Central Illustration
Key Words: bioactive molecules, myocardial infarction, myocardial tissue regeneration, nanofiber cardiac patch, synthetic and/or natural or biological materials
Highlights
-
•
Adverse cardiac remodeling following myocardial infarction is a leading cause of morbidity and mortality worldwide.
-
•
Several impediments exist with current cell therapy approaches to repair damaged myocardium following injury, highlighting the need for alternative approaches.
-
•
This review highlights a promising new approach of using biomaterial electrospun nanofiber patches for promoting tissue regeneration.
Summary
Because the adult heart has only minimal regenerative capacity, its inability to induce regeneration is well-known in patients with myocardial infarction. However, based on multidisciplinary approaches, it is possible to restore myocardial capability with regenerative medicine via living cardiac patches seeded with therapeutic ingredients ranging from multiple cell types to bioactive molecules, including growth factors, microRNA, and extracellular vesicles to the affected site. Biomaterials, natural and/or synthesized polymers, or in vivo sources such as collagen, fibrin, and decellularized extracellular matrix are used to form these cardiac patches. Herein, we review various techniques where seeded cells and bioactive agents are incorporated within porous nanofibers to create functional cardiac patches that provide myocardial extracellular matrix–like features, mechanical support, and a large surface-to-volume ratio for promoting cellular metabolism as well as compensation for the loss of cardiomyocytes in the infarcted region. We summarize recent advances through electrospinning-generated nanofibers of synthetic and/or natural polymers combined with biological material to create cardiac patches to repair and improve the function of infarcted myocardium. As tailoring designs on cardiac patches have been shown to exhibit deformation mechanisms and enhanced myocardial tissue regeneration, significant roles of various patterns and associated parameters are also discussed. The enhanced delivery of therapeutics offered by tailored nanofiber cardiac patches to treat myocardial infarction and overcome challenges of existing cardiac regeneration therapies such as low stability, short half-lifetime, and delivery methods may promote the potential for their clinical impact on myocardial regeneration.
Myocardial infarction (MI) is a consequence of the lack of oxygen and nutrients supply in the myocardium. It is usually associated with collagen fiber proliferation and scar tissue formation, which block electrical pulse signals in the infarcted area. It is well known that infract area thinning occurs in patients who survive MI, and almost 20% of the heart muscle is damaged if MI lasts 6 to 30 minutes. As a result, a sufficient number of cardiomyocytes undergo apoptosis or necrosis during the occurrence of cardiac remodeling. Because cardiomyocytes lose their regenerative potential early after birth in mammals, restoring myocardial structure and function in MI is the primary obstacle. Additionally, the nonmyocyte population of the heart contributes toward the effective functioning of the myocardium because of the involvement of the cardiac extracellular matrix (ECM) in the remodeling of the infarcted heart and myocardial stiffness. It is known that structural and biochemical remodeling of the heart occurs with enhanced collagen gene expression in cardiac fibroblasts. The hostile environment during the development of MI is unfavorable for natural repair and regenerative mechanisms, affecting the ejection fraction and loss of contractility in the infarcted area, eventually leading to arrhythmias, cardiac damage, and heart failure.1, 2, 3, 4, 5 The prevalence of MI in subjects <60 and >60 years of age has been reported to be 3.8% and 9.5%, respectively. Because the mortality and morbidity rates in MI-induced heart failure are high, heart transplantation in MI patients is a challenging proposition due to limited availability of donor organ supply and high risk of organ rejection.6,7
Active proliferation of cardiomyocytes and nonmyocyte cells occurs during cardiac development but such a mechanism in the adult heart seems to be impaired. However, some investigators have shown the ability of adult cardiomyocytes to proliferate, whereas others have provided evidence regarding the involvement of stem cells in myocardial regeneration after severe cardiac injury.8, 9, 10 The capability of the heart to regenerate myocardial tissue has also been reported under different conditions, including MI.11,12 A cardiac patch made of biomaterials and biological materials or cell-seeded materials has been used for myocardial regeneration and treatment of MI (Figure 1). Such a patch is implanted onto the surface of the infarcted region of the heart by glue and suture or using a suture-free procedure to renew ischemic cells and repair infarcted myocardium to recover normal function.13,14 The native myocardium is anisotropic, consisting of aligned cardiomyocytes at an angle of <45° and aligned capillaries organized in the direction of muscle fibers to fulfil interstitial oxygen and nutrient diffusion requirements for cell metabolism. Thus, wrapping the myocardium by mural cells ensures sufficient support for substrate transport and blood flow.15 Because the myocardium has an interwoven architecture in which coiled perimysial fibers offer the mechanical qualities for effective cardiac muscle contraction by expanding and recoiling the fibers, electrospun nanofiber cardiac patches preserve the biomimetic nanofibrous topography and provide a biological niche for activities of native ECM. These lightweight patches offer an extensive surface-to-volume ratio, interconnected pores, and permeability for myocardial regeneration after MI.16 Because the heart is a complex 3-dimensional (3D) anisotropic structure with a helically organized myocardial band and slender interlayer configuration shifting from the endocardium to the epicardium, an anisotropic aligned fibrous and micro-grooved cardiac patch can provide contact direction for cellular reorganization and functional modulation. Cardiomyocytes cultured on the anisotropic cardiac patches stretched in the structural direction to obtain a higher sarcomere length, faster Ca2+ propagation speed, and synchronous contraction rate have been shown to exhibit excellent electrophysiological properties in comparison to those revealed by 2-dimensional (2D) flat plate patches.17, 18, 19, 20 There is also evidence to show that anisotropy impacts electrical signal propagation by stimulating the polarization of extracellular gap junctions to the longitudinal ends of the cardiomyocytes.21 In this regard, electrospinning-generated in vitro nanofiber cardiac patches with anisotropic microstructures, which resemble the natural ECM, have attracted significant attention.22 In fact, the potentials of various electrospun cardiac patches made from synthetic and/or natural23 and electroactive biomaterials loaded with bioactive molecules or drugs have been actively investigated for the regeneration of the infarcted regions of the heart.24 Such patches do not limit later systolic activity but simulate the unique physical structure of native tissue with the same anisotropy and mechanical strength for myocardial regeneration. Their effectiveness has been observed in the delivery of loaded mass cells, nano-micro sized drugs, molecules, proteins, microRNA, and extracellular vesicles at the diseased site.14,25, 26, 27, 28 These patches reinforce the matrix directly by binding to the material surface or covalently linking cell adhesion molecules to facilitate angiogenesis and cell survival.29 As these cardiac patches promote a physical, structural, biochemical, and extracellular matrix-rich microenvironment, they encourage cell-cell and cell-matrix interactions and weave a regulatory network of cell signaling, compatible with elastic behavior during heart beating after implantation. By providing sufficient mechanical strength to the heart (left ventricle [LV]), these constructs reduce elevated heart wall stress, support healthy functional cardiomyocytes in the infarct areas of the heart, decrease scar size, and alter the cardiac remodeling for myocardial regeneration in addition to limiting post-infarct ventricular dilation and preventing post-infarction progression.27,30, 31, 32 We have analyzed the existing information and discussed the role of patterned cardiac patches with respect to their efficiency, closely compared to that of natural myocardial tissue in myocardial regeneration.
Figure 1.
A Schematic Biomaterial-Based Strategy for Myocardial Tissue Regeneration After Myocardial Infarction
Cardiac Patches for Myocardial Regeneration
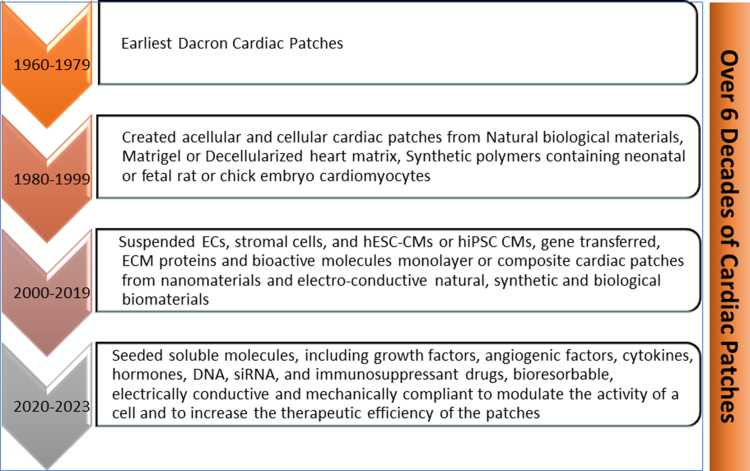
Over the past decade, diverse biomaterial cardiac patches have been used as carriers of stem cells, genes, growth factors, drugs, exosomes, and extracellular vesicles in the form of particles, injectable hydrogels, sprays, and nanofibers. These approaches have been used for the delivery of the bioactive agents at the infarcted zone for treating MI and reducing ventricular remodeling to promote cardiac repair, improve cardiac restoration, prevent arrhythmias, preserve ventricular function, and preclude heart failure.27,33, 34, 35, 36 As shown in Figure 2, in the 1960s, a milestone in the history of synthetic and natural or biological biomaterial patches in cardiac tissue regeneration emerged with the placement of a cardiac graft over a wounded heart. In the recent past, engineered cardiac patches loaded with cell types, including embryonic stem cells, induced pluripotent stem cells, endothelial cells (ECs) in mono- or multi-cell–layered form, as well as decellularized cardiac patches from animal products that are re-cellularized with therapeutic cells or agents have been successfully integrated with the cardiac tissue regeneration. Seeding the infarcted region with cells before implantation, a cardiac patch allows their growth and maturation in culture or biological reactors. These synthetic and/or natural or biological biomaterial patches have not only shown high customizability and competency after MI-challenged situations but have also overcome the challenges of limited availability of hearts for transplantation and organ rejection.37, 38, 39, 40, 41, 42, 43, 44
Figure 2.
Milestones in the History of Cardiac Patches for Myocardial Infarction Repair and Regeneration
CM = cardiomyocyte; EC = endothelial cells; ECM = extracellular matrix; hESC = human embryonic stem cell; hiPSC = human induced pluripotent stem cell; siRNA = small-interfering RNA.
In this regard, synthetic polymers poly(ε-caprolactone) (PCL), poly(glycerol sebacate), poly(lactic-co-glycolic acid), biodegradable polyurethane, poly(l-lactide), poly(vinyl alcohol), and polyethylene glycol and/or natural materials such as chitosan, collagen, gelatin, fibrin, and silk fibroin cardiac patches, have shown potential for use in myocardial regeneration.24 Cardiac patches formed from PCL/gelatin seeded with mesenchymal stem cells (MSCs) have shown effectiveness in restricting ventricular dilation and reducing adverse remodeling of the ventricular wall while protecting MSCs from the hypoxic conditions in the infarct zone in murine MI models.45 Incorporated alginate microparticles in a collagen-based patch can provide a controlled release of hepatocyte growth factor and insulin-like growth factor-1 to enhance endogenous cardiac stem cell migration and proliferation.46 Cardiac patches co-injected with cardiomyocytes have been shown to improve cell engraftment, myocardial wall stress, apoptosis, vascularization, metabolism, contractile function, cell viability, partial remuscularization, and LV function in MIanimal models.47,48 Decellularized ECM bio-inks simulate tissue-specific ECM components and/or resident cytokines, providing biological and biochemical cues required for remodeling and enhancing cellular functions such as survival, maturation, differentiation, migration, and intercellular interactions, as well as a cardiac niche–like microenvironment that augments expression of endogenous vascular endothelial growth factor (VEGF) and upregulate angiogenesis-related genes promoting cardiac regeneration.49 Likewise, PCL-based medium-chain-length polyhydroxy alkenoate porous composite myocardial patches seeded with cardiac stem cells increased delivery of cells in infarcted mice, and have been found to exhibit good biocompatibility, enhanced mechanical and physical properties, as well as 30% detectable cells even on the day 9 after transplantation.50 Cardiac patches bridge electrical and/or mechanical stimulation across the infarct to maintain and improve cardiac function51 and myocardial regeneration. Cardiac patches with the combination of bioactive nano- and biomaterials with MSC ECM/silk proteins incorporated with gold nanoparticles indicate a promising approach for cardiac repair in MI.52 Three-dimensional printed gelatin/hyaluronic acid patches that mimic the myocardium’s particular 3D architecture preserved cardiac function and regenerated the intrinsically anisotropic myocardium after MI,53 in addition to promoting in vitro and in vivo proliferation, differentiation, and contractility of cardiomyocytes. Using biomaterial patches directly facilitated paracrine interactions with the host myocardium to promote growth factors such as VEGF54 and basic fibroblast growth factor (bFGF)55 or therapeutic agents such as adenosine.30 Although implementation of transplanted cells limits the therapeutic efficacy or are entirely rejected (depending on cell types) by the host immune system, there is evidence that the cells infused on the site can create an appropriate environment to develop new cardiac cells by secreting growth factors before rejection.56
Nanofiber Cardiac Patches for Myocardial Regeneration
The electrospun biomaterial nanofiber cardiac patches have been shown to be a valuable tool for myocardial regeneration after MI. The exceptional capability of the electrospinning technique to convert polymeric biomaterials into nanofibers with tunable mixtures and different dimensions to produce cardiac patches is very well-known among several other procedures.26,57 With a simple equipment setup of electrospinning (Central Illustration), different types of nano- and/or microscale component-loaded (growth factors, endothelial cells, stem cells, cytokines, chemokines, and ECM proteins) nanofiber cardiac patches can be produced by optimizing parameters, including solution concentration, viscosity, voltage, conductivity, collecting layout, flow rate, and nozzle diameter.58 Consisting of a highly interconnected porous network of nanofibers, large surface area, light weight, and permeability that mimic myocardial ECM-like and in vivo cell microenvironment, nanofiber cardiac patches allow cells to fully metabolize and interact for the transportation of nutrients and metabolic wastes effectively.26,59 Because the electrospun nano- and/or microfibers from the polymers or electrically charged polymer solution or melt60 are oriented parallel to each other along one specific direction, the anisotropic structure of cardiac patches similar to that of the myocardium has shown to be significantly functionalized with other components to improve cell adhesion, survival, differentiation, diffusion, and mechanical support that imitates the natural environment to regenerate diseased myocardium and restore its function.22,23,61,62
Central Illustration.
A Schematic Electrospinning Process to Fabricate Biomaterial Based Nanofibers Patch
Moreover, the capacity of nanofibers is well known in MI for targeted drug delivery encapsulated with bioactive compounds for reversing adverse effects and myocardial tissue regeneration.35,63, 64, 65 In this regard, the large surface area of nanofibers facilitates efficient drug loading, while their intricate pore structure enhances drug loading capacity and controlled release66 Their stimuli-responsive ability under specific conditions such as patterning, magnetic fields, pH, or reactive oxygen species, has been shown to be efficient for localized, sustained, or on-demand drug release to accelerate the repair of infarcted tissue.67 Tuned with topological features, cardiac patches have been shown to enhance mechanical parameters and deformation mechanisms similar to native tissue in a model of MI in vivo by responding to external and internal stimuli to induce nanofibers for drug release.51,68 In fact, targeted delivery of nanomedicines for controllable modulation of the disease microenvironment has been shown to be beneficial by supramolecular nanofibers.69 Also, significantly sustained delivery of exosomes at acute MI sites, expanding angiogenesis in the infarcted area, diminishing myocardial fibrosis, and preserving the function of the heart has been shown through incorporation of exosomes and transforming growth factor-β3 with a PCL/type I collagen nanofiber patch.64 However, although the mechanisms that underlie drug-release through nanofibers may include diffusion, swelling, and degradation, they hold benefits through their structural and morphological characteristics, specifically surface area, porosity, fiber entanglements, and orientation. The surface drug molecules diffuse into the release media from the nanofibrous patch, occupying the interfibrous pores, and the polymer matrix swells, allowing drug molecules diffusion through nanofibers. This is followed by polymer degradation and enzymatic hydrolysis, which occurs at the surface, and assisted diffusion of the remaining molecules.70,71 Nanofiber fabrication with water and nontoxic solvents can overcome the challenges of toxicity of cardiac patch material72,73 as well as enhance angiogenesis following MI in a safe and nontoxic way.74,75 Because of these advantages, the corresponding roles of these cardiac patches in the repair and regeneration of damaged myocardium in MI have been extensively studied (Table 1).26,31,36,44,62,76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87
Table 1.
Nanofibers Cardiac Patches for Myocardial Regeneration
| Biomaterials | Cellular Nanofiber Cardiac Patch | First Author |
|---|---|---|
| PCL-gelatin | hiPSC-CMs | Kumar et al26 |
| PCL/GelMA-Ppy nanoparticles | Cardiomyocytes/fibroblasts | He et al31 |
| PCL/gelatin | hiPSC-CMs | Sridharan et al36 |
| Alginate/PCL | Cardiac progenitor cells | Karimi et al44 |
| PCL-FN-immobilized nanofibers | UCB-MSC | Kang et al62 |
| PLA, PEG, and PCL/collagen | HGF and IGF | Kerignard et al83 |
| PCL | Sacrificial particles (PEO) | Wanjare et al84 |
| Xylan (polysaccharides)/PVA | Acellular patch | Venugopal et al85 |
| PLGA | Endothelial cells, VEGF | Fleischer et al86 |
| β-PVDF | TiO2 | Arumugam et al87 |
| PEO | ECM | Shah et al76 |
| PLA/PCL | Cardiomyocytes | Wei et al77 |
| Alginate | Cardiomyocytes | Lee et al78 |
| CNT/silk | Cardiomyocytes | Zhao et al79 |
| PLA/PANI | Cardiomyoblast (H9c2) | Wang et al80 |
| PCL/NO | NO2 | Zhu et al81 |
| PCL | hiPSC-CMs | Liu et al82 |
β-PVDF = β-phase polyvinylidene fluoride; CM = cardiomyocyte; CNT = carbon nanotubes; ECM = extracellular matrix; ECs = endothelial cells; FN = fibronectin; GelMA = methyl acrylic anhydride-gelatin; HGF = hepatocyte growth factor; hiPSC = human induced pluripotent stem cells; IGF = insulin-like growth factor; MSC = mesenchymal stem cell; NO = nitric oxide; PANI = polyaniline; PCL = polycaprolactone; PEG = poly (ethylene) glycol; PEO = polyethylene oxide; PLA = poly(lactic acid); PLGA = poly(lactic-co-glycolic acid); Ppy = polypyrrole; PVA = polyvinyl alcohol; PVDF = poly (vinylidene fluoride); TiO2 = titanium dioxide; UCB-MSC = umbilical cord blood-derived mesenchymal stem cells; VEGF = vascular endothelial growth factor.
Hybrid cardiac patches composed of polymer nanofibers loaded with insulin-like growth factor have shown 89% to 92% release of insulin-like growth factor in 2 days and up to 50% to 58% release of hepatocyte growth factor in 35 days, which promotes cell proliferation for cardiac muscle regeneration.83 Matrix metalloproteinase-9–instructed assembly of bFGF nanofibers and slowly releasing bFGF in ischemic myocardium has been shown to promote cardiac repair, which can be applied clinically.55 An implantable β-phase polyvinylidene fluoride-poly(methyl methacrylate-hydroxyapatite-titanium dioxide–based seeded cardiac patch developed mechanically is a durable electrospun nanocomposite for replacing the scar tissue, which can match the extracellular matrix of cardiac cells and favor the adherence, proliferation, and maturation of the cells.87 Their capacity to generate a surface charge density when subjected to the pumping action of the heart muscles can enhance vascularization and thus support the development of newly seeded cells.87 Improved survival rate of stem cells in repairing myocardial injury has been reported by PCL-gelatin coaxial nanofiber cardiac patches, providing better mechanical properties and mimicking ECM structure for the enhancement of cell adhesion, viability, and regeneration.26 These electrospun nanofiber cardiac patches have shown improved cell attachment and biomimetic properties of myocardial tissue. The cells aligned on these patches exhibited synchronous contraction and thereby a quick response to cardiac drugs.26 Prominent maturation of the myocardium and a significantly higher velocity of maximum contraction uniaxially aligned were observed in PCL nanofibers seeded with human-induced pluripotent stem cell–derived cardiomyocytes.84 Xylan (a plant-based polysaccharide beech wood)/polyvinyl alcohol glutaraldehyde vapor cross-linked nanofibers for the culture of neonatal rat cardiac cells in infarcted regions show better mechanical strength and are suitable for the cardiac cells proliferation and use as a cardiac patch for MI.85 Better maturation and vital contractility of cardiomyocytes mimic structural organization, mechanical support, and controlled release of different biofactors have also been shown to effectively accommodate cardiac cells and organization of endothelial cells into blood vessels and contracting tissue emulating native architecture of myocardium thereby improving cardiac function.86,88 Mechanical properties, such as stress-strain and elastic modulus of the cardiac patch, are particularly critical in cardiac regeneration to withstand and contribute to the contractile activity of the heart. Decellularized porcine cardiac ECM–polyethylene oxide nanofibers have been shown to preserve ECM composition, to self-assemble into the same microstructure of native cardiac ECM, and to retain essential mechanical properties. Upon using a decellularized porcine myocardium slice as an acellular patch in a rat acute MI model, increased cell infiltration, macrophage accumulation, and prevention of thinning of the LV wall as well as a significant number of vessel structures on the patch and infarcted area were observed.76 Mechanical support that facilitates cell migration, angiogenesis, and ventricular function has been reported in post-MI–induced cardiac remodeling.30 Similarly, a PLA [poly(lactic acid]/PCL nanofiber patch was shown to improve cell viability and mechanical status to withstand the severe pumping effect of the myocardium.77 An alginate-based hierarchical structured electrospun cardiac patch has also been observed to promote cardiomyocyte repair due to the tensile strength, as well as repair of the infarcted myocardial region, without limiting the cardiac-systolic activity.78 Furthermore, electrical signals to cardiac tissue cells were directed by doping nanofibrous cardiac patches with conductive materials such as gold nanoparticles, carbon nanotubes, or graphene.79,89, 90, 91 Improved electrical conductivity promoted cardiomyocyte maturation and reduction in infarct size were demonstrated by silk nanofiber encapsulated with carbon nanotubes.79 In this regard, a reduction of 50% infarct size, an increase of 20% LV ejection fraction, and a 9-fold increase in the density of neovascularization in the infarcted area in a rat MI model was achieved by mussel-inspired conductive nanofibrous patches after 4 weeks of patch transplantation on the infarcted heart.31 Implanted blended PLA and polyaniline conductive nanofibrous patch in rats showed maturation and spontaneous beating of primary cardiomyocytes, differentiation of cardiomyoblasts, and the functional recovery of the myocardium.80 The beating frequency of cardiomyocyte depends on the thickness of the cardiac patch, and cardiomyocytes loaded into thick multifunctional nanofiber cardiac patch were seen to beat spontaneously at a much higher frequency.58,86 Good survival and infiltration of human cardiac stem cells, as well as efficient mechanical support, have been shown with use of a nanofiber cardiac patch of 50-μm thickness.92 Because remodeling of the myocardium post-injury correlates with distorted excitation-contraction coupling, nanofiber cardiac patches have been reported to mimic the anisotropic native tissue, enhance the cell assembly into contractile functional myocardial tissues, restore the contractile function of the LV, and repair the myocardium after MI.22,84,88,93 Elongated cardiomyocytes and endothelialized myocardium were seen with a PCL nanofiber/silk/carbon nanotube network enclosed in a hydrogel matrix by providing 3D cardiac anisotropy.94 Furthermore, upon implantation into the myocardium, a biodegradable PCL–nitric oxide cardiac patch has been shown to enhance cardiac repair in a porcine model of MI due to its efficacy in mitochondria-targeted cardioprotection.81
Also, the antibacterial, antimicrobial, and anti-inflammatory activities of electrospun nanofibers with incorporated therapeutic agents in various platforms have been reported.95 Therapies targeting inflammation benefit patients with cardio-inflammatory phenotype suffering from ischemic heart disease. In the case of MI, necrosis of cardiomyocytes triggers the activation of the adaptive and innate immune system, leading to an inflammatory response.96 Immune response modulation in post-infarction myocardial regeneration controls the inflammatory environment and significantly influences the overall outcome in repairing the damaged cardiac tissue.97, 98, 99, 100 Since the immune response to implanted devices is critical in determining their success or failure, immunomodulation may effectively mitigate adverse foreign body reactions.101 Therefore, the ability of biomaterial cardiac patch–mediated myocardium regeneration has been explored for their capacity to modulate immunological reactions of overlapping phases (inflammation, proliferation, and resolution) and support cardiac tissue regeneration in myocardial infarction.102,103 Whereas natural biological materials tend to have low immunogenicity due to their limited mechanical characteristics, synthetic polymers with highly controllable mechanical properties are more likely to induce immune response. Immunomodulatory hybrid micro-nanofiber constructs have been suggested as candidates for enhancing cardiac regeneration.104,105 Composites of nanofibers in conjunction with electrical stimulation or release of bioactive enhanced functionality and regeneration of cardiac tissue offer great potential for enhancing material-cell interactions and modulation of the immune system.106,107 Studies have shown immunomodulatory effects of carbon-based nanofiber patches, such as graphene, in regenerating cardiac tissue after myocardial injury.108,109 Releasing cellular components and growth factors that interact with host tissue cells influence the immune response and cardiac repair.26 Incorporating dexamethasone-peptide amphiphile into nanofibers could reduce inflammation and protect the cardiac tissue from oxidative stress during regeneration, as demonstrated in vitro using a human inflammatory reporter cell line.110
In addition, there have been promising results in studies on immunomodulatory therapies that use dexamethasone-incorporated peptide nanofibers to induce antigen-specific responses, leading to better tolerogenic immunotherapies.111 Moreover, aligned nanofiber patches with a balanced porousness and smooth surface have been shown to reduce foreign body response and immunomodulatory capacity, which helps modulate the host response in vivo.112 Hydrophilic nanofibers with an aligned topography explored for their immunomodulatory properties have shown that activation of the NOD-like receptor thermal protein domain-associated protein 3 inflammasome facilitates the maturation and release of inflammatory factors by activating caspase-1. This mechanism regulates the macrophage-mediated foreign body response and implant integration, thereby shaping the peri-implantation immune microenvironment.113 In a preclinical study conducted on animals, a cardiac patch made of cardiomyocytes and human fibroblasts on a bioresorbable matrix was beneficial in treating ischemia-induced decreases in LV function caused by a loss of functioning cardiomyocytes. The subjects who received the treatment showed improved LV contractility/function, partially reversed cardiac remodeling, and enhanced exercise activity without any increase in ventricular arrhythmias or myocardial energy use. The mechanism of action was related to localized immune-mediated changes in gene expression at the injury site, which repaired and polarized macrophages from their pro-inflammatory state to their anti-inflammatory reparative state.104 Furthermore, studies have shown that incorporating signaling molecules directly into nanofibers can lead to local immunosuppression,114 controlled drug delivery that responds to changes in temperature,115 and increased cellular proliferation, adhesion, and attachment, which promotes a biomimetic matrix116 and enhances biological activity for myocardium regeneration.
Thus, nanofiber cardiac patches can be seen to assist in replacing scar tissue effectively and repair the infarcted area of the heart. Together, these observations show the ability of various biomaterial-based electrospun nanofibrous patches to promote cell proliferation, adhesion, differentiation, and preservation of their phenotypic shape. Their anisotropic structure, high porosity, low density, antibacterial activities, and capacity to deliver cells or bioactive factors make them suitable tools for infarcted hearts and clinical translation. These nanofiber-based cardiac patches have the potential for clinical application as they may maintain structural and functional complexity within the human heart.
Topological Features of Nanofiber Cardiac Patches
It is well known that the cardiac patch must have a proper design, thickness, and size relevant to the typical infarct size in addition to its mechanical, physicochemical properties and electrical conductivity to improve cardiac function and myocardial regeneration in MI.51,117 The significant roles of designed nanofiber patches to deliver seeded material are actively studied in tissue engineering. Tailored with square, rectangular, or honeycomb conventional patterns on nanofiber, patches have been shown to simulate anisotropic architecture similar to myocardium, elevate cardiomyocyte viability, promote penetration of cells, and induce expression of cardiac-related genes.118,119 Likewise, in coiled, helical, or spring-like nanofiber patches, increased elasticity and extensibility for supporting the assembly of functional cardiac tissue with strong contraction forces have been observed.91,120,121 Albumin electrospun nanofiber patches with laser-patterned microscale grooves and holes have been reported to exhibit anisotropic electrical signal propagation, as the microchannels pattern within the patch seeded with ECs has been shown to form closed lumens.32,86,92 Moreover, the cage-like structures patterned on poly(lactic-co-glycolic acid) multilayered nanofibrous cardiac patch have been shown to control the release of VEGF to promote vascularization and anti-inflammatory drugs into the exterior microenvironment and integrate suitably with the native heart muscle.86
Because most biomaterial cardiac patches have conventional patterns when stretched longitudinally, they tend to contract transversally causing mismatch with native cardiac tissue behavior during the regeneration of infarcted myocardial tissue. Thus, these patches are limited in their use due to inadequate flexibility, elongation, and poor cellular infiltration.122,123 On the other hand, the tailored (auxetic) design of cardiac patches promotes flexibility, mechanical strength, and deformation mechanisms, enabling them to withstand stresses, improve cellular infiltration, and impart cell differentiation. Such outcomes are considered to augment the compatibility of cardiac patches to the demanding mechanics of the heart and enhance their use in myocardial regeneration after MI. The expansion of nanofiber cardiac patches with tailored designs in multiple directions that concurrently increase the surface area to promote cell adhesion and growth are essential for their use in cardiac regeneration.124 A variety of geometries, such as re-entrant, rotating, chiral, crumpled and sinusoidal structures have been identified, which promotes a deformation mechanism allowing expansion in multiple directions simultaneously.125 Thus, it is difficult to ensure that the cardiac patch matches with the behavior of myocardial tissue. However, it has been shown that an angled solid square geometry on an electrospun polymer nanofibrous patch coated with gold nanoparticle for conductivity was easily stretched with small tensile load compared to an electrospun nanofiber patch.126 Expansion in multiple directions simultaneously and retaining the original shape upon forces dissipated has been shown during the cycles of applied stress and strain to these patches (Figure 3). There was an increase in tensile capacity of this designed nanofibrous patch compared to a nonpatterned nanofibrous patch, which confirms the tuned flexible nature due to tailored design. Also, the total elongation capacity of the thick patch is significantly reduced compared to the thin patch, highlighting reduced deformation ability due to increased patch thickness.127 It indicates that patterned cardiac patches can be modified according to infarct thickness and size to support the infarcted region. In this regard, sinuous cardiac patches of poly-ethylene-glycol diacrylate film casting, embedded silver nanowires, and microarchitectures closed to chiral pattern cardiac patches using photopolymer resin via ink-jet printing have been shown to function for strain detection in cardiac muscle.128 An excimer-lasered microablation micropatterned “re-entrant honeycomb shape” on a conducive composite of polyaniline and phytic acid cardiac patch was found to stretch according to the movement of the native heart tissue without detrimental effects.51
Figure 3.
An Angled, Square-Patterned PCL-Nanofiber Patch Provides Multidirectional Expansion and Increased Surface Area Under Stretched Conditions
PCL = poly(ε-caprolactone).
It is indicated that a patch with a missing rib design fabricated by melt electrospinning writing can overcome the limited elasticity capacity seen in the conventional square patch design and adapt the strains and stresses experienced by the human myocardium during both diastole and systole. Such patches reflect anisotropic mechanical properties, as well as the anisotropic ratio of effective stiffness, and are thus compatible with the directionally dependent mechanics of the heart.128 It has been shown that the functionality of cardiac patches for their use in myocardial regeneration is dependent on structural geometry82 such as nanofiber interlocking and patterned design.129 These observations suggest that designed nanofibrous patches have the advantage of deformation behavior matching the stretchable mechanism of the native tissue, that is, the ability to be tailored and optimized according to the severity of targeted infracted region to serve as a potent cardiac patch for cardiac repair or myocardial regeneration. However, the understanding of tailoring patterns into cardiac patches for treating MI remains in the theoretical stage. Overall, although the beneficial effects of cardiac patches in animal models and their clinical feasibility by successful transplantation are supported,130, 131, 132 there are still considerable difficulties in their use.133,134
Conclusions
It is well known that MI is a severe medical condition, and the limited regenerative potential of cardiomyocytes restricts the capacity for self-repair of cardiac tissue. Because myocardium is comprised of electroactive tissues, electrospun nanofiber cardiac patches have been actively explored as a strategy for myocardium regeneration. This targeted approach may complement pharmacological interventions, revascularization procedures, lifestyle modifications, and other existing myocardial injury and tissue repair treatments. This review highlights promising results of biomaterial electrospun nanofibers with or without tailored-design–enabled cardiac patches through controllable delivery of embedded cell loads and bioactive molecules or therapeutic agents at the infarcted region for promoting myocardial tissue regeneration.
Directly applied to damaged heart tissue, nanofiber cardiac patches mimic the mechanical properties of the native ECM and provide structural support and scaffolding for cell attachment, growth, and migration to the injury site. Their large surface area of nanofibers facilitates efficient drug loading. At the same time, their intricate pore structure enhances drug loading capacity and benefits localized, measured, and sustained delivery of therapeutic compounds to the diseased site, whereas the selection of drug-polymer–solvent systems or electrospinning techniques promotes reduced initial burst release. Their stimuli-responsive ability to release targeted and controllable drugs accelerates the repair of infarcted tissue, while their tuned topological features according to infarct size can achieve deformation mechanisms similar to native tissue. These cardiac patches provide adequate conductive and mechanical strength for maintaining the myocardium's ability to contract and relax, making them suitable platforms for the regeneration of infarcted myocardium. Combined with cellular components and growth factors, they interact with host tissue cells to influence the immune response, impacting cardiac repair. Their immunomodulatory effects, potentially reducing inflammation and scar formation, are particularly noteworthy. These patches also offer the potential for in vitro drug efficacy and toxicity testing before clinical trials.
Although the beneficial effects of cardiac patches in animal models and preclinical studies showed promising performance, their clinical feasibility is progressing, and ongoing clinical trials explore their potential and new insights into infarcted myocardial repair and regeneration.54,83,135,136 However, there are still many challenges with their clinical implications. For instance, the risks of open-chest surgery for implantation increases the patient's risk of death, inflammation, a lengthy recovery period, and the heavy burden of sutures for cardiac patch transplantations for an already injured heart. Without suture-free engraftment, ensuring seamless patch integration with the host tissue remains challenging. Long-term safety studies are still required because all patches are external foreign substances that may cause relevant immune rejection due to the materials used or their metabolic derivatives. Furthermore, there is a lack of large animal and human clinical studies. Their applications are limited to the laboratory scale, necessitating more in-depth preclinical and clinical evaluation to validate their effectiveness in mitigating myocardial injury and for the translation process.
Despite the challenges, with the combination of a growing understanding of the biological pathways underlying the infarcted region, nanofiber cardiac patches that can generate all cardiac cell types with appropriate electrical and calcium signal transduction events would become an increasingly feasible and transformative choice for patients suffering from MI and heart failure. However, the clinical translation is currently at a halt, like other emerging treatments, such as cell therapy for heart failure.99,137 Therefore, it is crucial to advance research and development in this area to fully realize the potential of nanofiber cardiac patches and their benefits for patients.
Funding Support and Author Disclosures
This work was supported by a foundation grant to Dr Kirshenbaum from the Canadian Institute for Health Research (CIHR) and St Boniface Hospital Research Foundation. Dr Rabinovich-Nikitin has received grants from the St Boniface Hospital Research Foundation, Manitoba Medical Services Foundation, Winnipeg Foundation. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Pfeffer M.A., Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 2.Chapman A.R., Shah A.S.V., Lee K.K., et al. Long-term outcomes in patients with type 2 myocardial infarction and myocardial injury. Circulation. 2018;137:1236–1245. doi: 10.1161/CIRCULATIONAHA.117.031806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciucci G., Colliva A., Vuerich R., Pompilio G., Zacchigna S. Biologics and cardiac disease: challenges and opportunities. Trends Pharmacol Sci. 2022;43:894–905. doi: 10.1016/j.tips.2022.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Robledo M. Myocardial regeneration in young rats. Am J Pathol. 1956;32:1215. [PMC free article] [PubMed] [Google Scholar]
- 5.Uygur A., Lee R.T. Mechanisms of cardiac regeneration. Dev Cell. 2016;36:362–374. doi: 10.1016/j.devcel.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awad M.A., Shah A., Griffith B.P. Current status and outcomes in heart transplantation: a narrative review. Rev Cardiovasc Med. 2022;23:11. doi: 10.31083/j.rcm2301011. [DOI] [PubMed] [Google Scholar]
- 7.Salari N., Morddarvanjoghi F., Abdolmaleki A., et al. The global prevalence of myocardial infarction: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2023;23:206. doi: 10.1186/s12872-023-03231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kajstura J., Leri A., Finato N., Di Loreto C., Beltrami C.A., Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci USA. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beltrami A.P., Barlucchi L., Torella D., et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 10.Regula K.M., Rzeszutek M.J., Baetz D., Seneviratne C., Kirshenbaum L.A. Therapeutic opportunities for cell cycle re-entry and cardiac regeneration. Cardiovasc Res. 2004;64:395–401. doi: 10.1016/j.cardiores.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Laflamme M.A., Murry C.E. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vu T.V.A., Lorizio D., Vuerich R., Lippi M., Nascimento D.S., Zacchigna S. Extracellular matrix-based approaches in cardiac regeneration: challenges and opportunities. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232415783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimatsu T., Oosawa H., Takai F., et al. Patch-and-glue repair in combination with or without direct suture for cardiac rupture after myocardial infarction. Gen Thorac Cardiovasc Surg. 2007;55:345–350. doi: 10.1007/s11748-007-0144-4. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann W.-H., Melnychenko I., Wasmeier G., et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 15.Oosthoek P.W., Moorman A.F.M., Sauer U., Gittenberger-de Groot A.C. Capillary distribution in the ventricles of hearts with pulmonary atresia and intact ventricular septum. Circulation. 1995;91:1790–1798. doi: 10.1161/01.cir.91.6.1790. [DOI] [PubMed] [Google Scholar]
- 16.Kai D., Prabhakaran M.P., Jin G., Ramakrishna S. Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering. J Biomed Mater Res Part A. 2011;99A:376–385. doi: 10.1002/jbm.a.33200. [DOI] [PubMed] [Google Scholar]
- 17.Kocica M.J., Corno A.F., Carreras-Costa F., et al. The helical ventricular myocardial band: global, three-dimensional, functional architecture of the ventricular myocardium. Eur J Cardiothoracic Surg. 2006;29:S21–S40. doi: 10.1016/j.ejcts.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Rao C., Prodromakis T., Kolker L., et al. The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials. 2013;34:2399–2411. doi: 10.1016/j.biomaterials.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salick M.R., Napiwocki B.N., Sha J., et al. Micropattern width dependent sarcomere development in human ESC-derived cardiomyocytes. Biomaterials. 2014;35:4454–4464. doi: 10.1016/j.biomaterials.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bursac N., Parker K.K., Iravanian S., Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ Res. 2002;91:e45–e54. doi: 10.1161/01.res.0000047530.88338.eb. [DOI] [PubMed] [Google Scholar]
- 21.López-Canosa A., Perez-Amodio S., Yanac-Huertas E., et al. A microphysiological system combining electrospun fibers and electrical stimulation for the maturation of highly anisotropic cardiac tissue. Biofabrication. 2021;13 doi: 10.1088/1758-5090/abff12. [DOI] [PubMed] [Google Scholar]
- 22.Han J., Wu Q., Xia Y., Wagner M.B., Xu C. Cell alignment induced by anisotropic electrospun fibrous scaffolds alone has limited effect on cardiomyocyte maturation. Stem Cell Res. 2016;16:740–750. doi: 10.1016/j.scr.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kai D., Jin G., Prabhakaran M.P., Ramakrishna S. Electrospun synthetic and natural nanofibers for regenerative medicine and stem cells. Biotechnol J. 2013;8:59–72. doi: 10.1002/biot.201200249. [DOI] [PubMed] [Google Scholar]
- 24.Huang W., Huo M., Cheng N., Wang R. New forms of electrospun nanofibers applied in cardiovascular field. Front Cardiovasc Med. 2022;8:1–8. doi: 10.3389/fcvm.2021.801077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thakkar S., Misra M. Electrospun polymeric nanofibers: new horizons in drug delivery. Eur J Pharm Sci. 2017;107:148–167. doi: 10.1016/j.ejps.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Kumar N., Sridharan D., Palaniappan A., et al. Scalable biomimetic coaxial aligned nanofiber cardiac patch: a potential model for “clinical trials in a dish.”. Front Bioeng Biotechnol. 2020;8:1–17. doi: 10.3389/fbioe.2020.567842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 28.Li Z., Mei S., Dong Y., et al. Functional nanofibrous biomaterials of tailored structures for drug delivery — a critical review. Pharmaceutics. 2020;12:1–23. doi: 10.3390/pharmaceutics12060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyagi Y., Chiu L.L.Y., Cimini M., Weisel R.D., Radisic M., Li R.K. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials. 2011;32:1280–1290. doi: 10.1016/j.biomaterials.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Serpooshan V., Zhao M., Metzler S.A., et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials. 2013;34:9048–9055. doi: 10.1016/j.biomaterials.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He Y., Ye G., Song C., et al. Mussel-inspired conductive nanofibrous membranes repair myocardial infarction by enhancing cardiac function and revascularization. Theranostics. 2018;8:5159. doi: 10.7150/thno.27760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y., Zeng D., Ding L., et al. Three-dimensional poly-(ε-caprolactone) nanofibrous scaffolds directly promote the cardiomyocyte differentiation of murine-induced pluripotent stem cells through Wnt/β-catenin signaling. BMC Cell Biol. 2015;16:1–13. doi: 10.1186/s12860-015-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiekh P.A., Mohammed S.A., Gupta S., et al. Oxygen releasing and antioxidant breathing cardiac patch delivering exosomes promotes heart repair after myocardial infarction. Chem Eng J. 2022;428 [Google Scholar]
- 34.Bassat E., Mutlak Y.E., Genzelinakh A., et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature. 2017;547:179–184. doi: 10.1038/nature22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garbayo E., Ruiz-Villalba A., Hernández S.C., et al. Delivery of cardiovascular progenitors with biomimetic microcarriers reduces adverse ventricular remodeling in a rat model of chronic myocardial infarction. Acta Biomater. 2021;126:394–407. doi: 10.1016/j.actbio.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Sridharan D., Palaniappan A., Blackstone B.N., Powell H.M., Khan M. Electrospun aligned coaxial nanofibrous scaffold for cardiac repair. Wound Regen Methods Protoc. 2021:129–140. doi: 10.1007/978-1-0716-0845-6_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson R.F., Bassett J.S. Penetrating wounds of the pericardium or its contents. JAMA. 1966;195:513–518. [PubMed] [Google Scholar]
- 38.Bader D., Oberpriller J.O. Repair and reorganization of minced cardiac muscle in the adult newt (Notophthalmus viridescens) J Morphol. 1978;155:349–357. doi: 10.1002/jmor.1051550307. [DOI] [PubMed] [Google Scholar]
- 39.Li R.K., Jia Z.Q., Weisel R.D., Mickle D.A., Choi A., Yau T.M. Survival and function of bioengineered cardiac grafts. Circulation. 1999;100(suppl 19):II63–II69. doi: 10.1161/01.cir.100.suppl_2ii-e63. [DOI] [PubMed] [Google Scholar]
- 40.Leor J., Aboulafia-Etzion S., Dar A., et al. Bioengineered cardiac grafts: a new approach to repair the infarcted myocardium? Circulation. 2000;102(suppl 19):III56–III61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 41.Bel A., Planat-Bernard V., Saito A., et al. Composite cell sheets: a further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells. Circulation. 2010;122:S118–S123. doi: 10.1161/CIRCULATIONAHA.109.927293. [DOI] [PubMed] [Google Scholar]
- 42.Ravichandran R., Venugopal J.R., Sundarrajan S., Mukherjee S., Sridhar R., Ramakrishna S. Expression of cardiac proteins in neonatal cardiomyocytes on PGS/fibrinogen core/shell substrate for cardiac tissue engineering. Int J Cardiol. 2013;167:1461–1468. doi: 10.1016/j.ijcard.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 43.Ryu H., Wang X., Xie Z., et al. Materials and design approaches for a fully bioresorbable, electrically conductive and mechanically compliant cardiac patch technology. Adv Sci. 2023 doi: 10.1002/advs.202303429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karimi S.N.H., Aghdam R.M., Ebrahimi S.A.S., Chehrehsaz Y. Tri-layered alginate/poly (ε -caprolactone) electrospun scaffold for cardiac tissue engineering. Polym Int. 2022;71:1099–1108. [Google Scholar]
- 45.Wang Q., Wang H., Li Z., Wang Y., Wu X., Tan Y. Mesenchymal stem cell-loaded cardiac patch promotes epicardial activation and repair of the infarcted myocardium. J Cell Mol Med. 2017;21:1751–1766. doi: 10.1111/jcmm.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Neill H.S., O’Sullivan J., Porteous N., et al. A collagen cardiac patch incorporating alginate microparticles permits the controlled release of hepatocyte growth factor and insulin-like growth factor-1 to enhance cardiac stem cell migration and proliferation. J Tissue Eng Regen Med. 2018;12:e384–e394. doi: 10.1002/term.2392. [DOI] [PubMed] [Google Scholar]
- 47.Gao L., Kupfer M.E., Jung J.P., et al. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold. Circ Res. 2017;120:1318–1325. doi: 10.1161/CIRCRESAHA.116.310277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Querdel E., Reinsch M., Castro L., et al. Human engineered heart tissue patches remuscularize the injured heart in a dose-dependent manner. Circulation. 2021;143:1991–2006. doi: 10.1161/CIRCULATIONAHA.120.047904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jang J., Park H.-J., Kim S.-W., et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017;112:264–274. doi: 10.1016/j.biomaterials.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 50.Constantinides C., Basnett P., Lukasiewicz B., et al. In vivo tracking and 1H/19F magnetic resonance imaging of biodegradable polyhydroxyalkanoate/polycaprolactone blend scaffolds seeded with labeled cardiac stem cells. ACS Appl Mater Interfaces. 2018;10:25056–25068. doi: 10.1021/acsami.8b06096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kapnisi M., Mansfield C., Marijon C., et al. Auxetic cardiac patches with tunable mechanical and conductive properties toward treating myocardial infarction. Adv Funct Mater. 2018;28 doi: 10.1002/adfm.201800618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong Y., Hong M., Dai R., Wu H., Zhu P. Engineered bioactive nanoparticles incorporated biofunctionalized ECM/silk proteins based cardiac patches combined with MSCs for the repair of myocardial infarction: In vitro and in vivo evaluations. Sci Total Environ. 2020;707 doi: 10.1016/j.scitotenv.2019.135976. [DOI] [PubMed] [Google Scholar]
- 53.Gaetani R., Feyen D.A.M., Verhage V., et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015;61:339–348. doi: 10.1016/j.biomaterials.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Lakshmanan R., Kumaraswamy P., Krishnan U.M., Sethuraman S. Engineering a growth factor embedded nanofiber matrix niche to promote vascularization for functional cardiac regeneration. Biomaterials. 2016;97:176–195. doi: 10.1016/j.biomaterials.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y., Wang D., Wu C., et al. MMP 9-instructed assembly of bFGF nanofibers in ischemic myocardium to promote heart repair. Theranostics. 2022;12:7237. doi: 10.7150/thno.77345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park J., Kim B., Han J., et al. Graphene oxide flakes as a cellular adhesive: prevention of reactive oxygen species mediated death of implanted cells for cardiac repair. ACS Nano. 2015;9:4987–4999. doi: 10.1021/nn507149w. [DOI] [PubMed] [Google Scholar]
- 57.Jun I., Han H.-S., Edwards J.R., Jeon H. Electrospun fibrous scaffolds for tissue engineering: viewpoints on architecture and fabrication. Int J Mol Sci. 2018;19:745. doi: 10.3390/ijms19030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fleischer S., Miller J., Hurowitz H., Shapira A., Dvir T. Effect of fiber diameter on the assembly of functional 3D cardiac patches. Nanotechnology. 2015;26 doi: 10.1088/0957-4484/26/29/291002. [DOI] [PubMed] [Google Scholar]
- 59.Kitsara M., Agbulut O., Kontziampasis D., Chen Y., Menasché P. Fibers for hearts: a critical review on electrospinning for cardiac tissue engineering. Acta Biomater. 2017;48:20–40. doi: 10.1016/j.actbio.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 60.Castilho M., Feyen D., Flandes-Iparraguirre M., et al. Melt electrospinning writing of poly-hydroxymethylglycolide-co-ε-caprolactone-based scaffolds for cardiac tissue engineering. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201700311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kai D., Wang Q.-L., Wang H.-J., et al. Stem cell-loaded nanofibrous patch promotes the regeneration of infarcted myocardium with functional improvement in rat model. Acta Biomater. 2014;10:2727–2738. doi: 10.1016/j.actbio.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 62.Kang B.-J., Kim H., Lee S.K., et al. Umbilical-cord-blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized polycaprolactone nanofiber improve cardiac function. Acta Biomater. 2014;10:3007–3017. doi: 10.1016/j.actbio.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 63.Duan X., Chen H., Guo C. Polymeric nanofibers for drug delivery applications: a recent review. J Mater Sci Mater Med. 2022;33:78. doi: 10.1007/s10856-022-06700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ping P., Guan S., Ning C., et al. Fabrication of blended nanofibrous cardiac patch transplanted with TGF-β3 and human umbilical cord MSCs-derived exosomes for potential cardiac regeneration after acute myocardial infarction. Nanomed Nanotech Biol Med. 2023;54 doi: 10.1016/j.nano.2023.102708. [DOI] [PubMed] [Google Scholar]
- 65.Zhuang J., Zhang X., Liu Q., Zhu M., Huang X. Targeted delivery of nanomedicines for promoting vascular regeneration in ischemic diseases. Theranostics. 2022;12:6223. doi: 10.7150/thno.73421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaydhane M.K., Sharma C.S., Majumdar S. Electrospun nanofibres in drug delivery: advances in controlled release strategies. RSC Adv. 2023;13:7312–7328. doi: 10.1039/d2ra06023j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katz B., Rosenberg A., Frishman W.H. Controlled-release drug delivery systems in cardiovascular medicine. Am Heart J. 1995;129:359–368. doi: 10.1016/0002-8703(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 68.Kai D., Prabhakaran M.P., Stahl B., Eblenkamp M., Wintermantel E., Ramakrishna S. Mechanical properties and in vitro behavior of nanofiber–hydrogel composites for tissue engineering applications. Nanotechnology. 2012;23 doi: 10.1088/0957-4484/23/9/095705. [DOI] [PubMed] [Google Scholar]
- 69.Shang Y., Zhi D., Feng G., et al. Supramolecular nanofibers with superior bioactivity to insulin-like growth factor-I. Nano Lett. 2019;19:1560–1569. doi: 10.1021/acs.nanolett.8b04406. [DOI] [PubMed] [Google Scholar]
- 70.Hiwrale A., Bharati S., Pingale P., Rajput A. Nanofibers: a current era in drug delivery system. Heliyon. 2023;9(9) doi: 10.1016/j.heliyon.2023.e18917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu J., Zhang Z., Zhou W., et al. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. J Control Release. 2020;320:337–346. doi: 10.1016/j.jconrel.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 72.Séon-Lutz M., Couffin A.-C., Vignoud S., Schlatter G., Hébraud A. Electrospinning in water and in situ crosslinking of hyaluronic acid/cyclodextrin nanofibers: towards wound dressing with controlled drug release. Carbohydr Polym. 2019;207:276–287. doi: 10.1016/j.carbpol.2018.11.085. [DOI] [PubMed] [Google Scholar]
- 73.Torres-Martínez E.J., Cornejo Bravo J.M., Serrano Medina A., Pérez González G.L., Villarreal Gómez L.J. A summary of electrospun nanofibers as drug delivery system: drugs loaded and biopolymers used as matrices. Curr Drug Deliv. 2018;15:1360–1374. doi: 10.2174/1567201815666180723114326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kai D., Prabhakaran M.P., Jin G., Ramakrishna S. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J Biomed Mater Res Part B Appl Biomater. 2011;98:379–386. doi: 10.1002/jbm.b.31862. [DOI] [PubMed] [Google Scholar]
- 75.Chung H.-J., Kim J.-T., Kim H.-J., et al. Epicardial delivery of VEGF and cardiac stem cells guided by 3-dimensional PLLA mat enhancing cardiac regeneration and angiogenesis in acute myocardial infarction. J Control release. 2015;205:218–230. doi: 10.1016/j.jconrel.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 76.Shah M., Kc P., Zhang G. In vivo assessment of decellularized porcine myocardial slice as an acellular cardiac patch. ACS Appl Mater Interfaces. 2019;11:23893–23900. doi: 10.1021/acsami.9b06453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei X., Gao Q., Xie C., et al. Extracellular recordings of bionic engineered cardiac tissue based on a porous scaffold and microelectrode arrays. Analytic Methods. 2019;11:5872–5879. [Google Scholar]
- 78.Lee J.-S., Chae S., Yoon D., Yoon D., Chun W., Kim G.H. Angiogenic factors secreted from human ASC spheroids entrapped in an alginate-based hierarchical structure via combined 3D printing/electrospinning system. Biofabrication. 2020;12 doi: 10.1088/1758-5090/abaf9a. [DOI] [PubMed] [Google Scholar]
- 79.Zhao G., Zhang X., Li B., Huang G., Xu F., Zhang X. Solvent-free fabrication of carbon nanotube/silk fibroin electrospun matrices for enhancing cardiomyocyte functionalities. ACS Biomater Sci Eng. 2020;6:1630–1640. doi: 10.1021/acsbiomaterials.9b01682. [DOI] [PubMed] [Google Scholar]
- 80.Wang L., Wu Y., Hu T., Guo B., Ma P.X. Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater. 2017;59:68–81. doi: 10.1016/j.actbio.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 81.Zhu D., Hou J., Qian M., et al. Nitrate-functionalized patch confers cardioprotection and improves heart repair after myocardial infarction via local nitric oxide delivery. Nat Commun. 2021;12:1–16. doi: 10.1038/s41467-021-24804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu L., Xu F., Jin H., et al. Integrated manufacturing of suspended and aligned nanofibrous scaffold for structural maturation and synchronous contraction of HiPSC-derived cardiomyocytes. Bioengineering. 2023;10:702. doi: 10.3390/bioengineering10060702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kerignard E., Bethry A., Falcoz C., Nottelet B., Pinese C. Design of hybrid polymer nanofiber/collagen patches releasing IGF and HGF to promote cardiac regeneration. Pharmaceutics. 2022;14:1854. doi: 10.3390/pharmaceutics14091854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wanjare M., Hou L., Nakayama K.H., et al. Anisotropic microfibrous scaffolds enhance the organization and function of cardiomyocytes derived from induced pluripotent stem cells. Biomater Sci. 2017;5:1567–1578. doi: 10.1039/c7bm00323d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Venugopal J., Rajeswari R., Shayanti M., et al. Xylan polysaccharides fabricated into nanofibrous substrate for myocardial infarction. Mater Sci Eng C. 2013;33:1325–1331. doi: 10.1016/j.msec.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 86.Fleischer S., Shapira A., Feiner R., Dvir T. Modular assembly of thick multifunctional cardiac patches. Proc Natl Acad Sci USA. 2017;114:1898–1903. doi: 10.1073/pnas.1615728114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arumugam R., Srinadhu E.S., Subramanian B., Nallani S. β-PVDF based electrospun nanofibers — a promising material for developing cardiac patches. Med Hypotheses. 2019;122:31–34. doi: 10.1016/j.mehy.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 88.Ayaz H.G.Ş., Perets A., Ayaz H., et al. Textile-templated electrospun anisotropic scaffolds for regenerative cardiac tissue engineering. Biomaterials. 2014;35:8540–8552. doi: 10.1016/j.biomaterials.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 89.Haraguchi Y., Shimizu T., Yamato M., Kikuchi A., Okano T. Electrical coupling of cardiomyocyte sheets occurs rapidly via functional gap junction formation. Biomaterials. 2006;27:4765–4774. doi: 10.1016/j.biomaterials.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 90.Kanjwal M.A., Al Ghaferi A. Graphene incorporated electrospun nanofiber for electrochemical sensing and biomedical applications: a critical review. Sensors. 2022;22:8661. doi: 10.3390/s22228661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fleischer S., Shevach M., Feiner R., Dvir T. Coiled fiber scaffolds embedded with gold nanoparticles improve the performance of engineered cardiac tissues. Nanoscale. 2014;6:9410–9414. doi: 10.1039/c4nr00300d. [DOI] [PubMed] [Google Scholar]
- 92.Chen W.L., Kan C.D. Using cell-seeded electrospun patch for myocardial injury: in vitro and in rat model. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:5338–5341. doi: 10.1109/EMBC.2018.8513557. [DOI] [PubMed] [Google Scholar]
- 93.Gaharwar A.K., Nikkhah M., Sant S., Khademhosseini A. Anisotropic poly (glycerol sebacate)-poly (-caprolactone) electrospun fibers promote endothelial cell guidance. Biofabrication. 2015;7 doi: 10.1088/1758-5090/7/1/015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu Y., Wang L., Guo B., Ma P.X. Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy. ACS Nano. 2017;11:5646–5659. doi: 10.1021/acsnano.7b01062. [DOI] [PubMed] [Google Scholar]
- 95.Mostafavi E., Dubey A.K., Walkowiak B., Kaushik A., Ramakrishna S., Teodori L. Antimicrobial surfaces for implantable cardiovascular devices. Curr Opin Biomed Eng. 2022 [Google Scholar]
- 96.Adamo L., Rocha-Resende C., Prabhu S.D., Mann D.L. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17:269–285. doi: 10.1038/s41569-019-0315-x. [DOI] [PubMed] [Google Scholar]
- 97.Nakkala J.R., Li Z., Ahmad W., Wang K., Gao C. Immunomodulatory biomaterials and their application in therapies for chronic inflammation-related diseases. Acta Biomater. 2021;123:1–30. doi: 10.1016/j.actbio.2021.01.025. [DOI] [PubMed] [Google Scholar]
- 98.Mokarram N., Bellamkonda R.V. A perspective on immunomodulation and tissue repair. Ann Biomed Eng. 2014;42:338–351. doi: 10.1007/s10439-013-0941-0. [DOI] [PubMed] [Google Scholar]
- 99.Perin E.C., Borow K.M., Henry T.D., et al. Randomized trial of targeted transendocardial mesenchymal precursor cell therapy in patients with heart failure. J Am Coll Cardiol. 2023;81:849–863. doi: 10.1016/j.jacc.2022.11.061. [DOI] [PubMed] [Google Scholar]
- 100.Salybekov A.A., Kawaguchi A.T., Masuda H., Vorateera K., Okada C., Asahara T. Regeneration-associated cells improve recovery from myocardial infarction through enhanced vasculogenesis, anti-inflammation, and cardiomyogenesis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morais J.M., Papadimitrakopoulos F., Burgess D.J. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. AAPS J. 2010;12:188–196. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rosellini E., Cascone M.G., Guidi L., Schubert D.W., Roether J.A., Boccaccini A.R. Mending a broken heart by biomimetic 3D printed natural biomaterial-based cardiac patches: a review. Front Bioeng Biotechnol. 2023;11 doi: 10.3389/fbioe.2023.1254739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Farache Trajano L., Smart N. Immunomodulation for optimal cardiac regeneration: insights from comparative analyses. NPJ Regen Med. 2021;6:8. doi: 10.1038/s41536-021-00118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lancaster J.J., Grijalva A., Fink J., et al. Biologically derived epicardial patch induces macrophage mediated pathophysiologic repair in chronically infarcted swine hearts. Commun Biol. 2023;6:1203. doi: 10.1038/s42003-023-05564-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu S., Yao L., Wang Y., et al. Immunomodulatory hybrid micro-nanofiber scaffolds enhance vascular regeneration. Bioact Mater. 2023;21:464–482. doi: 10.1016/j.bioactmat.2022.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Idrees H., Zaidi S.Z.J., Sabir A., Khan R.U., Zhang X., Hassan S. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials. 2020;10:1970. doi: 10.3390/nano10101970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guo B., Ma P.X. Conducting polymers for tissue engineering. Biomacromolecules. 2018;19:1764–1782. doi: 10.1021/acs.biomac.8b00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gazzi A., Fusco L., Orecchioni M., et al. Graphene, other carbon nanomaterials and the immune system: toward nanoimmunity-by-design. J Phys Mater. 2020;3 [Google Scholar]
- 109.Nazari H., Azadi S., Hatamie S., et al. Fabrication of graphene-silver/polyurethane nanofibrous scaffolds for cardiac tissue engineering. Polym Adv Technol. 2019;30:2086–2099. [Google Scholar]
- 110.Webber M.J., Matson J.B., Tamboli V.K., Stupp S.I. Controlled release of dexamethasone from peptide nanofiber gels to modulate inflammatory response. Biomaterials. 2012;33:6823–6832. doi: 10.1016/j.biomaterials.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lau C.Y.J., Benne N., Lou B., et al. Tuning surface charges of peptide nanofibers for induction of antigen-specific immune tolerance: an introductory study. J Pharm Sci. 2022;111:1004–1011. doi: 10.1016/j.xphs.2022.01.030. [DOI] [PubMed] [Google Scholar]
- 112.Ma Q., Wang X., Feng B., et al. Fiber configuration determines foreign body response of electrospun scaffolds: in vitro and in vivo assessments. Biomed Mater. 2024;19 doi: 10.1088/1748-605X/ad1c99. [DOI] [PubMed] [Google Scholar]
- 113.Ren Y., Chen Y., Chen W., et al. Hydrophilic nanofibers with aligned topography modulate macrophage-mediated host responses via the NLRP3 inflammasome. J Nanobiotechnology. 2023;21:269. doi: 10.1186/s12951-023-02024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holan V., Chudickova M., Trosan P., et al. Cyclosporine A-loaded and stem cell-seeded electrospun nanofibers for cell-based therapy and local immunosuppression. J Control Release. 2011;156:406–412. doi: 10.1016/j.jconrel.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 115.Loh X.J., Peh P., Liao S., Sng C., Li J. Controlled drug release from biodegradable thermoresponsive physical hydrogel nanofibers. J Control Release. 2010;143:175–182. doi: 10.1016/j.jconrel.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 116.Jiang Q., Reddy N., Yang Y. Cytocompatible cross-linking of electrospun zein fibers for the development of water-stable tissue engineering scaffolds. Acta Biomater. 2010;6:4042–4051. doi: 10.1016/j.actbio.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 117.Lin Y.-D., Ko M.-C., Wu S.-T., et al. A nanopatterned cell-seeded cardiac patch prevents electro-uncoupling and improves the therapeutic efficacy of cardiac repair. Biomater Sci. 2014;2:567–580. doi: 10.1039/c3bm60289c. [DOI] [PubMed] [Google Scholar]
- 118.Baranski J.D., Chaturvedi R.R., Stevens K.R., et al. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc Natl Acad Sci USA. 2013;110:7586–7591. doi: 10.1073/pnas.1217796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Y., Xu G., Wei J., Wu Q., Li X. Cardiomyocyte coculture on layered fibrous scaffolds assembled from micropatterned electrospun mats. Mater Sci Eng C. 2017;81:500–510. doi: 10.1016/j.msec.2017.08.042. [DOI] [PubMed] [Google Scholar]
- 120.Xu T., Miszuk J.M., Zhao Y., Sun H., Fong H. Electrospun polycaprolactone 3D nanofibrous scaffold with interconnected and hierarchically structured pores for bone tissue engineering. Adv Healthc Mater. 2015;4:2238–2246. doi: 10.1002/adhm.201500345. [DOI] [PubMed] [Google Scholar]
- 121.Fleischer S., Feiner R., Shapira A., et al. Spring-like fibers for cardiac tissue engineering. Biomaterials. 2013;34:8599–8606. doi: 10.1016/j.biomaterials.2013.07.054. [DOI] [PubMed] [Google Scholar]
- 122.Chaturvedi R.R., Herron T., Simmons R., et al. Passive stiffness of myocardium from congenital heart disease and implications for diastole. Circulation. 2010;121:979–988. doi: 10.1161/CIRCULATIONAHA.109.850677. [DOI] [PubMed] [Google Scholar]
- 123.Ribeiro M.C., Slaats R.H., Schwach V., et al. A cardiomyocyte show of force: a fluorescent alpha-actinin reporter line sheds light on human cardiomyocyte contractility versus substrate stiffness. J Mol Cell Cardiol. 2020;141:54–64. doi: 10.1016/j.yjmcc.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 124.Clausen A., Wang F., Jensen J.S., Sigmund O., Lewis J.A. Topology optimized architectures with programmable Poisson’s ratio over large deformations. Adv Mater. 2015;27:5523–5527. doi: 10.1002/adma.201502485. [DOI] [PubMed] [Google Scholar]
- 125.Yanping L., Hong H. A review on auxetic structures and polymeric materials. Sci Res Essays. 2010;5:1052–1063. [Google Scholar]
- 126.Ko J., Bhullar S., Cho Y., Lee P.C., Jun M.B.-G. Design and fabrication of auxetic stretchable force sensor for hand rehabilitation. Smart Mater Struct. 2015;24 [Google Scholar]
- 127.Bhullar S.K., Rana D., Lekesiz H., et al. Design and fabrication of auxetic PCL nanofiber membranes for biomedical applications. Mater Sci Eng C. 2017;81:334–340. doi: 10.1016/j.msec.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 128.Olvera D., Sohrabi Molina M., Hendy G., Monaghan M.G. Electroconductive melt electrowritten patches matching the mechanical anisotropy of human myocardium. Adv Funct Mater. 2020;30 [Google Scholar]
- 129.Pang C., Lee G.Y., Kim T., et al. A flexible and highly sensitive strain-gauge sensor using reversible interlocking of nanofibres. Nat Mater. 2012;11:795–801. doi: 10.1038/nmat3380. [DOI] [PubMed] [Google Scholar]
- 130.Chachques J.C., Trainini J.C., Lago N., et al. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM clinical trial): one year follow-up. Cell Transplant. 2007;16:927–934. doi: 10.3727/096368907783338217. [DOI] [PubMed] [Google Scholar]
- 131.Chachques J.C., Trainini J.C., Lago N., Cortes-Morichetti M., Schussler O., Carpentier A. Myocardial Assistance by Grafting a New Bioartificial Upgraded Myocardium (MAGNUM trial): clinical feasibility study. Ann Thorac Surg. 2008;85:901–908. doi: 10.1016/j.athoracsur.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 132.Ju Y.M., Ahn H., Arenas-Herrera J., et al. Electrospun vascular scaffold for cellularized small diameter blood vessels: a preclinical large animal study. Acta Biomater. 2017;59:58–67. doi: 10.1016/j.actbio.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 133.Stoddard R.J., Steger A.L., Blakney A.K., Woodrow K.A. In pursuit of functional electrospun materials for clinical applications in humans. Ther Deliv. 2016;7:387–409. doi: 10.4155/tde-2016-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ogle B.M., Bursac N., Domian I., et al. Distilling complexity to advance cardiac tissue engineering. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aad2304. 342ps13-342ps13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mei X., Chang K. Recent developments in therapeutic cardiac patches. Front Cardiovasc Med. 2020;7 doi: 10.3389/fcvm.2020.610364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mehrabi A., Baheiraei N., Adabi M., Amirkhani Z. Development of a novel electroactive cardiac patch based on carbon nanofibers and gelatin encouraging vascularization. Appl Biochem Biotechnol. 2020;190:931–948. doi: 10.1007/s12010-019-03135-6. [DOI] [PubMed] [Google Scholar]
- 137.Bolli R., Tang X.-L. The sad plight of cell therapy for heart failure: causes and consequences. J Cardiovasc Aging. 2022;2:16. doi: 10.20517/jca.2022.02. [DOI] [PMC free article] [PubMed] [Google Scholar]