Abstract
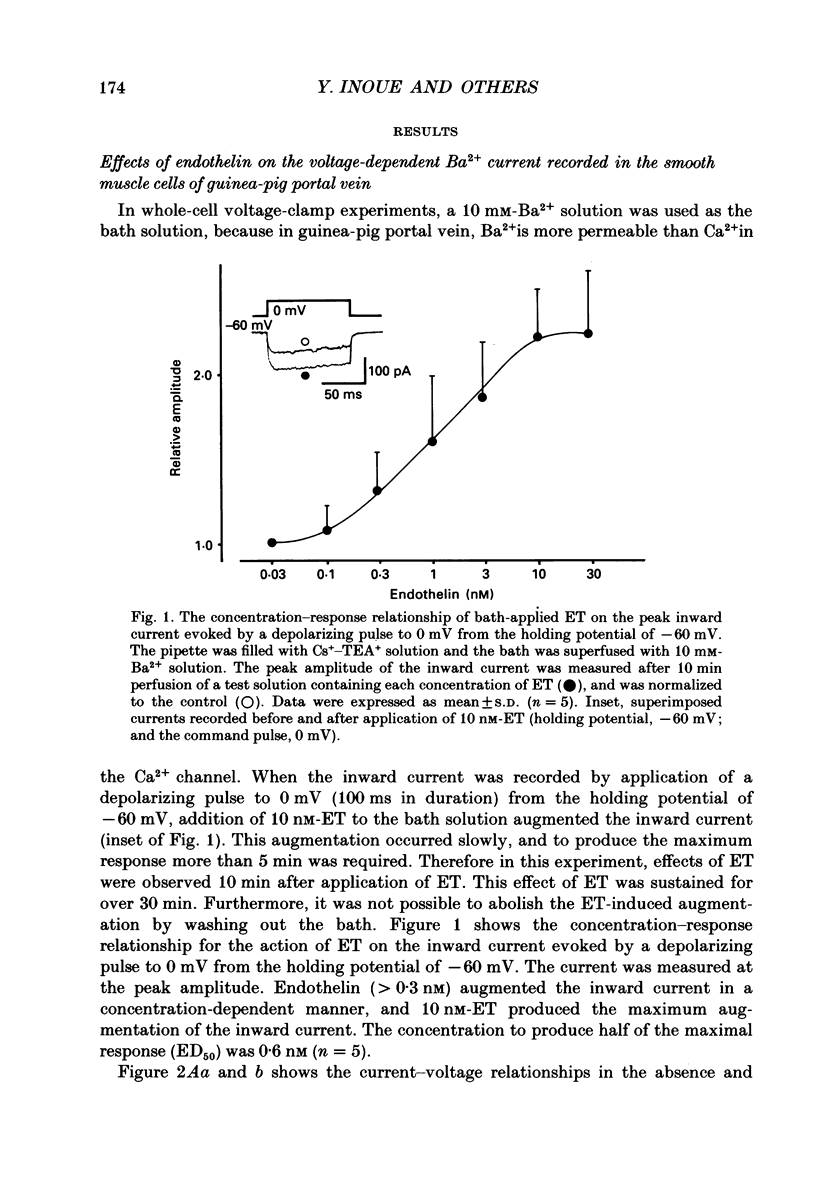
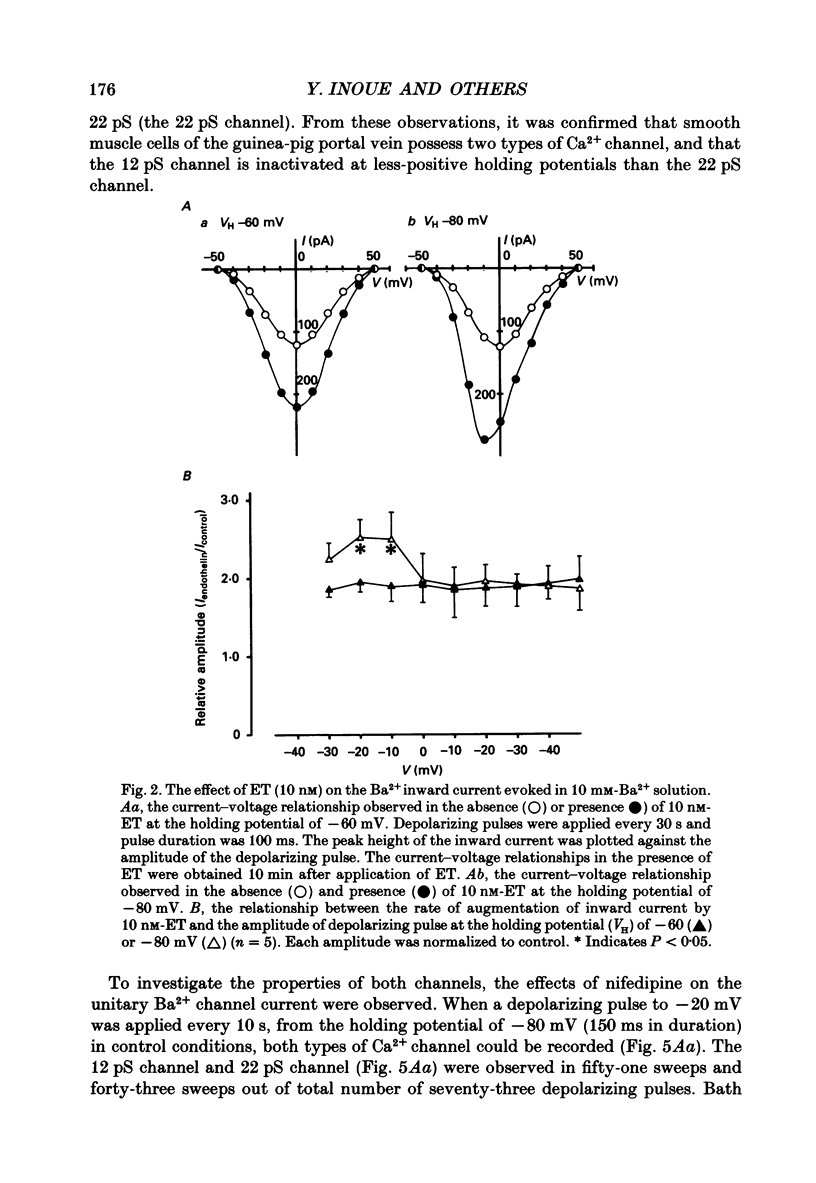
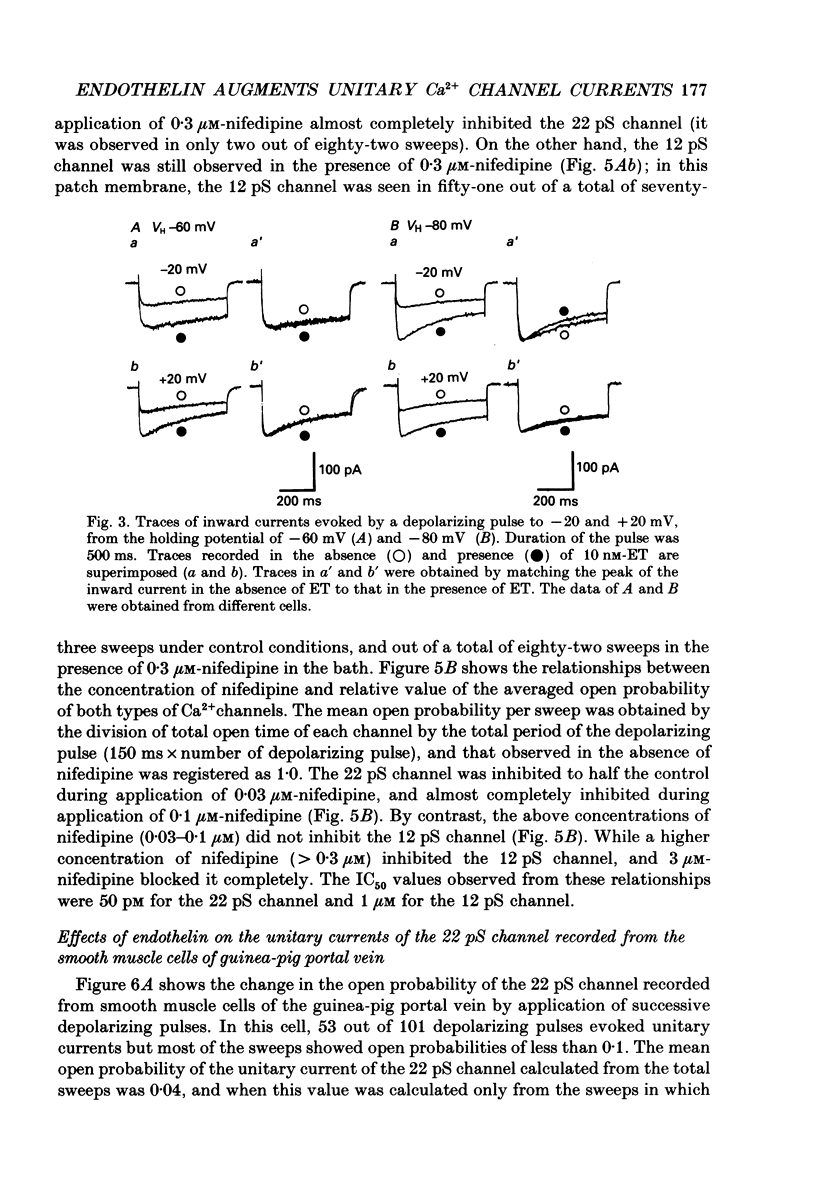
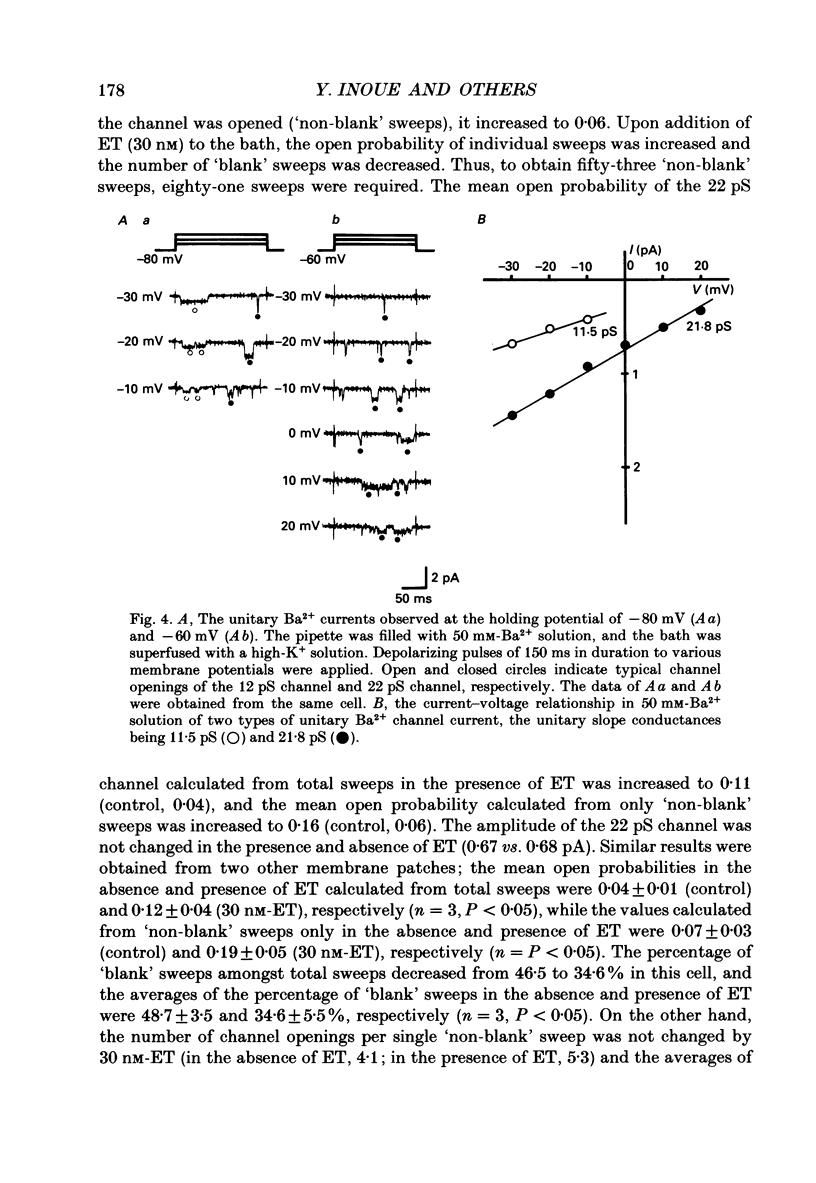
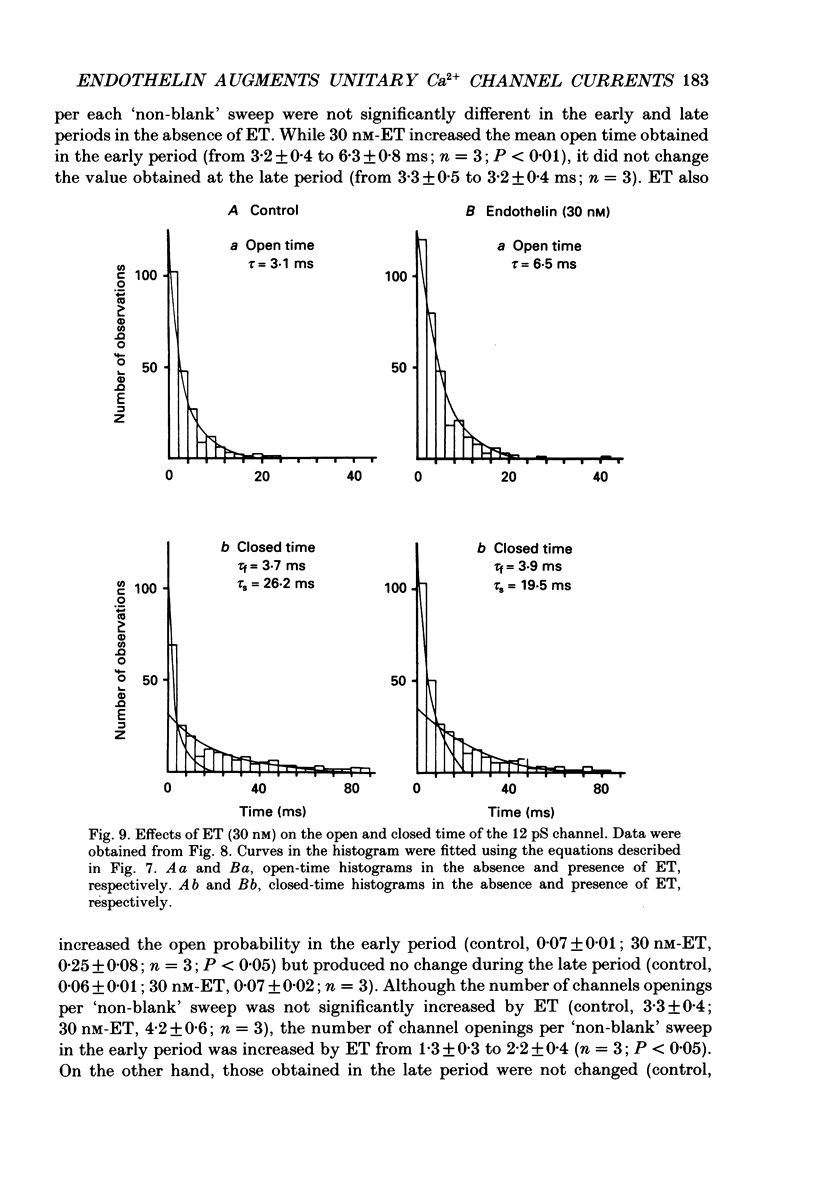
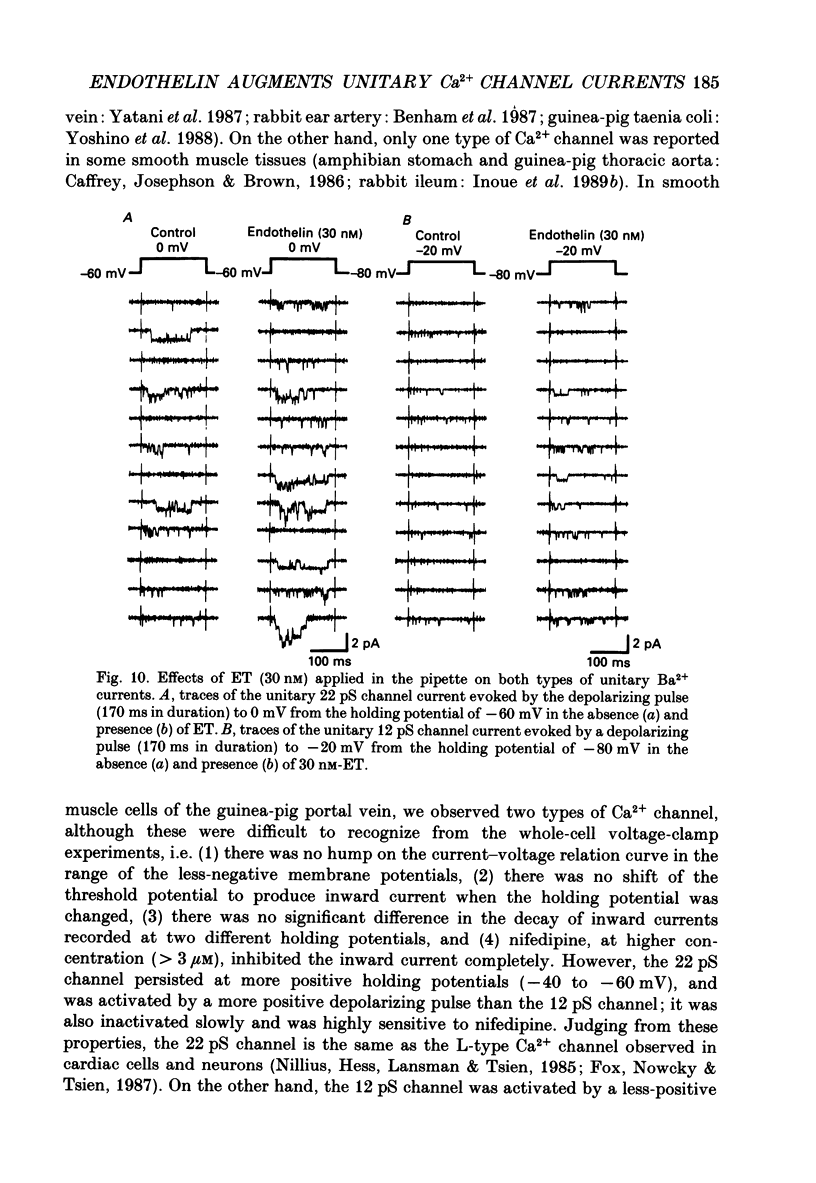
1. The effects of endothelin (ET) on the Ca2+ channel current in smooth muscle cells of the guinea-pig portal vein were investigated using the patch-clamp technique with whole-cell and cell-attached configurations. 2. ET augmented the macroscopic Ba2+ current in a dose-dependent manner; this effect was inhibited by nifedipine or Cd2+. Augmentation of the inward current by ET did not depend on the amplitude of the depolarizing pulse. Further, when the membrane potential was held at -60 mV, ET increased the amplitude of the Ba2+ inward current measured at the peak and end of the depolarizing pulse to the same extent. 3. By contrast, when the membrane potential was held at -80 mV, depolarizing pulses to potentials more negative than 0 mV produced greater augmentation of the inward current than did those more positive than 0 mV. Moreover, when a depolarizing pulse to below 0 mV was applied, ET increased the peak amplitude of the inward current more than the amplitude measured at the end of pulse. 4. Using the patch-clamp technique with cell-attached configuration, two types of unitary Ba2+ current with conductances of 22 and 12 pS were obtained in 50 mM-Ba2+ solution. Nifedipine inhibited both types of unitary channel current, but the sensitivity of the 22 pS Ca2+ channel to nifedipine was 20-fold higher than the 12 pS Ca2+ channel. 5. Bath application of ET prolonged the mean open time, reduced the number of sweeps in which no Ca2+ channel was opened ('blank' sweep), and increased the number of channel openings evoked by each depolarizing pulse without changes of conductance. As a consequence, ET increased the open probability of both channels. 6. Augmentation of the 12 pS channels by ET was seen only in the early phase of a depolarizing pulse (57 ms from the onset of 170 ms pulse), while augmentation of the 22 pS channels was seen during the entire period of a depolarizing pulse. 7. When ET was added to the pipette solution, the activity of both Ca2+ channels was increased. However, this effect was less frequently observed than when ET was applied in the bath. 8. These results suggest that ET augments both the nifedipine-sensitive and resistant Ca2+ channels in the smooth muscle cell membrane of the guinea-pig portal vein, but in different ways. Presumably, ET acts indirectly on the voltage-dependent Ca2+ channel.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auguet M., Delaflotte S., Chabrier P. E., Pirotzky E., Clostre F., Braquet P. Endothelin and Ca++ agonist Bay K 8644: different vasoconstrictive properties. Biochem Biophys Res Commun. 1988 Oct 14;156(1):186–192. doi: 10.1016/s0006-291x(88)80822-2. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Sturek M., Puga A., Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ Res. 1986 Aug;59(2):229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Kunze D. L., Yatani A. Dual effects of dihydropyridines on whole cell and unitary calcium currents in single ventricular cells of guinea-pig. J Physiol. 1986 Oct;379:495–514. doi: 10.1113/jphysiol.1986.sp016266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey J. M., Josephson I. R., Brown A. M. Calcium channels of amphibian stomach and mammalian aorta smooth muscle cells. Biophys J. 1986 Jun;49(6):1237–1242. doi: 10.1016/S0006-3495(86)83753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Anoxia and endothelium-dependent reactivity of the canine femoral artery. J Physiol. 1983 Feb;335:65–74. doi: 10.1113/jphysiol.1983.sp014519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish R. D., Sperti G., Colucci W. S., Clapham D. E. Phorbol ester increases the dihydropyridine-sensitive calcium conductance in a vascular smooth muscle cell line. Circ Res. 1988 May;62(5):1049–1054. doi: 10.1161/01.res.62.5.1049. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y., Hirata Y., Yoshimi H., Kojima T., Kobayashi Y., Yanagisawa M., Masaki T. Endothelin is a potent secretagogue for atrial natriuretic peptide in cultured rat atrial myocytes. Biochem Biophys Res Commun. 1988 Aug 30;155(1):167–172. doi: 10.1016/s0006-291x(88)81064-7. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gillespie M. N., Owasoyo J. O., McMurtry I. F., O'Brien R. F. Sustained coronary vasoconstriction provoked by a peptidergic substance released from endothelial cells in culture. J Pharmacol Exp Ther. 1986 Feb;236(2):339–343. [PubMed] [Google Scholar]
- Goto K., Kasuya Y., Matsuki N., Takuwa Y., Kurihara H., Ishikawa T., Kimura S., Yanagisawa M., Masaki T. Endothelin activates the dihydropyridine-sensitive, voltage-dependent Ca2+ channel in vascular smooth muscle. Proc Natl Acad Sci U S A. 1989 May;86(10):3915–3918. doi: 10.1073/pnas.86.10.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey K. A., Rubanyi G., Paul R. J., Highsmith R. F. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am J Physiol. 1985 May;248(5 Pt 1):C550–C556. doi: 10.1152/ajpcell.1985.248.5.C550. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Yoshimi H., Takata S., Watanabe T. X., Kumagai S., Nakajima K., Sakakibara S. Cellular mechanism of action by a novel vasoconstrictor endothelin in cultured rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1988 Aug 15;154(3):868–875. doi: 10.1016/0006-291x(88)90220-3. [DOI] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Acetylcholine activates single sodium channels in smooth muscle cells. Pflugers Arch. 1987 Sep;410(1-2):69–74. doi: 10.1007/BF00581898. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Xiong Z. L., Kitamura K., Kuriyama H. Modulation produced by nifedipine of the unitary Ba current of dispersed smooth muscle cells of the rabbit ileum. Pflugers Arch. 1989 Sep;414(5):534–542. doi: 10.1007/BF00580988. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Yanagisawa M., Kimura S., Goto K., Masaki T. Positive inotropic action of novel vasoconstrictor peptide endothelin on guinea pig atria. Am J Physiol. 1988 Oct;255(4 Pt 2):H970–H973. doi: 10.1152/ajpheart.1988.255.4.H970. [DOI] [PubMed] [Google Scholar]
- Kai H., Kanaide H., Nakamura M. Endothelin-sensitive intracellular Ca2+ store overlaps with caffeine-sensitive one in rat aortic smooth muscle cells in primary cultures. Biochem Biophys Res Commun. 1989 Jan 16;158(1):235–243. doi: 10.1016/s0006-291x(89)80203-7. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guinea pig). Pflugers Arch. 1985 Dec;405(4):340–348. doi: 10.1007/BF00595686. [DOI] [PubMed] [Google Scholar]
- Marsden P. A., Danthuluri N. R., Brenner B. M., Ballermann B. J., Brock T. A. Endothelin action on vascular smooth muscle involves inositol trisphosphate and calcium mobilization. Biochem Biophys Res Commun. 1989 Jan 16;158(1):86–93. doi: 10.1016/s0006-291x(89)80180-9. [DOI] [PubMed] [Google Scholar]
- Miasiro N., Yamamoto H., Kanaide H., Nakamura M. Does endothelin mobilize calcium from intracellular store sites in rat aortic vascular smooth muscle cells in primary culture? Biochem Biophys Res Commun. 1988 Oct 14;156(1):312–317. doi: 10.1016/s0006-291x(88)80841-6. [DOI] [PubMed] [Google Scholar]
- Momose K., Gomi Y. [Studies on isolated smooth muscle cells. VI. Dispersion procedures for acetylcholine-sensitive smooth muscle cells of guinea pig (author's transl)]. Nihon Heikatsukin Gakkai Zasshi. 1980 Mar;16(1):29–36. doi: 10.1540/jsmr1965.16.29. [DOI] [PubMed] [Google Scholar]
- Nakaki T., Nakayama M., Yamamoto S., Kato R. Endothelin-mediated stimulation of DNA synthesis in vascular smooth muscle cells. Biochem Biophys Res Commun. 1989 Feb 15;158(3):880–883. doi: 10.1016/0006-291x(89)92804-0. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. F., Robbins R. J., McMurtry I. F. Endothelial cells in culture produce a vasoconstrictor substance. J Cell Physiol. 1987 Aug;132(2):263–270. doi: 10.1002/jcp.1041320210. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Modulation of ionic currents in smooth muscle balls of the rabbit intestine by intracellularly perfused ATP and cyclic AMP. Pflugers Arch. 1987 May;408(5):465–473. doi: 10.1007/BF00585070. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Regulation of calcium current by intracellular calcium in smooth muscle cells of rabbit portal vein. Circ Res. 1988 Feb;62(2):375–383. doi: 10.1161/01.res.62.2.375. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Terada K., Yamaguchi K., Inoue R., Okabe K., Kitamura K., Hirata M., Kuriyama H. Effects of inositol phosphates on the membrane activity of smooth muscle cells of the rabbit portal vein. Pflugers Arch. 1988 Sep;412(4):382–389. doi: 10.1007/BF01907556. [DOI] [PubMed] [Google Scholar]
- Rakugi H., Nakamaru M., Saito H., Higaki J., Ogihara T. Endothelin inhibits renin release from isolated rat glomeruli. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1244–1247. doi: 10.1016/s0006-291x(88)81273-7. [DOI] [PubMed] [Google Scholar]
- Resink T. J., Scott-Burden T., Bühler F. R. Endothelin stimulates phospholipase C in cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1360–1368. doi: 10.1016/s0006-291x(88)81025-8. [DOI] [PubMed] [Google Scholar]
- Silberberg S. D., Poder T. C., Lacerda A. E. Endothelin increases single-channel calcium currents in coronary arterial smooth muscle cells. FEBS Lett. 1989 Apr 10;247(1):68–72. doi: 10.1016/0014-5793(89)81242-6. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Inagami T., Hare G. M., Johns J. A. Endothelin action: Inhibition by a protein kinase C inhibitor and involvement of phosphoinositols. Biochem Biophys Res Commun. 1989 Jan 16;158(1):170–176. doi: 10.1016/s0006-291x(89)80193-7. [DOI] [PubMed] [Google Scholar]
- Takagi M., Matsuoka H., Atarashi K., Yagi S. Endothelin: a new inhibitor of renin release. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1164–1168. doi: 10.1016/s0006-291x(88)80996-3. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Vigne P., Barhanin J., Schmid-Alliana A., Frelin C., Lazdunski M. Molecular mechanism of action of the vasoconstrictor peptide endothelin. Biochem Biophys Res Commun. 1988 Dec 30;157(3):977–985. doi: 10.1016/s0006-291x(88)80970-7. [DOI] [PubMed] [Google Scholar]
- Worley J. F., 3rd, Deitmer J. W., Nelson M. T. Single nisoldipine-sensitive calcium channels in smooth muscle cells isolated from rabbit mesenteric artery. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5746–5750. doi: 10.1073/pnas.83.15.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yatani A., Seidel C. L., Allen J., Brown A. M. Whole-cell and single-channel calcium currents of isolated smooth muscle cells from saphenous vein. Circ Res. 1987 Apr;60(4):523–533. doi: 10.1161/01.res.60.4.523. [DOI] [PubMed] [Google Scholar]
- Yoshino M., Someya T., Nishio A., Yabu H. Whole-cell and unitary Ca channel currents in mammalian intestinal smooth muscle cells: evidence for the existence of two types of Ca channels. Pflugers Arch. 1988 Feb;411(2):229–231. doi: 10.1007/BF00582322. [DOI] [PubMed] [Google Scholar]



