H., Yang J., Cho J. m6A RNA demethylase AtALKBH9B promotes mobilization of a heat-activated long terminal repeat retrotransposon in Arabidopsis. Sci. Adv. 2023;9:eadf3292. doi: 10.1126/sciadv.adf3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field S., Jang G.-J., Dean C., Strader L.C., Rhee S.Y. Plants use molecular mechanisms mediated by biomolecular condensates to integrate environmental cues with development. Plant Cell. 2023;35:3173–3186. doi: 10.1093/plcell/koad062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye M., Harada B.T., Behm M., He C. RNA modifications modulate gene expression during development. Science. 2018;361:1346–1349. doi: 10.1126/science.aau1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan G., Sharma B., Li Y.-F., Armstrong C.D., Merum P., Rohila J.S., Gregory B.D., Sunkar R. mRNA N6-methyladenosine is critical for cold tolerance in Arabidopsis. Plant J. 2022;111:1052–1068. doi: 10.1111/tpj.15872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C., Begley T.J., Dedon P.C. tRNA modifications regulate translation during cellular stress. FEBS Lett. 2014;588:4287–4296. doi: 10.1016/j.febslet.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Liu C., Meng F., Hu L., Fu X., Yang Z., Wang N., Jiang Q., Zhang X., Ma F. The m6A reader MhYTP2 regulates MdMLO19 mRNA stability and antioxidant genes translation efficiency conferring powdery mildew resistance in apple. Plant Biotechnol. J. 2022;20:511–525. doi: 10.1111/pbi.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R., Shoaib Y., Cai J., Kang H. ALKBH10B-mediated m6A demethylation is crucial for drought tolerance by affecting mRNA stability in Arabidopsis. Environ. Exp. Bot. 2023;209 [Google Scholar]
- Han X., Wang J., Zhang Y., Kong Y., Dong H., Feng X., Li T., Zhou C., Yu J., Xin D., et al. Changes in the m6A RNA methylome accompany the promotion of soybean root growth by rhizobia under cadmium stress. J. Hazard Mater. 2023;441 doi: 10.1016/j.jhazmat.2022.129843. [DOI] [PubMed] [Google Scholar]
- Hou N., Li C., He J., Liu Y., Yu S., Malnoy M., Mobeen Tahir M., Xu L., Ma F., Guan Q. MdMTA-mediated m6A modification enhances drought tolerance by promoting mRNA stability and translation efficiency of genes involved in lignin deposition and oxidative stress. New Phytol. 2022;234:1294–1314. doi: 10.1111/nph.18069. [DOI] [PubMed] [Google Scholar]
- Hou Y., Sun J., Wu B., Gao Y., Nie H., Nie Z., Quan S., Wang Y., Cao X., Li S. CPSF30-L-mediated recognition of mRNA m6A modification controls alternative polyadenylation of nitrate signaling-related gene transcripts in Arabidopsis. Mol. Plant. 2021;14:688–699. doi: 10.1016/j.molp.2021.01.013. [DOI] [PubMed] [Google Scholar]
- Hu J., Cai J., Park S.J., Lee K., Li Y., Chen Y., Yun J.Y., Xu T., Kang H. N6-methyladenosine mRNA methylation is important for salt stress tolerance in Arabidopsis. Plant J. 2021;106:1759–1775. doi: 10.1111/tpj.15270. [DOI] [PubMed] [Google Scholar]
- Hu J., Cai J., Xu T., Kang H. Epitranscriptomic mRNA modifications governing plant stress responses: underlying mechanism and potential application. Plant Biotechnol. J. 2022;20:2245–2257. doi: 10.1111/pbi.13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Xu T., Kang H. Crosstalk between RNA m6A modification and epigenetic factors in plant gene regulation. Plant Commun. 2024;5 doi: 10.1016/j.xplc.2024.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S.M., Leonardi A., Dedon P.C., Begley T.J. The versatile roles of the tRNA epitranscriptome during cellular responses to toxic exposures and environmental stress. Toxics. 2019;7:17. doi: 10.3390/toxics7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huong T.T., Ngoc L.N.T., Kang H. Functional characterization of a putative RNA demethylase ALKBH6 in Arabidopsis growth and abiotic stress responses. Int. J. Mol. Sci. 2020;21:6707. doi: 10.3390/ijms21186707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huong T.T., Yang Z., Ngoc L.N.T., Kang H. ALKBH8B, a putative RNA demethylase, plays a role in the response of Arabidopsis to salt stress and abscisic acid. J. Plant Biol. 2022;65:319–330. [Google Scholar]
- Janssen K.A., Xie Y., Kramer M.C., Gregory B.D., Garcia B.A. Data-independent acquisition for the detection of mononucleoside RNA modifications by mass spectrometry. J. Am. Soc. Mass Spectrom. 2022;33:885–893. doi: 10.1021/jasms.2c00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha U.C., Nayyar H., Jha R., Khurshid M., Zhou M., Mantri N., Siddique K.H.M. Long non-coding RNAs: Emerging players regulating plant abiotic stress response and adaptation. BMC Plant Biol. 2020;20:466–520. doi: 10.1186/s12870-020-02595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q., Zong X., Mao Y., Qian S.-B. A heat shock–responsive lncRNA Heat acts as a HSF1-directed transcriptional brake via m6A modification. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2102175118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Zhong Z., Gu L., Zhang X., Wei J., Ye C., Lin G., Qu G., Xiang X., Wen C., et al. Light-induced LLPS of the CRY2/SPA1/FIO1 complex regulating mRNA methylation and chlorophyll homeostasis in Arabidopsis. Nat. Plants. 2023;9:2042–2058. doi: 10.1038/s41477-023-01580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H., Xu T. N6-methyladenosine RNA methylation modulates liquid‒liquid phase separation in plants. Plant Cell. 2023;35:3205–3213. doi: 10.1093/plcell/koad103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M.C., Janssen K.A., Palos K., Nelson A.D.L., Vandivier L.E., Garcia B.A., Lyons E., Beilstein M.A., Gregory B.D. N6-methyladenosine and RNA secondary structure affect transcript stability and protein abundance during systemic salt stress in Arabidopsis. Plant Direct. 2020;4 doi: 10.1002/pld3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.G., Kim J., Seo P.J. N6-methyladenosine-modified RNA acts as a molecular glue that drives liquid–liquid phase separation in plants. Plant Signal. Behav. 2022;17 doi: 10.1080/15592324.2022.2079308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.P., Liu K., Kim E.Y., Medina-Puche L., Dong H., Di M., Singh R.M., Li M., Qi S., Meng Z., et al. The m6A reader ECT1 drives mRNA sequestration to dampen salicylic acid-dependent stress responses in Arabidopsis. Plant Cell. 2024;36:746–763. doi: 10.1093/plcell/koad300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Zhang M., Sun W., Yue D., Ma Y., Zhang B., Duan L., Wang M., Lindsey K., Nie X., et al. N6-methyladenosine RNA modification regulates cotton drought response in a Ca2+ and ABA-dependent manner. Plant Biotechnol. J. 2023;21:1270–1285. doi: 10.1111/pbi.14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Li D., Cai L., Zhou Q., Liu C., Lin J., Li Y., Zhao X., Li L., Liu X., et al. Transcriptome-wide profiling of RNA N4-cytidine acetylation in Arabidopsis thaliana and Oryza sativa. Mol. Plant. 2023;16:1082–1098. doi: 10.1016/j.molp.2023.04.009. [DOI] [PubMed] [Google Scholar]
- Li J., Ahmad M., Sang L., Zhan Y., Wang Y., Yan Y., Liu Y., Mi W., Lu M., Dai Y., et al. O-GlcNAcylation promotes the cytosolic localization of the m6A reader YTHDF1 and colorectal cancer tumorigenesis. J. Biol. Chem. 2023;299 doi: 10.1016/j.jbc.2023.104738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Zhou Q., Cai L., Li L., Xie C., Li D., Zhu F., Li X., Zhao X., Liu X., et al. TMK4-mediated FIP37 phosphorylation regulates auxin-triggered N6-methyladenosine modification of auxin biosynthetic genes in Arabidopsis. Cell Rep. 2024;43 doi: 10.1016/j.celrep.2024.114597. [DOI] [PubMed] [Google Scholar]
- Liang Q., Zhang J., Lam H.-M., Chan T.-F. Nanopore direct RNA sequencing reveals N6-methyladenosine and polyadenylation landscapes on long non-coding RNAs in Arabidopsis thaliana. BMC Plant Biol. 2024;24:1126. doi: 10.1186/s12870-024-05845-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Wang J., Hou X. Transcriptome-wide N6-methyladenosine (m6A) methylome profiling of heat stress in pak-choi (Brassica rapa ssp. chinensis) Plants. 2020;9:1080. doi: 10.3390/plants9091080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Zhang Y., He Q., Qi Z., Zhang G., Xu W., Yi T., Wu G., Li R. MTA, an RNA m6A methyltransferase, enhances drought tolerance by regulating the development of trichomes and roots in poplar. Int. J. Mol. Sci. 2020;21:2462. doi: 10.3390/ijms21072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J.-H., Wang Y., Wang M., Zhang L.-Y., Peng H.-R., Zhou Y.-Y., Jia G.F., He Y. Natural variation in RNA m6A methylation and its relationship with translational status. Plant Physiol. 2020;182:332–344. doi: 10.1104/pp.19.00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Tang Y., Li S., Zhang L., Liu Y., Zhang R., Diao X., Yu J. The m6A reader SiYTH1 enhances drought tolerance by affecting the messenger RNA stability of genes related to stomatal closure and reactive oxygen species scavenging in Setaria italica. J. Integr. Plant Biol. 2023;65:2569–2586. doi: 10.1111/jipb.13575. [DOI] [PubMed] [Google Scholar]
- Manavella P.A., Godoy Herz M.A., Kornblihtt A.R., Sorenson R., Sieburth L.E., Nakaminami K., Seki M., Ding Y., Sun Q., Kang H., et al. Beyond transcription: compelling open questions in plant RNA biology. Plant Cell. 2023;35:1626–1653. doi: 10.1093/plcell/koac346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Pérez M., Aparicio F., López-Gresa M.P., Bellés J.M., Sánchez-Navarro J.A., Pallás V. Arabidopsis m6A demethylase activity modulates viral infection of a plant virus and the m6A abundance in its genomic RNAs. Proc. Natl. Acad. Sci. USA. 2017;114:10755–10760. doi: 10.1073/pnas.1703139114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z., Zhang T., Qi Y., Song J., Han Z., Ma C. Evolution of the RNA N6-methyladenosine methylome mediated by genomic duplication. Plant Physiol. 2020;182:345–360. doi: 10.1104/pp.19.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoc L.N.T., Park S.J., Cai J.,, Huong T.T., Lee K., Kang H. RsmD, a chloroplast rRNA m2G methyltransferase, plays a role in cold stress tolerance by possibly affecting chloroplast translation in Arabidopsis. Plant Cell Physiol. 2021;62:948–958. doi: 10.1093/pcp/pcab060. [DOI] [PubMed] [Google Scholar]
- Nguyen T.K.H., Kang H. Reading m6A marks in mRNA: a potent mechanism of gene regulation in plants. J. Integr. Plant Biol. 2024;66:2586–2599. doi: 10.1111/jipb.13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya K., Iwakiri J., Aly M.K., Sakaguchi Y., Adachi S., Natsume T., Terai G., Asai K., Suzuki T., Hirose T. m6A modification of HSATIII lncRNAs regulates temperature-dependent splicing. EMBO J. 2021;40 doi: 10.15252/embj.2021107976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikanov Y.S., Melnikov S.V., Söll D., Steitz T.A. Structural insights into the role of rRNA modifications in protein synthesis and ribosome assembly. Nat. Struct. Mol. Biol. 2015;22:342–344. doi: 10.1038/nsmb.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prall W., Ganguly D.R., Gregory B.D. The covalent nucleotide modifications within plant mRNAs: What we know, how we find them, and what should be done in the future. Plant Cell. 2023;35:1801–1816. doi: 10.1093/plcell/koad044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez G.F., Cesaro B., Fatica A. Multiple roles of m6A RNA modification in translational regulation in cancer. Int. J. Mol. Sci. 2022;11:8971. doi: 10.3390/ijms23168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růžička K., Zhang M., Campilho A., Bodi Z., Kashif M., Saleh M., Eeckhout D., El-Showk S., Li H., Zhong S., et al. Identification of factors required for m6A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol. 2017;215:157–172. doi: 10.1111/nph.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scutenaire J., Deragon J.M., Jean V., Benhamed M., Raynaud C., Favory J.J., Merret R., Bousquet-Antonelli C. The YTH domain protein ECT2 is an m6A reader required for normal trichome branching in Arabidopsis. Plant Cell. 2018;30:986–1005. doi: 10.1105/tpc.17.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergiev P.V., Aleksashin N.A., Chugunova A.A., Polikanov Y.S., DOntsova O.A. Structural and evolutionary insights into ribosomal RNA methylation. Nat. Chem. Biol. 2018;14:226–235. doi: 10.1038/nchembio.2569. [DOI] [PubMed] [Google Scholar]
- Sharma B., Prall W., Bhatia G., Gregory B.D. The diversity and functions of plant RNA modifications: what we know and where we go from here. Annu. Rev. Plant Biol. 2023;74:53–85. doi: 10.1146/annurev-arplant-071122-085813. [DOI] [PubMed] [Google Scholar]
- Shen H., Zhou Y., Liao C., Xie Q., Chen G., Hu Z., Wu T. The AlkB homolog SlALKBH10B negatively affects drought and salt tolerance in Solanum lycopersicum. Int. J. Mol. Sci. 2023;25:173. doi: 10.3390/ijms25010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Zou W., Liu X., Zhang H., Li X., Fu G., Fei Q., Qian Q., Shang L. Programmable RNA N6-methyladenosine editing with CRISPR/dCas13a in plants. Plant Biotechnol. J. 2024;22:1867–1880. doi: 10.1111/pbi.14307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilpa, Thakur R., Prasad P. Epigenetic regulation of abiotic stress responses in plants. Biochim. Biophy. Acta–General Subjects. 2024;1868 doi: 10.1016/j.bbagen.2024.130661. [DOI] [PubMed] [Google Scholar]
- Shriram V., Kumar V., Devarumath R.M., Khare T.S., Wani S.H. MicroRNAs as potential targets for abiotic stress tolerance in plants. Front. Plant Sci. 2016;7:817. doi: 10.3389/fpls.2016.00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib Y., Hu J., Manduzio S., Kang H. Alpha-ketoglutarate-dependent dioxygenase homolog 10B, an N6-methyladenosine mRNA demethylase, plays a role in salt stress and abscisic acid responses in Arabidopsis thaliana. Physiol. Plantarum. 2021;173:1078–1089. doi: 10.1111/ppl.13505. [DOI] [PubMed] [Google Scholar]
- Shuai P., Liang D., Tang S., Zhang Z., Ye C.-Y., Su Y., Xia X., Yin W. Genome-wide identification and functional prediction of novel and drought-responsive lincRNAs in Populus trichocarpa. J. Exp. Bot. 2014;65:4975–4983. doi: 10.1093/jxb/eru256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis-Miranda J., Chodasiewicz M., Skirycz A., Fernie A.R., Moschou P.N., Bozhkov P.V., Gutierrez-Beltran E. Stress-related biomolecular condensates in plants. Plant Cell. 2023;35:3187–3204. doi: 10.1093/plcell/koad127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P., Tian E., Cai Z., Chen X., Chen S., Yu K., Bian H., He K., Jia G. Methyltransferase ATMETTL5 writes m6A on 18S ribosomal RNA to regulate translation in Arabidopsis. New Phytol. 2024;244:571–587. doi: 10.1111/nph.20034. [DOI] [PubMed] [Google Scholar]
- Song P., Wei L., Chen Z., Cai Z., Lu Q., Wang C., Tian E., Jia G. m6A readers ECT2/ECT3/ECT4 enhance mRNA stability through direct recruitment of the poly(A) binding proteins in Arabidopsis. Genome Biol. 2023;24:103. doi: 10.1186/s13059-023-02947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P., Yang J., Wang C., Lu Q., Shi L., Tayier S., Jia G. Arabidopsis N6-methyladenosine reader CPSF30-L recognizes FUE signals to control polyadenylation site choice in liquid-like nuclear bodies. Mol. Plant. 2021;14:571–587. doi: 10.1016/j.molp.2021.01.014. [DOI] [PubMed] [Google Scholar]
- Song X., Li Y., Cao X., Qi Y. MicroRNAs and their regulatory roles in plant–environment interactions. Annu. Rev. Plant Biol. 2019;70:489–525. doi: 10.1146/annurev-arplant-050718-100334. [DOI] [PubMed] [Google Scholar]
- Su T., Fu L., Kuang L., Chen D., Zhang G., Shen Q., Wu D. Transcriptome-wide m6A methylation profile reveals regulatory networks in roots of barley under cadmium stress. J. Hazard Mater. 2022;423 doi: 10.1016/j.jhazmat.2021.127140. [DOI] [PubMed] [Google Scholar]
- Sugiokto F.G., Saiada F., Zhang K., Li R. SUMOylation of the m6A reader YTHDF2 by PIAS1 promotes viral RNA decay to restrict EBV replication. mBio. 2024;15 doi: 10.1128/mbio.03168-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.-L., Zhu A.C., Gao Y., Terajima H., Fei Q., Liu S., Zhang L., Zhang Z., Harada B.T., He Y.Y., et al. Stabilization of ERK-phosphorylated METTL3 by USP5 increases m6A methylation. Mol. Cell. 2020;80:633–647.e7. doi: 10.1016/j.molcel.2020.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinehart W.E., Jackman J.E. Diversity in mechanism and function of tRNA methytransferases. RNA Biol. 2015;12:398–411. doi: 10.1080/15476286.2015.1008358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Chen S., Jia G. Detection, regulation, and functions of RNA N6-methyladenosine modification in plants. Plant Comm. 2023;4 doi: 10.1016/j.xplc.2023.100546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Gao C.-C., Gao Y., Yang Y., Shi B., Yu J.-L., Lyu C., Sun B.F., Wang H.L., Xu Y., et al. OsNSUN2-mediated 5-methylcytosine mRNA modification enhances rice adaptation to high temperature. Dev. Cell. 2020;53:272–286.e7. doi: 10.1016/j.devcel.2020.03.009. [DOI] [PubMed] [Google Scholar]
- Tang J., Jia P., Xin P., Chu J., Shi D.-Q., Yang W.-C. The Arabidopsis TRM61/TRM6 complex is a bona fide tRNA N1-methyladenosine methyltransferase. J. Exp. Bot. 2020;71:3024–3036. doi: 10.1093/jxb/eraa100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Yang J., Duan H., Jia G. ALKBH10B, an mRNA m6A demethylase, modulates ABA response during seed germination in Arabidopsis. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.712713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Yang J., Lu Q., Tang Q., Chen S., Jia G. The RNA N6-methyladenosine demethylase ALKBH9B modulates ABA responses in Arabidopsis. J. Integr. Plant Biol. 2022;64:2361–2373. doi: 10.1111/jipb.13394. [DOI] [PubMed] [Google Scholar]
- Tang J., Lei D., Yang J., Chen S., Wang X., Huang X., Zhang S., Cai Z., Zhu S., Wan J., et al. OsALKBH9-mediated m6A demethylation regulates tapetal PCD and pollen exine accumulation in rice. Plant Biotechnol. J. 2024;22:2410–2423. doi: 10.1111/pbi.14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.-M., Ye T.-T., You X.-J., Yin X.-M., Ding J.-H., Shao W.-X., Chen M.Y., Yuan B.F., Feng Y.Q. Mass spectrometry profiling analysis enables the identification of new modifications in ribosomal RNA. Chin. Chem. Lett. 2023;34 [Google Scholar]
- Tang Z., Xu M., Ito H., Cai J., Ma X., Qin J., Yu D., Meng Y. Deciphering the non-coding RNA-level response to arsenic stress in rice (Oryza sativa) Plant Signal. Behav. 2019;14 doi: 10.1080/15592324.2019.1629268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Hu J., Gao C., Chen G., Wang B., Lin C., Song L., Ding Y., Zhou G. Genome-wide analysis of long non-coding RNAs reveals the regulatory roles in the heat tolerance of Chinese cabbage (Brassica rapa ssp. chinensis) Sci. Rep. 2019;9:5002–5014. doi: 10.1038/s41598-019-41428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Jiang B., Gu L., Chen Y., Mora M., Zhu M., Noory E., Wang Q., Lin C. A photoregulatory mechanism of the circadian clock in Arabidopsis. Nat. Plants. 2021;7:1397–1408. doi: 10.1038/s41477-021-01002-z. [DOI] [PubMed] [Google Scholar]
- Wang Y., Pang C., Li X., Hu Z., Lv Z., Zheng B., Chen P. Identification of tRNA nucleoside modification genes critical for stress response and development in rice and Arabidopsis. BMC Plant Biol. 2017;17:261. doi: 10.1186/s12870-017-1206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li D., Gao J., Li X., Zhang R., Jin X., Hu Z., Zheng B., Persson S., Chen P. The 2′-O-methyladenosine nucleoside modification gene OsTRM13 positively regulates salt stress tolerance in rice. J. Exp. Bot. 2017;68:1479–1491. doi: 10.1093/jxb/erx061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Yang J., Song P., Zhang W., Lu Q., Yu Q., Jia G. FIONA1 is an RNA N6-methyladenosine methyltransferase affecting Arabidopsis photomorphogenesis and flowering. Genome Biol. 2022;23:40. doi: 10.1186/s13059-022-02612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Li W., Cheng Z., Sun J., Gao J., Li J., Niu X., Amjid M.W., Yang H., Zhu G., et al. Transcriptome-wide N6-methyladenosine profiling of cotton root provides insights for salt stress tolerance. Environ. Exp. Bot. 2022;194 [Google Scholar]
- Wang L., Zhuang H., Fan W., Zhang X., Dong H., Yang H., Cho J. m6A RNA methylation impairs gene expression variability and reproductive thermotolerance in Arabidopsis. Genome Biol. 2022;23:244. doi: 10.1186/s13059-022-02814-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Sun J., Zu X., Gong J., Deng H., Hang R., Zhang X., Liu C., Deng X., Luo L., et al. Pseudouridylation of chloroplast ribosomal RNA contributes to low temperature acclimation in rice. New Phytol. 2022;236:1708–1720. doi: 10.1111/nph.18479. [DOI] [PubMed] [Google Scholar]
- Wang S., Wang H., Xu Z., Jiang S., Shi Y., Xie H., Wang S., Hua J., Wu Y. m6A mRNA modification promotes chilling tolerance and modulates gene translation efficiency in Arabidopsis. Plant Physiol. 2023;192:1466–1482. doi: 10.1093/plphys/kiad112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Liu H., Wang F., Liu X., Sun Y., Zhao J., Zhu C., Gan L., Yu J., Witte C.P., et al. N4-acetylation of cytidine in mRNA plays essential roles in plants. Plant Cell. 2023;35:3739–3756. doi: 10.1093/plcell/koad189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L.-H., Song P., Wang Y., Lu Z., Tang Q., Yu Q., Xiao Y., Zhang X., Duan H.C., Jia G. The m6A reader ECT2 controls trichome morphology by affecting mRNA stability in Arabidopsis. Plant Cell. 2018;30:968–985. doi: 10.1105/tpc.17.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C., Chen P.J., Miao Z., Liu D.R. Programmable m6A modification of cellular RNAs with a Cas13-directed methyltransferase. Nat. Biotechnol. 2020;38:1431–1440. doi: 10.1038/s41587-020-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Wang Y., Wang J., Li X., Li J., Ye K. Profiling of RNA ribose methylation in Arabidopsis thaliana. Nucleic Acids Res. 2021;49:4104–4119. doi: 10.1093/nar/gkab196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Su T., Zhang S., Zhang Y., Wong C.E., Ma J., Shao Y., Hua C., Shen L., Yu H. N6-methyladenosine-mediated feedback regulation of abscisic acid perception via phase-separated ECT8 condensates in Arabidopsis. Nat. Plants. 2024;10:469–482. doi: 10.1038/s41477-024-01638-7. [DOI] [PubMed] [Google Scholar]
- Xu C., Wu Z., Duan H.C., Fang X., Jia G., Dean C. R-loop resolution promotes co-transcriptional chromatin silencing. Nat. Commun. 2021;12:1790. doi: 10.1038/s41467-021-22083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Xu H., Liu Y., Li M., Ali M., Xu X., Lu G. RNA N6-methyladenosine responds to low-temperature stress in tomato anthers. Front. Plant Sci. 2021;12:687826. doi: 10.3389/fpls.2021.687826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Cui Y., Feng Y., Hu Y., Liu L., Duan L. Long non-coding RNAs of plants in response to abiotic stresses and their regulating roles in promoting environmental adaptation. Cells. 2023;12:729. doi: 10.3390/cells12050729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Li L., Li X., Zhong M., Li X., Qu L., Zhang H., Tang D., Liu X., He C., et al. The blue light receptor CRY1 interacts with FIP37 to promote N6-methyladenosine RNA modification and photomorphogenesis in Arabidopsis. New Phytol. 2023;237:840–854. doi: 10.1111/nph.18583. [DOI] [PubMed] [Google Scholar]
- Yang W., Meng J., Liu J., Ding B., Tan T., Wei Q., Yu Y. The N1-methyladenosine methylome of petunia mRNA. Plant Physiol. 2020;183:1710–1724. doi: 10.1104/pp.20.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Alariqi M., Li B., Hussain A., Zhou H., Wang Q., Wang F., Wang G., Zhu X., Hui F., et al. CRISPR/dCas13 (Rx) derived RNA N6-methyladenosine (m6A) dynamic modification in plant. Adv. Sci. 2024;11 doi: 10.1002/advs.202401118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Lv Z., Diao S., Liu H., Duan A., He C., Zhang J. Unique features of the m6A methylome and its response to drought stress in sea buckthorn (Hippophae rhamnoides Linn.) RNA Biol. 2021;18:794–803. doi: 10.1080/15476286.2021.1992996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zhang S., Wu Y., Li Y., Kong L., Wu R., Zhao M., Liu W., Yu H. Defining context-dependent m6A RNA methylomes in Arabidopsis. Dev. Cell. 2024;59:2772–2786.e3. doi: 10.1016/j.devcel.2024.06.012. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhu J., Gong Z., Zhu J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022;23:104–119. doi: 10.1038/s41576-021-00413-0. [DOI] [PubMed] [Google Scholar]
- Zhang M., Bodi Z., Mackinnon K., Zhong S., Archer N., Mongan N.P., Simpson G.G., Fray R.G. Two zinc finger proteins with functions in m6A writing interact with HAKAI. Nat. Commun. 2022;13:1127. doi: 10.1038/s41467-022-28753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Zeng Y., Peng R., Dong J., Lan Y., Duan S., Chang Z., Ren J., Luo G., Liu B., et al. N6-methyladenosine RNA modification regulates photosynthesis during photodamage in plants. Nat. Commun. 2022;13:7441. doi: 10.1038/s41467-022-35146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xu J., Li R., Ge Y., Li Y., Li R. Plants’ response to abiotic stress: mechanism and strategies. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241310915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Guo T., Li J., Jiang L., Wang N. Tomato (Solanum lycopersicum L.) YTH domain- containing RNA-binding protein (YTP) family members participate in low-temperature treatment and waterlogging stress responses. Horticulturae. 2024;10:522. [Google Scholar]
- Zhong S., Li H., Bodi Z., Button J., Vespa L., Herzog M., Fray R.G. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20:1278–1288. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S., Li X., Li C., Bai H., Chen J., Gan L., Zhu J., Oh T., Yan X., Zhu J., et al. SERRATE drives phase separation behaviors to regulate m6A modification and miRNA biogenesis. Nat. Cell Biol. 2024;26:2129–2143. doi: 10.1038/s41556-024-01530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Tian S., Qin G. RNA methylomes reveal the m6A-mediated regulation of DNA demethylase gene SlDML2 in tomato fruit ripening. Genome Biol. 2019;20:156. doi: 10.1186/s13059-019-1771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Gao G., Tang R., Wang W., Wang Y., Tian S., Qin G. m6A-mediated regulation of crop development and stress responses. Plant Biotechnol. J. 2022;20:1447–1455. doi: 10.1111/pbi.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Tang R., Li X., Tian S., Li B., Qin G. N6-methyladenosine RNA modification regulates strawberry fruit ripening in an ABA-dependent manner. Genome Biol. 2021;22:168. doi: 10.1186/s13059-021-02385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]