Abstract
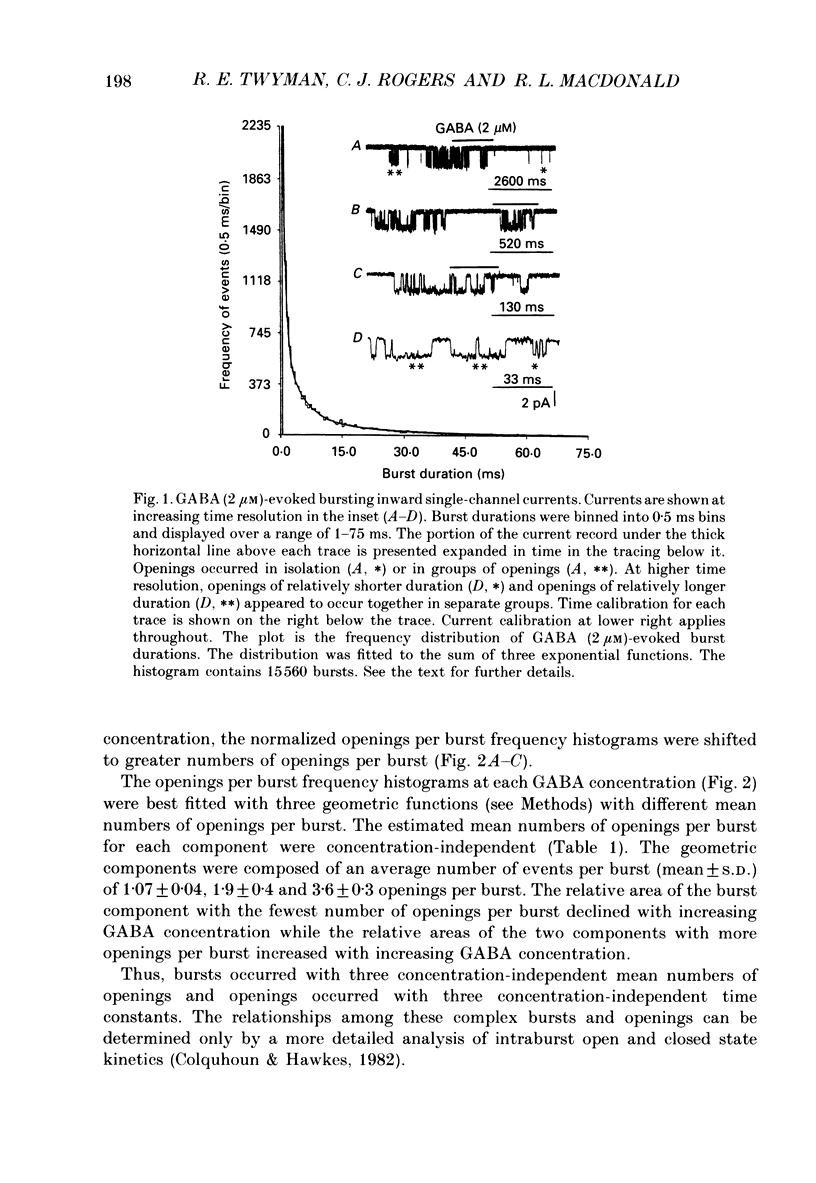
1. The intraburst kinetic properties of the main conductance state of gamma-aminobutyric acidA (GABAA) receptor channels in excised outside-out patches obtained from somata of mouse spinal cord neurones in cell culture were investigated using the patch clamp single-channel recording technique. 2. At 2 microM-GABA, the burst duration distribution was fitted by four exponential functions with time constants of 0.5, 2.4, 8.3 and 31.8 ms. 3. At 0.5, 1 and 2 microM-GABA, frequency distribution histograms of the number of apparent openings per burst were best fitted by three geometric functions with similar mean numbers (1.1, 1.9 and 3.6) of openings per burst. The proportion of bursts with a mean of 1.1 openings per burst decreased with increased GABA concentration while the proportion of bursts with means of 1.9 and 3.6 openings per burst increased with GABA concentration. 4. Analyses of GABA receptor channel intraburst kinetics were performed at all three GABA concentrations. The results were similar for all concentrations, but detailed results are presented only for 2 microM-GABA. 5. The open time distribution for all intraburst openings was best fitted by three exponential functions with time constants of 0.6, 2.9 and 8.9 ms. 6. Intraburst open time and total open time distributions for bursts with one to five openings were fitted with two or three exponential functions or gamma distributions, respectively. The number of components, time constants and relative areas were similar for both distributions. 7. The distributions of open times for the nth opening within bursts of k openings were similar for bursts containing two to five openings. The distributions of open times for the nth opening of all bursts varied with position within the burst. The distributions shifted to longer openings as the opening number increased from one to five. 8. The distributions of all closings within all bursts or within bursts with two to five openings and of closings relative to position in all bursts could be fitted by two exponential functions with time constants of about 0.20 and 3.1 ms and relative proportions of 0.55 and 0.45 at all GABA concentrations. 9. The total closed time distributions for bursts containing two to four closings, however, were all best fitted with only a single gamma distribution with a time constant of 1.3 ms.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
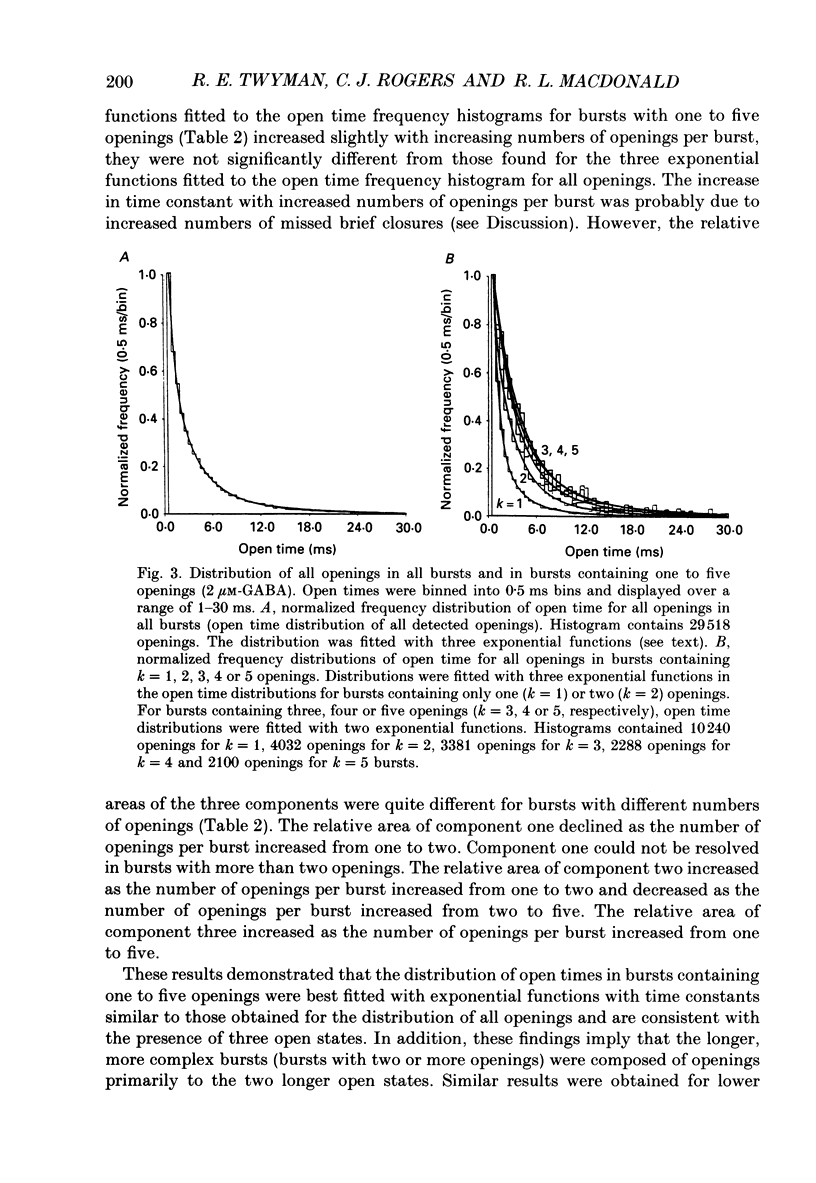
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Clapham D. E. gamma-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988 Mar;11(3):112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Leibowitz M. D. Acetylcholine receptor kinetics. A description from single-channel currents at snake neuromuscular junctions. Biophys J. 1982 Sep;39(3):253–261. doi: 10.1016/S0006-3495(82)84515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Steinbach J. H., Stevens C. F. An analysis of the dose-response relationship at voltage-clamped frog neuromuscular junctions. J Physiol. 1978 Aug;281:421–444. doi: 10.1113/jphysiol.1978.sp012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Sterz R. Determination of dose-response curves by quantitative ionophoresis at the frog neuromuscular junction. J Physiol. 1978 Aug;281:395–419. doi: 10.1113/jphysiol.1978.sp012430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Franke C. Single glutamate-gated synaptic channels at the crayfish neuromuscular junction. II. Dependence of channel open time on glutamate concentration. Pflugers Arch. 1987 Mar;408(3):307–314. doi: 10.1007/BF02181474. [DOI] [PubMed] [Google Scholar]
- Gration K. A., Lambert J. J., Ramsey R. L., Rand R. P., Usherwood P. N. Closure of membrane channels gated by glutamate receptors may be a two-step process. Nature. 1982 Feb 18;295(5850):599–603. doi: 10.1038/295599a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Korenbrot J. I., Maricq A. V. Kinetics of activation of acetylcholine receptors in a mouse muscle cell line under a range of acetylcholine concentrations. Biophys J. 1987 Mar;51(3):449–455. doi: 10.1016/S0006-3495(87)83366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Colquhoun D., Cull-Candy S. G. On the kinetics of large-conductance glutamate-receptor ion channels in rat cerebellar granule neurons. Proc R Soc Lond B Biol Sci. 1988 May 23;233(1273):407–422. doi: 10.1098/rspb.1988.0030. [DOI] [PubMed] [Google Scholar]
- Jackson M. B. Dependence of acetylcholine receptor channel kinetics on agonist concentration in cultured mouse muscle fibres. J Physiol. 1988 Mar;397:555–583. doi: 10.1113/jphysiol.1988.sp017019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A. On the application of "a plausible model" of allosteric proteins to the receptor for acetylcholine. J Theor Biol. 1967 Aug;16(2):306–320. doi: 10.1016/0022-5193(67)90011-2. [DOI] [PubMed] [Google Scholar]
- Kerry C. J., Kits K. S., Ramsey R. L., Sansom M. S., Usherwood P. N. Single channel kinetics of a glutamate receptor. Biophys J. 1987 Jan;51(1):137–144. doi: 10.1016/S0006-3495(87)83318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerry C. J., Ramsey R. L., Sansom M. S., Usherwood P. N. Glutamate receptor channel kinetics: the effect of glutamate concentration. Biophys J. 1988 Jan;53(1):39–52. doi: 10.1016/S0006-3495(88)83064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca P., Rice J. A., Fredkin D. R., Montal M. Kinetic analysis of channel gating. Application to the cholinergic receptor channel and the chloride channel from Torpedo californica. Biophys J. 1985 Apr;47(4):469–478. doi: 10.1016/S0006-3495(85)83939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Internal motions in proteins and gating kinetics of ionic channels. Biophys J. 1988 Jun;53(6):877–884. doi: 10.1016/S0006-3495(88)83168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Rogers C. J., Twyman R. E. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1989 Mar;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. J. gamma-Aminobutyric acid- and piperazine-activated single-channel currents from Ascaris suum body muscle. Br J Pharmacol. 1985 Feb;84(2):445–461. doi: 10.1111/j.1476-5381.1985.tb12929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden D. C., Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):329–355. doi: 10.1098/rspb.1985.0065. [DOI] [PubMed] [Google Scholar]
- Papke R. L., Millhauser G., Lieberman Z., Oswald R. E. Relationships of agonist properties to the single channel kinetics of nicotinic acetylcholine receptors. Biophys J. 1988 Jan;53(1):1–10. doi: 10.1016/S0006-3495(88)83059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom B. R., Neale E., Henkart M., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977 Sep;40(5):1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- Roux B., Sauvé R. A general solution to the time interval omission problem applied to single channel analysis. Biophys J. 1985 Jul;48(1):149–158. doi: 10.1016/S0006-3495(85)83768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F., Neil J., Barkakati N. The automated analysis of data from single ionic channels. Pflugers Arch. 1982 Dec;395(4):331–340. doi: 10.1007/BF00580798. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Hamill O. P., Bormann J. Patch-clamp measurements of elementary chloride currents activated by the putative inhibitory transmitter GABA and glycine in mammalian spinal neurons. J Neural Transm Suppl. 1983;18:83–95. [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by low concentrations of agonist. J Physiol. 1986 Apr;373:129–162. doi: 10.1113/jphysiol.1986.sp016039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Agonists block currents through acetylcholine receptor channels. Biophys J. 1984 Aug;46(2):277–283. doi: 10.1016/S0006-3495(84)84022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D. S., Barnes E. M., Jr, Hablitz J. J. Whole-cell and single-channel recordings of GABA-gated currents in cultured chick cerebral neurons. J Neurophysiol. 1988 Feb;59(2):495–513. doi: 10.1152/jn.1988.59.2.495. [DOI] [PubMed] [Google Scholar]
- Yamamoto D., Suzuki N. Blockage of chloride channels by HEPES buffer. Proc R Soc Lond B Biol Sci. 1987 Feb 23;230(1258):93–100. doi: 10.1098/rspb.1987.0011. [DOI] [PubMed] [Google Scholar]



