Abstract
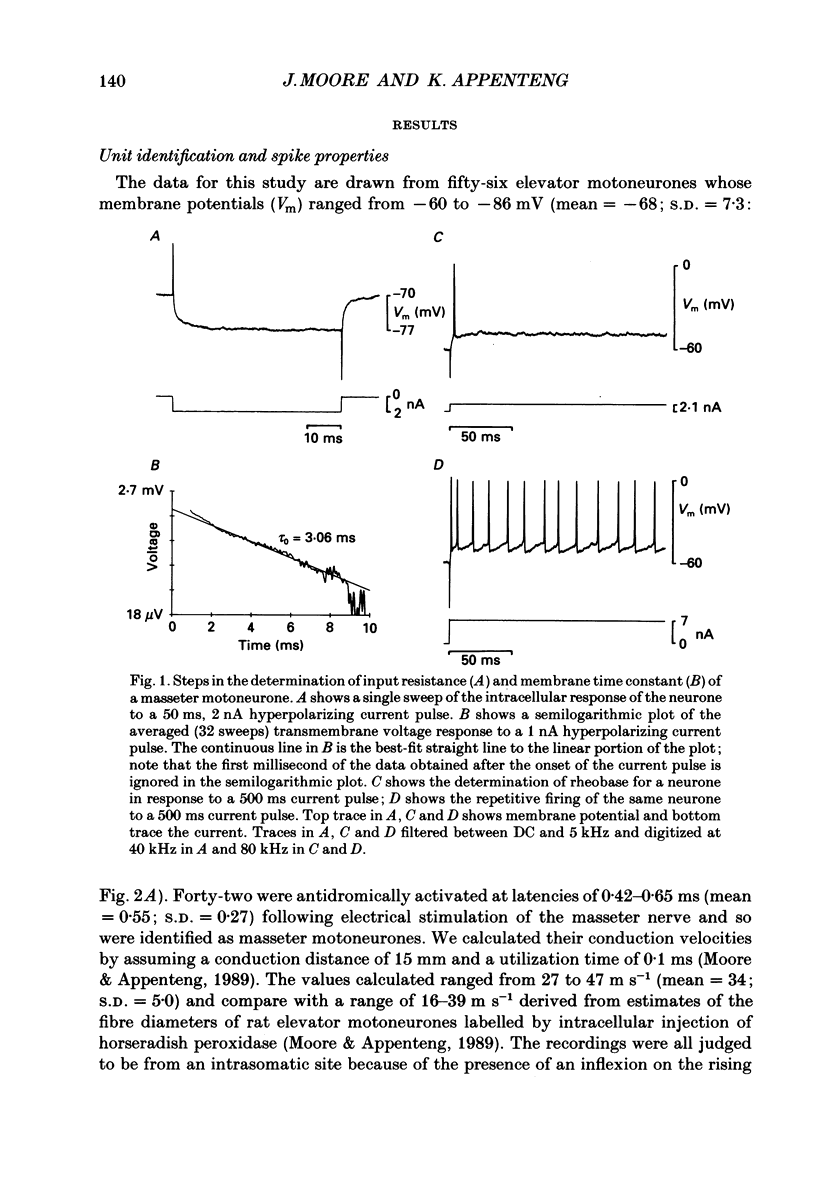
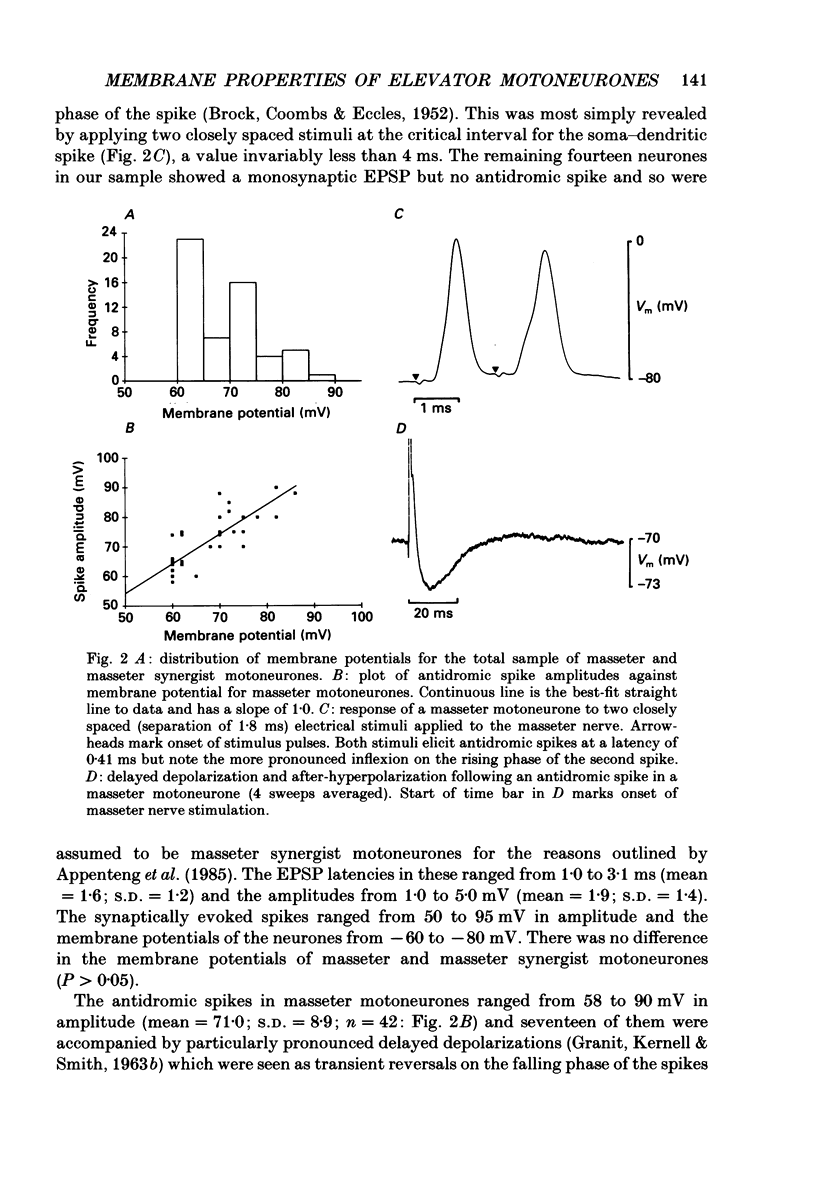
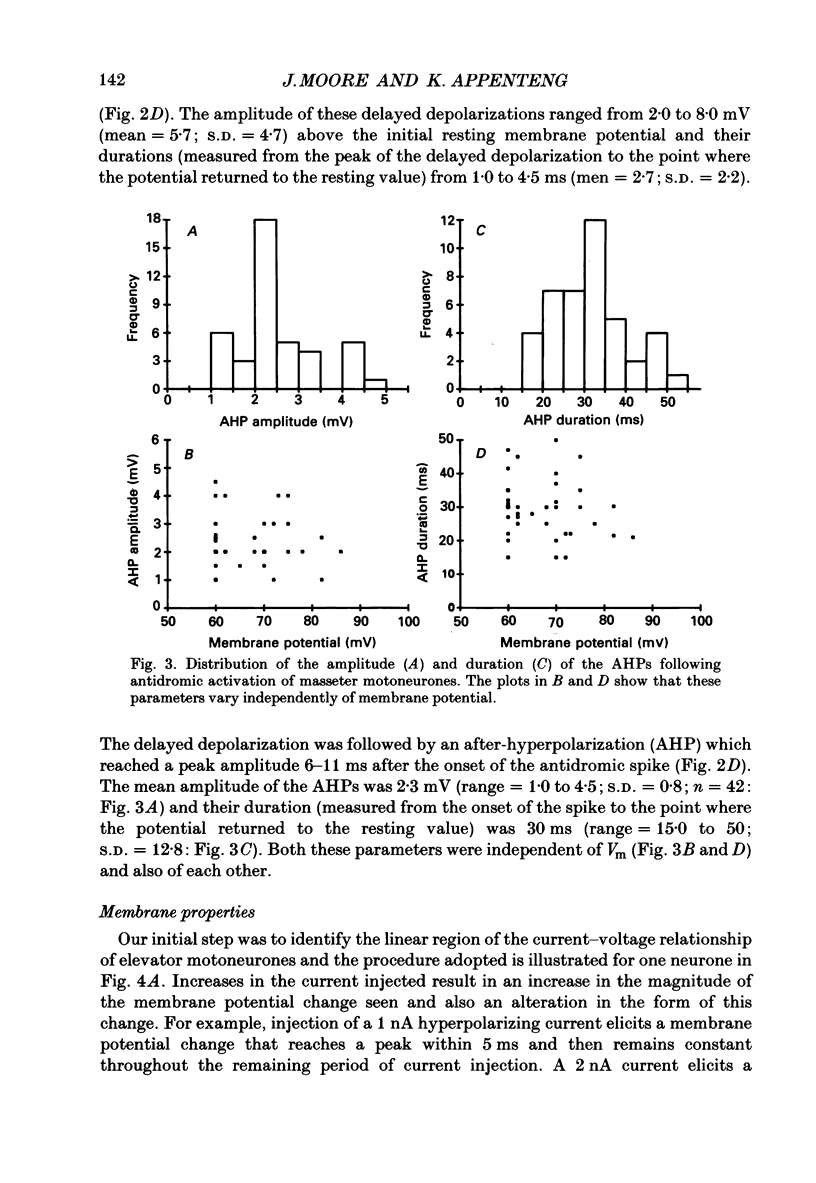
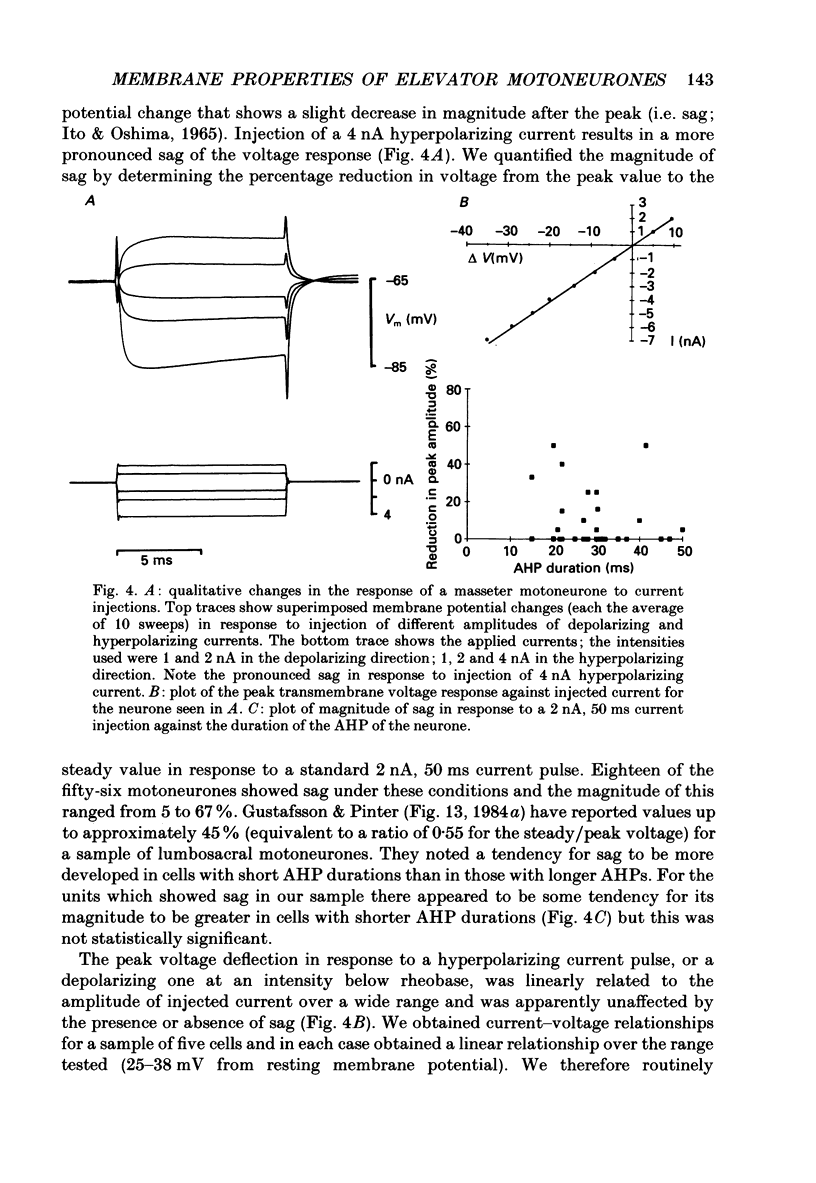
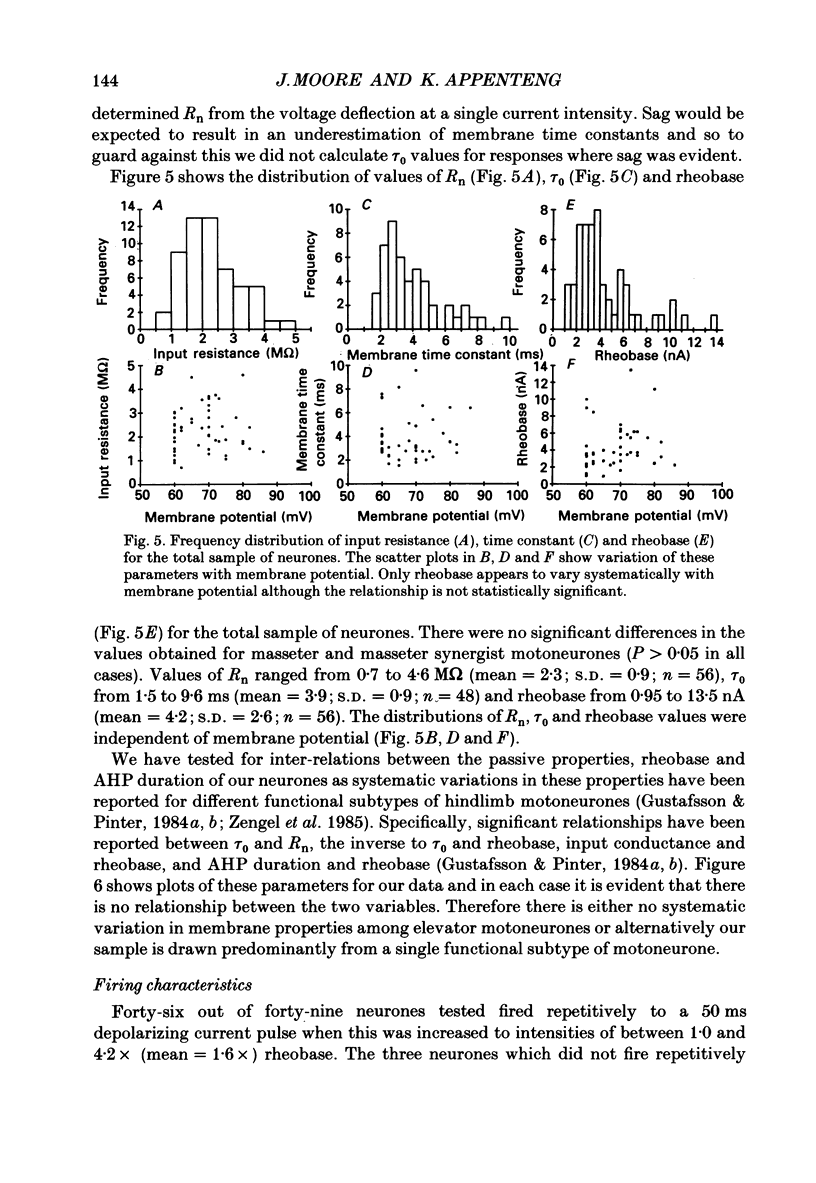
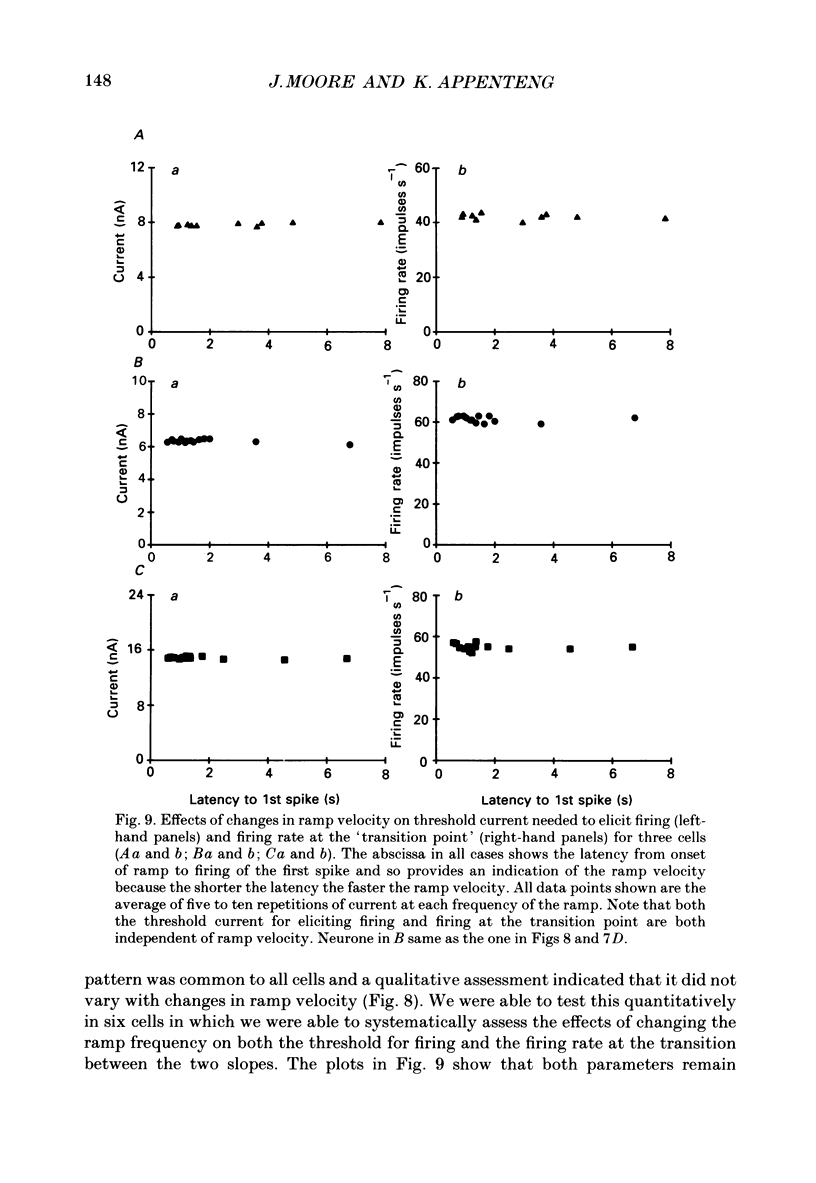
1. We have determined the membrane and firing properties of fifty-six jaw-elevator motoneurones in rats that were anaesthetized with pentobarbitone, paralysed and artificially ventilated. 2. Forty-two neurones were identified as masseter motoneurones and fourteen as masseter synergist motoneurones. The membrane potentials for the sample ranged from -60 to -86 (mean = -68; S.D. = 7.3; n = 56), and spike amplitudes from 50 to 95 mV. The duration of the after-hyperpolarization following antidromic spikes in masseter motoneurones ranged from 15 to 50 ms (mean = 30; S.D. = 12.8) and their amplitudes from 1.0 to 4.5 mV (mean = 2.7; S.D. = 2.2; n = 42). 3. The mean input resistance for the total sample was 2.3 M omega (S.D. = 0.9; n = 56), membrane time constant 3.9 ms (S.D. = 0.9; n = 48) and rheobase 4.2 nA (S.D. = 2.6; n = 56). The distribution of these parameters was independent of membrane potential. We found no significant interrelationships between the membrane properties and one interpretation of this is that our sample may be drawn from a homogenous population of motoneurones. We also suggest that elevator motoneurones may have a lower Rm (specific membrane resistivity) value than cat hindlimb motoneurones because they have a similar range of input resistance values but only half the total surface area. 4. Forty-six out of forty-nine neurones fired repetitively to a depolarizing current pulse at a mean threshold of 1.6 x rheobase. Current-frequency plots were constructed for thirteen neurones and all but one showed a primary and secondary range in the firing of the first interspike interval. The mean slope in the primary range was 31 impulses s-1 nA-1 and 77 impulses s-1 nA-1 for the secondary range. The mean minimal firing frequency for steady firing was 26 impulses s-1 and, in response to an increase of stimulation, the rate increased monotonically with a slope of 11 impulses s-1 nA-1. 5. The dynamic sensitivity of twelve neurones was assessed from their response to ramp waveforms of current of constant amplitude but varying frequencies (0.2-2 Hz). Firing initially increased along a steep slope up to a frequency of between 40 and 60 impulses s-1 and then increased along a much shallower slope. Both the threshold for eliciting firing and the firing at the transition point of the two slopes remained constant with changes in ramp frequency.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


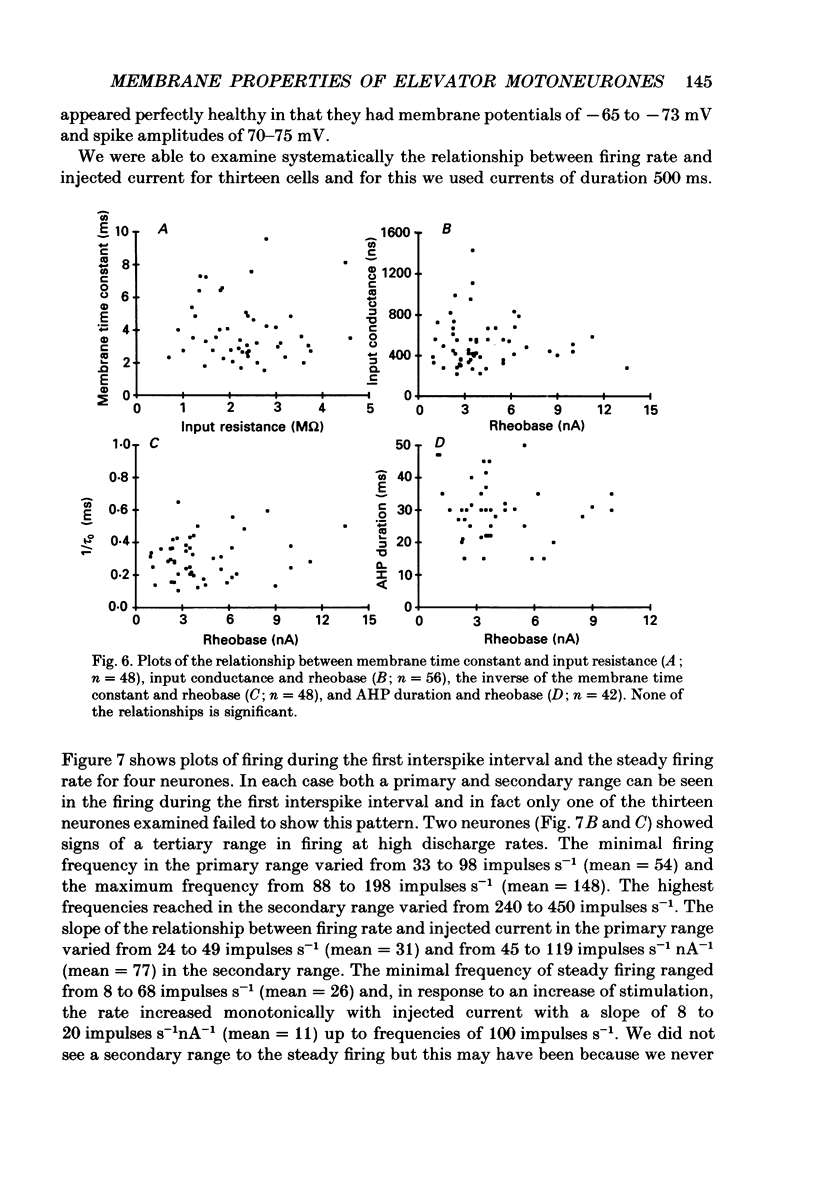
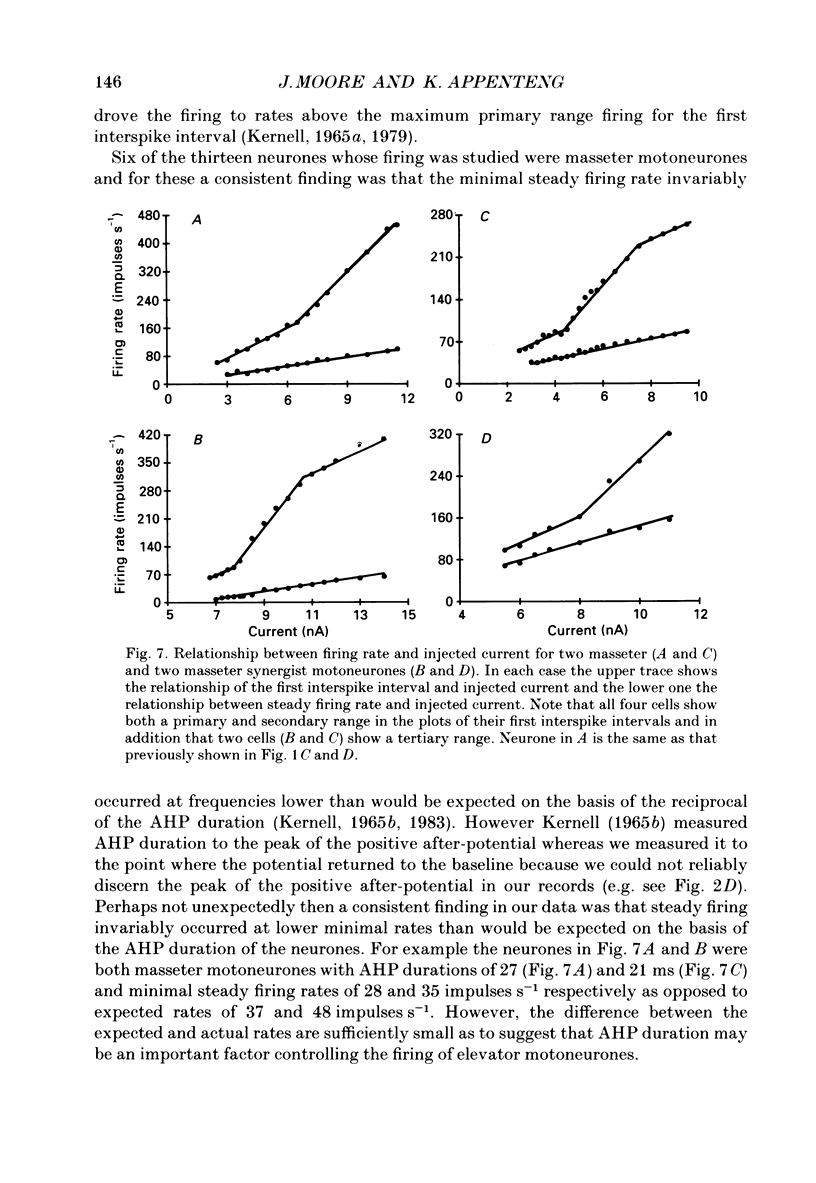





Selected References
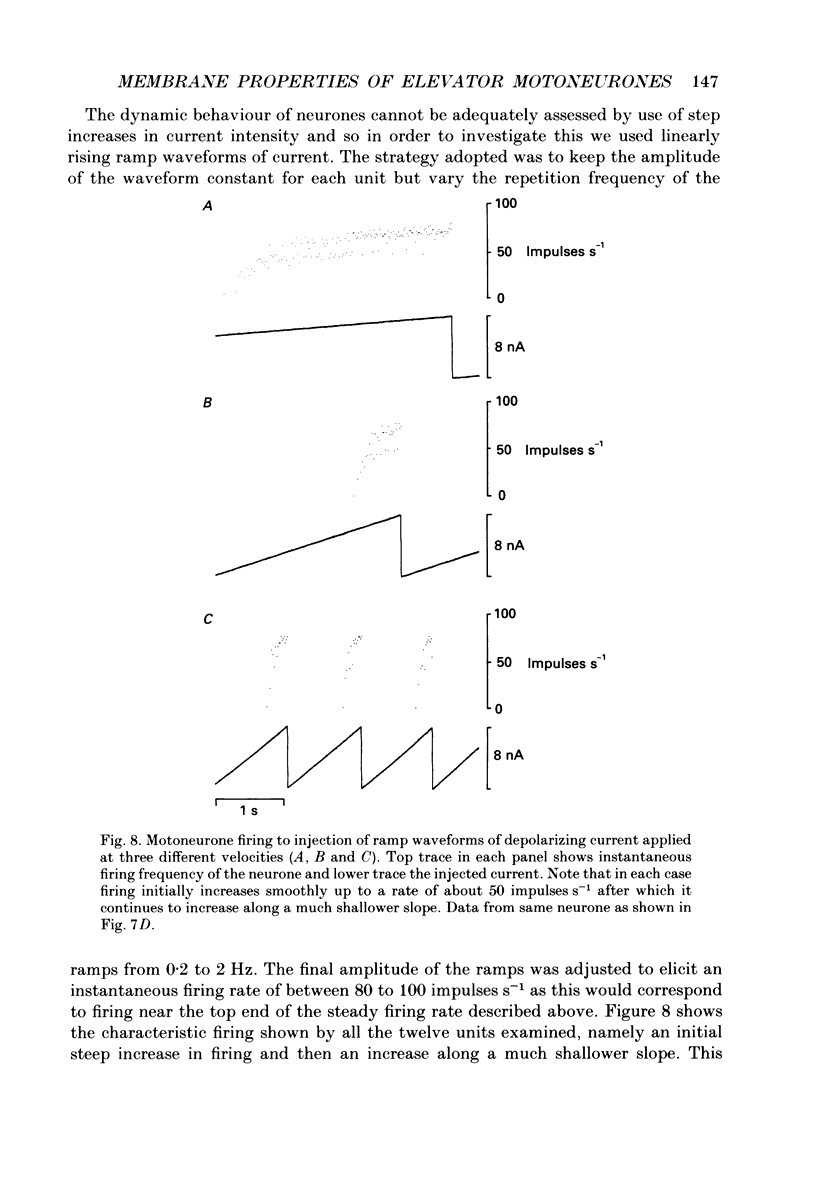
These references are in PubMed. This may not be the complete list of references from this article.
- Appenteng K., Conyers L., Moore J. A. The monosynaptic excitatory connections of single trigeminal interneurones to the V motor nucleus of the rat. J Physiol. 1989 Oct;417:91–104. doi: 10.1113/jphysiol.1989.sp017792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenteng K., Morimoto T., Taylor A. Fusimotor activity in masseter nerve of the cat during reflex jaw movements. J Physiol. 1980 Aug;305:415–431. doi: 10.1113/jphysiol.1980.sp013373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. The recording of potentials from motoneurones with an intracellular electrode. J Physiol. 1952 Aug;117(4):431–460. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F., Campadelli P., Piccinelli L. The dynamic response of cat alpha-motoneurones investigated by intracellular injection of sinusoidal currents. Exp Brain Res. 1984;54(2):275–282. doi: 10.1007/BF00236227. [DOI] [PubMed] [Google Scholar]
- Bradley K., Somjen G. G. Accommodation in motoneurones of the rat and the cat. J Physiol. 1961 Apr;156(1):75–92. doi: 10.1113/jphysiol.1961.sp006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras H., Gogan P., Tyc-Dumont S. The dendrites of single brain-stem motoneurons intracellularly labelled with horseradish peroxidase in the cat. Morphological and electrical differences. Neuroscience. 1987 Sep;22(3):947–970. doi: 10.1016/0306-4522(87)92972-1. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Fricke R. A., Perkel D. H. Passive electrical constants in three classes of hippocampal neurons. J Neurophysiol. 1981 Oct;46(4):812–827. doi: 10.1152/jn.1981.46.4.812. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Dum R. P., Fleshman J. W., Glenn L. L., Lev-Tov A., O'Donovan M. J., Pinter M. J. A HRP study of the relation between cell size and motor unit type in cat ankle extensor motoneurons. J Comp Neurol. 1982 Jul 20;209(1):17–28. doi: 10.1002/cne.902090103. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Redman S. J. Cable properties of cat spinal motoneurones measured by combining voltage clamp, current clamp and intracellular staining. J Physiol. 1989 Feb;409:63–87. doi: 10.1113/jphysiol.1989.sp017485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand J., Durand-Arczynska W., Wankmiller D. Coupling of active sodium transport to oxidative metabolism in the rabbit distal colon. J Physiol. 1988 Feb;396:55–64. doi: 10.1113/jphysiol.1988.sp016949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman J. W., Munson J. B., Sypert G. W., Friedman W. A. Rheobase, input resistance, and motor-unit type in medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1981 Dec;46(6):1326–1338. doi: 10.1152/jn.1981.46.6.1326. [DOI] [PubMed] [Google Scholar]
- GRANIT R., KERNELL D., SHORTESS G. K. QUANTITATIVE ASPECTS OF REPETITIVE FIRING OF MAMMALIAN MOTONEURONES, CAUSED BY INJECTED CURRENTS. J Physiol. 1963 Oct;168:911–931. doi: 10.1113/jphysiol.1963.sp007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., KERNELL D., SMITH R. S. DELAYED DEPOLARIZATION AND THE REPETITIVE RESPONSE TO INTRACELLULAR STIMULATION OF MAMMALIAN MOTONEURONES. J Physiol. 1963 Oct;168:890–910. doi: 10.1113/jphysiol.1963.sp007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S. J., Lennerstrand G., Hull C. D. Motor unit responses in the lateral rectus muscle of the cat: intracellular current injection of abducens nucleus neurons. Acta Physiol Scand. 1976 Jan;96(1):58–63. doi: 10.1111/j.1748-1716.1976.tb10170.x. [DOI] [PubMed] [Google Scholar]
- Grantyn R., Grantyn A. Morphological and electrophysiological properties of cat abducens motoneurons. Exp Brain Res. 1978 Feb 15;31(2):249–274. doi: 10.1007/BF00237603. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Pinter M. J. An investigation of threshold properties among cat spinal alpha-motoneurones. J Physiol. 1984 Dec;357:453–483. doi: 10.1113/jphysiol.1984.sp015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Pinter M. J. Relations among passive electrical properties of lumbar alpha-motoneurones of the cat. J Physiol. 1984 Nov;356:401–431. doi: 10.1113/jphysiol.1984.sp015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Oshima T. Electrical behaviour of the motoneurone membrane during intracellularly applied current steps. J Physiol. 1965 Oct;180(3):607–635. doi: 10.1113/jphysiol.1965.sp007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodkowski J. S., Viana F., Dick T. E., Berger A. J. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol. 1987 Jul;58(1):105–124. doi: 10.1152/jn.1987.58.1.105. [DOI] [PubMed] [Google Scholar]
- Jodkowski J. S., Viana F., Dick T. E., Berger A. J. Repetitive firing properties of phrenic motoneurons in the cat. J Neurophysiol. 1988 Aug;60(2):687–702. doi: 10.1152/jn.1988.60.2.687. [DOI] [PubMed] [Google Scholar]
- Kernell D. Functional properties of spinal motoneurons and gradation of muscle force. Adv Neurol. 1983;39:213–226. [PubMed] [Google Scholar]
- Kernell D. Rhythmic properties of motoneurones innervating muscle fibres of different speed in m. gastrocnemius medialis of the cat. Brain Res. 1979 Jan 5;160(1):159–162. doi: 10.1016/0006-8993(79)90612-7. [DOI] [PubMed] [Google Scholar]
- Moore J. A., Appenteng K. The morphology of the axons and axon collaterals of rat jaw-elevator motoneurones. Brain Res. 1989 Jun 12;489(2):383–386. doi: 10.1016/0006-8993(89)90876-7. [DOI] [PubMed] [Google Scholar]
- Nordstrom S. H., Yemm R. The relationship between jaw position and isometric active tension produced by direct stimulation of the rat masseter muscle. Arch Oral Biol. 1974 May;19(5):353–359. doi: 10.1016/0003-9969(74)90176-9. [DOI] [PubMed] [Google Scholar]
- RALL W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959 Nov;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- Rall W., Rinzel J. Branch input resistance and steady attenuation for input to one branch of a dendritic neuron model. Biophys J. 1973 Jul;13(7):648–687. doi: 10.1016/S0006-3495(73)86014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinzel J., Rall W. Transient response in a dendritic neuron model for current injected at one branch. Biophys J. 1974 Oct;14(10):759–790. doi: 10.1016/S0006-3495(74)85948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokx J. T., van Willigen J. D., Jüch P. J. Distribution of innervating neurons of masticatory muscle spindles in the rat: an HRP study. Exp Neurol. 1985 Jun;88(3):562–569. doi: 10.1016/0014-4886(85)90071-8. [DOI] [PubMed] [Google Scholar]
- Rowlerson A., Mascarello F., Barker D., Saed H. Muscle-spindle distribution in relation to the fibre-type composition of masseter in mammals. J Anat. 1988 Dec;161:37–60. [PMC free article] [PubMed] [Google Scholar]
- Takata M., Fujita S., Kanamori N. Repetitive firing in trigeminal mesencephalic tract neurons and trigeminal motoneurons. J Neurophysiol. 1982 Jan;47(1):23–30. doi: 10.1152/jn.1982.47.1.23. [DOI] [PubMed] [Google Scholar]
- Taylor A., Cody F. W., Bosley M. A. Histochemical and mechanical properties of the jaw muscles of the cat. Exp Neurol. 1973 Jan;38(1):99–109. doi: 10.1016/0014-4886(73)90011-3. [DOI] [PubMed] [Google Scholar]
- Vornov J. J., Sutin J. Noradrenergic hyperinnervation of the motor trigeminal nucleus: alterations in membrane properties and responses to synaptic input. J Neurosci. 1986 Jan;6(1):30–37. doi: 10.1523/JNEUROSCI.06-01-00030.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. D., Robert J. M., Shofner W. P. Regularity and latency of units in ventral cochlear nucleus: implications for unit classification and generation of response properties. J Neurophysiol. 1988 Jul;60(1):1–29. doi: 10.1152/jn.1988.60.1.1. [DOI] [PubMed] [Google Scholar]
- Zengel J. E., Reid S. A., Sypert G. W., Munson J. B. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1985 May;53(5):1323–1344. doi: 10.1152/jn.1985.53.5.1323. [DOI] [PubMed] [Google Scholar]


