Abstract
Study Design
Retrospective Study.
Objectives
Minimally invasive endoscopic spinal surgery is gaining popularity, but our understanding of the lumbar spine's microvascular geometry relies heavily on cadaver studies and textbook illustrations. Additionally, inconsistent nomenclature of vessels in the literature hampers effective communication among surgeons. This study aims to improve the clarity and comprehensibility of the lumbar spinal microvascular geometry under endoscopic view.
Methods
The study included 400 patients who underwent endoscopic spinal surgery for lumbar spinal canal stenosis and foraminal stenosis. The surgeries were performed by an experienced surgeon using either the interlaminar or transforaminal approach. Endoscopic video recordings were further analyzed to map the microvascular geometry and common bleeding foci. The observed results were cross-referenced with existing literature to reconstruct a comprehensive view of the vascular anatomy.
Results
The transforaminal approach commonly encounters bleeding foci originating from the major branches of the segmental lumbar artery and the emissary veins within the foramen. The interlaminar approach primarily encounters bleeding foci from the muscle vessels in the dorsal lamina, which are believed to be located near the ends of the three main branches. In the intracanal region, epidural vessels form a rotary loop above the disc, which can contribute to most of the bleeding during discectomy.
Conclusions
This study provides a comprehensive understanding of the microvascular anatomy in the lumbar spine during endoscopic spinal surgery. Recognizing the geometry will help surgeons anticipate and control bleeding, reducing the risk of complications. The findings contribute to the improvement of surgical techniques and patient safety in endoscopic spinal surgery.
Keywords: anatomy, lumbar, spinal canal, blood supply, endoscopic spine surgery
Introduction
The technique of spinal surgery has undergone significant evolution from traditional open spine surgery to minimally invasive spine surgery, with endoscopic surgery gaining popularity in recent decades. Although initially limited to lumbar disc herniations (PELD), 1 endoscopic techniques have expanded to include disc herniations at other levels, spinal canal stenosis, fusion surgeries, and numerous proposed applications.2-7
While literature exists on the gross surgical anatomy of vessels in the lumbar spine, information on microvascular vessels is limited. Prior studies have focused on vessels around the vertebral body or spinal cord and their anatomical variation between each level, mainly the lumbar segmental arteries and spinal arteries and veins, with little mention of vessels frequently encountered during endoscopic surgery within the interlaminar space, spinal canal, or foramen. The lack of detailed knowledge of microvascular structure has made it difficult to identify and control bleeding under endoscopic spine surgery. Massive oozing with an unknown source can be challenging for inexperienced surgeons, and bleeding conditions can be overlooked, leading to major complications, such as hematoma, neural injury and even death. Therefore, recognizing microvascular geometry under endoscopic vision has become increasingly important in preventing heavy bleeding and modifying surgical procedures.
Despite recent efforts in detailing microanatomy, the lack of standardized nomenclature for certain vessels has hindered comprehension and effective communication among surgeons. Furthermore, these studies often rely on illustrated concept figures or cadaver-based research, which can pose challenges in terms of practical application. In clinical settings of minimally invasive spinal surgery, the exact source of vascular structures become less crucial. Surgeons are primarily concerned and aim to understand where these structures are encountered during the procedure, whether they might cause complications, and how to prevent or address such issues. This shift from theoretical nomenclature to practical surgical considerations underlines the core of our study.
In this article, the primary objective is to improve the clarity and comprehensibility of the microvascular geometry in the lumbar spine under endoscopic surgery. Furthermore, we aim to bridge the gap between literature and clinical practice by cross-referencing the anatomical studies and endoscopic views so as to achieve easier application of these knowledge into real surgery. Through this process, we could facilitate the control and prevention of heavy bleeding during endoscopic surgery and decrease the related complications.
Material and Methods
A total of 400 patients who underwent endoscopic spinal surgery for lumbar spinal canal stenosis and foraminal stenosis were enrolled in this study, excluding revision and infection cases. The surgeries were conducted by the same experienced surgeon using either the interlaminar or transforaminal approach, with careful attention to avoiding any additional soft tissue damage. To map the microvascular geometry, endoscopic video recording clips from 200 cases of each approach were reviewed. The route of the vessels was traced, and common bleeding foci were recorded and analyzed. The microvascular geometry and bleeding foci were then cross-referenced with existing textbook figures and literature to reconstruct a comprehensive view of the vascular anatomy, which was paired with the endoscopic view. All procedures were recorded and verified by the operating surgeon, and reviewed by three additional observers to ensure accuracy and consistency.
The endoscopic procedure utilized in this study was the unilateral biportal endoscopic spinal surgery technique. For the extraforaminal microvascular geometry, the transforaminal approach was used with an incision made 3 cm lateral to the lateral pedicle line in line with the transverse process. For the interlaminar and epidural microvascular geometry, the interlaminar approach was used with an incision made at the interlaminar space. Details of the endoscopic spinal surgery technique are illustrated in previous articles.8,9 All procedures were performed in accordance with the ethical standards of the Soon Chun Hyang University Hospoital Institutional Review Board of Human Study Committee and with the 1964 Helsinki declaration and its later amendments. The IRB approval number is 2023-09-01. Informed consent was obtained from all participants included in the study.
Results
The observed results are categorized according to the approach methods and under the sequence of the operation flow:
Transforaminal Approach
Via the more common left side outside-in transforaminal approach, we often encounter five bleeding foci originating from the major branches of the segmental lumbar artery and the emissary veins within the foramen. As the procedure begins with the creation of endoscopic portals, a frequent docking site for the endoscope and instrument is the transverse process of the index vertebra. Excessive dissection within the soft tissue above can introduce the first common bleeding focus, which is the inferior articular artery originating from the proximal adjacent level. The bleeding is typically observed from the proximal transverse process in the 9-10 o’clock direction of the endoscopic view (Figure 1, Supplement 1).
Figure 1.
The bleeding from inferior articular artery is typically observed from the proximal transverse process in the 9-10 o’clock direction of the endoscopic view.
Moving on to the subsequent step, as we navigate from the transverse process towards Kambin’s triangle, we traverse the medial and distal portions of the accessory process, eventually reaching the isthmus. At the 12 to 2 o'clock direction, a frequently encountered bleeding focus arises, where the superior articular artery ascends over the facet joint from ventral to dorsal (Figure 2, Supplement 2). Typically, this artery remains concealed beneath the muscle bundle and is less directly observed. Based on our experience, it is less prone to injury compared to other bleeding foci. This is because the endoscope and instrument usually traverse the facet joint and directly access the ventral side without disturbing the dorsal soft tissue. Bleeding is more commonly observed when the lateral and dorsal aspects of the joint sustain injury.
Figure 2.
At the 12 to 2 o’clock direction, the superior articular artery ascends over the facet joint, causing a common bleeding focus.
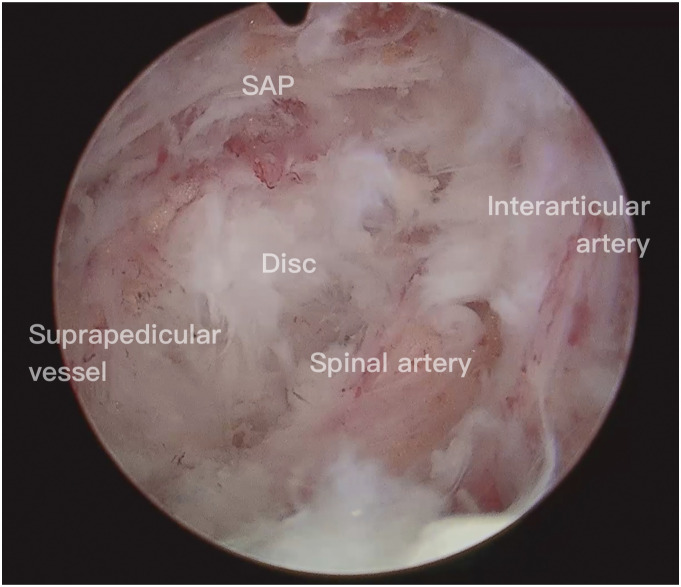
In order to access the neuroforamen and perform decompression of the exiting nerve root, a partial resection of the superior articular process (SAP) is typically required. To fully expose the tip of the SAP, capsulotomy at the craniolateral facet joint is sometimes mandatory. During the dissection process, caution must be exercised to avoid injury to the interarticular artery, which is situated between the craniolateral capsule and the dense fascia connecting the facet and isthmus (Figure 3, Supplement 3). Injury to this artery constitutes one of the frequently encountered bleeding foci.
Figure 3.
The interarticular artery is situated between the craniolateral capsule and the dense fascia connecting the facet and isthmus.
The two remaining common sources of bleeding are situated in the cranial and caudal regions of the intervertebral foramen. These bleeding sources consist of perineural vessels surrounding the exiting root and suprapedicular vessels running along the cranial edge of the lower pedicle. The suprapedicular vessels encompass the anterior spinal canal branch of the segmental lumbar artery and the suprapedicular emissary vein. These vessels can typically be identified after ventral plane resection of the SAP and adhesiolysis around the caudal portion of the neuroforamen, thereby exposing the cranial margin of the lower pedicle (Figure 4a, Supplement 4). By employing appropriate coagulation techniques, bleeding can be effectively prevented during exploration of this area. Lastly, bleeding from the perineural vessels represents the final commonly encountered bleeding focus. This bleeding occurs primarily when excessive or careless stretching is applied around the exiting root, particularly during perineural adhesiolysis and diskectomy. The perineural vessels comprise the spinal artery, its branching vessels, and the perineural emissary veins.
Figure 4.
Suprapedicular vessels are typically identifiable following ventral plane resection of the SAP, revealing the cranial margin of the lower pedicle.
Upon close observation, a rotary vessel loop or an anastomotic latticework of vessels above the disc space can be noticed (Figure 4(b)). Before performing any invasive procedures on the disc, such as diskectomy, it is suggested to perform a circular prophylactic coagulation on this structure to prevent bleeding. In cases where the axilla of the exiting nerve root will be further examined, such as in the case of diskectomy in an upper migrated disc, prophylactic coagulation around the axillary fat is also recommended, as the route of the perineural emissary veins is commonly concealed in this area.
Interlaminar Approach
The interlaminar space, located outside the spinal canal, is typically considered an avascular region primarily occupied by the ligamentum flavum and interspinous ligament. Therefore, any bleeding encountered during the interlaminar approach, prior to entering the spinal canal, is presumed to originate from the surrounding muscle vessels. Following the successful docking on the interlaminar space above the superficial ligamentum flavum using the left side interlaminar approach, three common bleeding foci are commonly observed, emanating from the 9, 7, and 5 o’clock directions.
The predominant source of bleeding during this stage is typically the muscle on the dorsal lamina at the 9 o'clock direction. It is believed that the bleeding originates from the interarticular artery, which spans a significant portion of the dorsal lamina area (Figure 5, Supplement 5). Comparatively, the bleeding foci at the 7 o’clock and 5 o'clock directions are relatively minor in nature when compared to the bleeding observed at the 9 o’clock direction. The 7 o'clock direction within the interlaminar space corresponds to the location of the facet joint, and it is believed that the bleeding arises from the superior articular artery (Figure 6, Supplement 6). As for the bleeding focus at the 5 o'clock direction, it is located in the recess between the inferior articular process (IAP) and the SAP of the distal adjacent level. Alternatively, it can be found at the base of the SAP. This is the specific location where the terminal branch of the inferior articular artery is typically located (Figure 7, Supplement 7).
Figure 5.
The main source of bleeding while exploring the interlaminar space is usually the muscle on the dorsal lamina at 9 o’clock, believed to originate from the interarticular artery.
Figure 6.
Bleeding at the 7 o’clock position is attributed to the superior articular artery.
Figure 7.
Regarding the bleeding focus at the 5 o’clock position, it is situated between the IAP and the SAP of the distal adjacent level, which is the location of the terminal branch of the inferior articular artery.
To gain access to the spinal canal, the deep ligamentum flavum is removed through a 3 mm cranial laminotomy of the lower vertebra, where the deep ligamentum flavum is attached. Despite the smaller vessel diameters within the spinal canal, the vessel structures are more clearly visible due to the absence of soft tissue obstruction within the vacant epidural space. By performing the 3 mm cranial laminotomy of the lower lamina, the crossing longitudinal antero-external epidural vein, intersecting with the traversing root at the lateral recess, can be exposed (Figure 8, Supplement 8). As the laminotomy is extended laterally, the suprapedicular vessels, including the bifurcated suprapedicular emissary veins from the longitudinal antero-external epidural vein, and the anterior spinal canal branch of the segmental lumbar artery, become visible along the cranial margin of the lower pedicle (Figure 9, Supplement 9). While we observed the ipsilateral side of the canal through the interlaminar approach, we are presented with a inferomedial side view of the structure that is observed in the transforaminal approach. From this viewpoint, a more direct observation of structures at the lateral recess becomes possible. These include the upper and lower laminar spur, crossing vessels, traversing root, and the herniated disc at the lateral recess. In contrast, the exiting root and perineural vessels are situated deeper in a cranial direction beneath the upper lamina, predominantly in the 9 o'clock direction, and are primarily concealed within the axillary fat (Figure 10). For the contralateral side of the epidural space, we can observe a mirror reflection of the rotary vessel loop as viewed from the transforaminal approach (Figure 11, Supplement 10). Prior to undertaking any invasive procedures, it is crucial to anticipate the location of the vessels and be prepared for the possibility of bleeding.
Figure 8.
Performing a 3 mm cranial laminotomy of the lower lamina exposes the crossing longitudinal antero-external epidural vein, which intersects with the traversing root at the lateral recess.
Figure 9.
Extending the laminotomy laterally reveals the suprapedicular vessels, including the bifurcated suprapedicular emissary veins from the longitudinal antero-external epidural vein, as well as the anterior spinal canal branch of the segmental lumbar artery, along the cranial margin of the lower pedicle.
Figure 10.
The ipsilateral view of the epidural space.
Figure 11.
The contralateral side of the epidural space, we can observe a mirror reflection of the rotary vessel loop as viewed from the transforaminal approach.
Discussion
The Microvascular Geometry
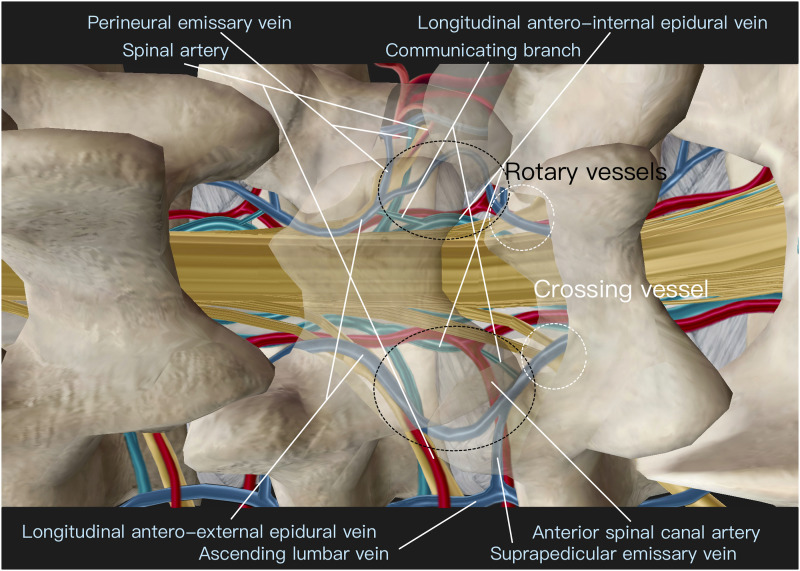
A range of vessel names has been used in the existing literature to refer to the vessels in the extraforaminal area and spinal canal. In an effort to enhance communication among researchers, we conducted a thorough review of the literature and proposed a unified nomenclature. Table 1 presents the vessel names we employed, along with the names found in other relevant studies. By integrating our observations from endoscopy with existing literature, we can envision and reconstruct the microvascular anatomy commonly encountered in the vertebral posterior column, both in the extra canal area (Figure 12) and the intracanal region (Figure 13). Following the bifurcation of the segmental lumbar artery from the Aorta, the first branch typically corresponds to the spinal artery, which supplies the exiting nerve root. In certain individuals, there may also be additional small ganglion branches that supply the root ganglion. 10 Following the bifurcation of the spinal artery branch, two additional arteries commonly run medially into the neuroforamen, known as the anterior and posterior spinal canal branches of the segmental lumbar artery. Subsequently, the segmental lumbar arteries of L1-L4 emit an anastomotic branch towards the lower adjacent neuroforamen. This anastomotic branch travels 4 mm ventral to the transverse process of the lower-level vertebra and subsequently enters the neuroforamen at a lower level, forming an anastomosis.11,12 Following the anastomotic branch, the segmental lumbar artery undergoes bifurcation into two primary branches: the lateral branch and the dorsal branch. The lateral branch courses along the caudal aspect of the transverse process, traversing through the psoas and quadratus lumborum muscles. Eventually, it supplies the lumbar plexus and the muscles of the abdominal wall. 13 This branch is less relevant to posterior-based endoscopic surgery.
Table 1.
Nomenclature of the Vessels in Current Literatures.
| Study | Year | Spinal Artery | Anterior Spinal Canal Branches | Posterior Spinal Canal Branches | Anastomotic Branch | Dorsal Branch | Lateral Branch | Longitudinal Antero-External Epidural Vein | Longitudinal Antero-Internal Epidural Vein |
|---|---|---|---|---|---|---|---|---|---|
| Crock et al | 1975 | Intermediate branches | Posterior branches | Anterior branches | |||||
| Nervous system branches | Anterior spinal canal branches | Posterior spinal canal branches | |||||||
| Roland et al | 1978 | Longitudinal antero-external epidural vein | Longitudinal antero-internal epidural vein | ||||||
| Ratcliffe et al | 1980 | Spinal artery | Communicating artery | ||||||
| Caglar et al | 2004 | Spinal branch | Anastomotic branch | ||||||
| Arslan et al | 2011 | Spinal artery | Anastomotic branch | Dorsal branch | Lateral trunk | ||||
| Clinical and Radiological anatomy of the lumbar spine 5th edition | 2012 | Medially directed branches | Posteriorly directed branch | Lateral branches | Posterior internal vertebral venous plexus | Anterior internal vertebral venous plexus | |||
| Radicular branch | Anterior spinal canal branches | Posterior spinal canal branches | |||||||
| Min et al | 2013 | Intertransverse artery | Anterior transverse artery | ||||||
| Benzel’s spine surgery 4th edition | 2014 | Spinal branch | Dorsal branch | Anterior transverse artery | |||||
| Nojiri et al | 2016 | Spinal artery | Anastomotic branch | ||||||
| Gregg et al | 2017 | Radicular artery | Dorsal branch | Lumbar artery | |||||
| Liu et al | 2018 | Intersegmental branch | |||||||
| Uchikado et al | 2019 | Spinal branch | Anastomotic branch | Dorsal branch | Lateral trunk | Longitudinal antero-external epidural vein | Longitudinal antero-internal epidural vein | ||
| Tatara et al | 2019 | Spinal nerve branch | Vertebral branch | Dorsal branch | Lateral branches | ||||
Figure 12.
The imagined and reconstructed microvascular anatomy of the extra-canal area.
Figure 13.
The imagined and reconstructed microvascular anatomy of the intra-canal area.
The dorsal branch then continues its course towards the dorsal lamina and plays a role in the endoscopic surgery. Upon reaching the lamina, the dorsal branch further divides into three branches: the interarticular artery, the superior articular artery, and the inferior articular artery.14-16 The interarticular artery follows a direct path towards the posterior lamina at the pars interarticularis, also known as the isthmus. It is typically concealed beneath the thick fascia located between the tip of the SAP and the isthmus. Upon reaching the dorsal side of the lamina, it proceeds medially towards the proximal interlaminar space, entering the dorsal muscles. From there, it continues its medial and posterior course, closely adhering to the middle of each spinous process. The interarticular artery forms an intricate network of interconnected vessels known as an open meshed plexus, which serves as the primary arterial supply to the paraspinal muscles located above the dorsal lamina. This network of vessels communicates with other main branches, ensuring adequate blood supply to the surrounding tissues.14,17 The superior and inferior articular arteries contribute significantly to the vascularization of the facet joint and surrounding tissues. The superior articular artery ascends directly over the facet joint and then penetrates into the dorsal muscle. On the other hand, the inferior articular artery travels distally along the lateral side of the facet, crosses the proximal portion of the transverse process, encircling the facet joint, and finally dives medially into the paraspinal muscle.
The vessels observed within the intracanal region are believed to originate from both the arterial and venous systems. However, distinguishing between these vessels can be challenging as they are often found at their terminal branches. The vessels that we observe and associate with the arterial system are assumed to be part of the anterior spinal canal branch of the segmental lumbar artery. These vessels enter the neuroforamen at the caudal region of the intervertebral foramen and follow along the cranial margin of the lower pedicle. They then bifurcate into ascending and descending branches, which form an anastomosis with the descending and ascending branches of the upper and lower levels, respectively, near the outer one-third of the intervertebral disc. In contrast, arteries within the posterior canal are typically not visible during endoscopic surgery. This is mainly due to their smaller size and tendency to penetrate into the intraosseous structure of the lamina at an earlier stage. 17
The veins observed during the surgery can be broadly categorized into longitudinal epidural veins and communicating emissary veins. The longitudinal epidural veins consist of two pairs: the longitudinal antero-external epidural veins in the posterior canal and the longitudinal antero-internal epidural veins in the anterior canal. The longitudinal antero-external epidural veins are typically located more laterally around the junction of the lateral recess and foramen. The longitudinal antero-internal epidural veins are situated more ventrally and medially. One is usually concealed under the dura, while the other is commonly observed next to the lateral margin of the dura. Additionally, there are multiple communicating branches between the longitudinal antero-external epidural veins and the antero-internal epidural veins.
The emissary veins are typically found at the cranial and caudal borders of the intervertebral foramen, namely the subpedicular region and the suprapedicular region, respectively. The emissary veins in the subpedicular region are referred to as perineural emissary veins. They branch off from the longitudinal epidural veins around the axillary region of the exiting root hidden inside the axillary fat, and run laterally through the neuroforamen alongside the exiting root. On the other hand, the suprapedicular emissary veins often bifurcate from the longitudinal antero-external epidural vein above the traversing root at the lateral recess, with this converging point usually hidden under the proximal margin of the lower lamina. They course along the cranial margin of the lower pedicle and exit the neuroforamen. The ascending lumbar vein receives drainage from these emissary veins via the neuroforamen, following a rostral path along the anterior aspect of the transverse processes. Eventually, these veins connect the iliac veins with the azygous system. Within the epidural space, these arterial and venous systems form a rotary vessel loop at the anterior spinal canal above the disc space at each level. 18
Clinical Meanings
Our understanding of the lumbar spinal artery and its branches has remained relatively unchanged for centuries. However, the microvascular geometry of these structures has not been well illustrated and exhibits slight variations depending on the studies. Most of the literature on the microvascular structure is based on angiography, phlebography, or cadaveric studies. In vivo imaging is less common and more challenging to obtain. With the advent of endoscopic surgery, we have entered a new era of exploring the microstructure. Initially, in uniportal endoscopic spine surgery for discectomy, such as percutaneous endoscopic discectomy (PED), the inside-out approach utilized Kambin's triangle, which is a relatively safe region as the main arterial branches are located more proximal to the disc space. However, as the field of endoscopic surgery expanded, the floating technique, known as the outside-in approach, gained more attention. This technique allows surgeons to access areas beyond Kambin's triangle. With the advancement of the surgical field, the risk of vessel injury increased.
In the extra canal area, arteries are often concealed within the paraspinal muscle. Their presence may only become apparent in the event of vessel rupture during surgery, resulting in significant bleeding that hampers the surgical field. During the interlaminar approach from the left side, the major branch of the arteries are typically situated at the 9, 7, and 5 o'clock positions, with the 9 o'clock position potentially being the primary source of bleeding. Avoiding interference with the dorsal muscle at the 9 o'clock direction can considerably reduce the bleeding rate associated with this approach. In the transforaminal approach, minimizing excessive soft tissue dissection around the proximal transverse process can decrease the likelihood of rupture of the inferior articular artery. Upon reaching the Kambin's triangle, bleeding from the 2 o’clock position can occur due to injury to the superior articular artery. Efficient control of bleeding can be achieved by cauterizing the upper edge of the lateral surface of the facet if the bleeding focus is not immediately identified. When accessing the isthmus and SAP tip, special care must be taken to avoid damaging the interarticular artery, as it can result in severe bleeding. The SAP can be resected while preserving the interarticular artery. However, it is important to note that the interarticular artery is often located just above the exiting root. Cauterization is frequently required to thoroughly inspect the exiting root and foramen. Prophylactic coagulation prior to accessing the deeper foramen is a recommended practice to mitigate the risk of inadvertent arterial rupture, thereby minimizing prolonged coagulation time, disorientation, and compromised surgical field visibility.
Uncontrolled intraoperative bleeding caused by mechanical injury to arterial branches can result in the formation of a symptomatic hematoma. An epidural hematoma predominantly occurs following an interlaminar approach. With the increasing utilization of endoscopic surgery for spinal stenosis and intervertebral discectomy, there has been a corresponding rise in the incidence of associated complications. Although the occurrence of symptomatic postoperative epidural hematoma is relatively rare (.02%–4.6%), its potential consequences are grave, encompassing cauda equina syndrome and even lower limb paralysis.19,20 Notably, albeit infrequent, hematomas can arise as a result of vessel injury during the full endoscopic transforaminal approach.21,22 Minor bleeding typically poses no significant issues and can be managed conservatively. However, when the main branches of the segmental artery sustain damage, the development of a substantial retroperitoneal hematoma becomes probable, precipitating the manifestation of symptomatic outcomes. To avoid blood vessel damage, it is important to carefully evaluate the trajectory of needle and inserted it into the relatively safe avascular area by touching the bone of the facet in the safety zone during the inside-out approach. 23 For the outside-in transforaminal approach, comprehending and recognizing the microvascular geometry will be even important to avoid unnecessary injury to the blood vessels and get to know when to do prophylactic cauterization to decrease the bleeding amount. In addition, because damaged blood vessels may not be seen well in the field of view of endoscopic surgery when the endoscope is removed after the surgery, it is necessary to perform a well bleeding control during surgery. In cases of severe bleeding with the endoscopic vision completely hindered by the oozing, cauterization blindly with prediction of the bleeding focus is possible with a good comprehension of microvascular geometry.
In the intracanal area, although the bleeding is comparatively less severe, it can still lead to persistent blurring of the endoscopic vision and prolong the duration of the operation, including anesthesia time. Having a thorough understanding of the vessel structure within the canal can further reduce the tendency for bleeding and enhance the efficiency and smoothness of the surgical procedure. Furthermore, the intracanal vessels can serve as valuable microstructural landmarks for reference during the operation. For instance, the presence of a crossing vessel can indicate that the surgeon has reached the lateral recess situated above the traversing root, medial to the lower pedicle. This finding suggests that a lower laminotomy is sufficient. Identifying a rotary vessel can provide a crucial clue that the intervertebral disc lies just beneath that specific region. Tracing the path of an emissary vein can guide the surgeon towards the direction of the neuroforamen. These landmarks, although relatively straightforward to navigate during a primary surgery, become even more valuable in cases of severe degeneration or revision surgery. They offer essential hints for correct orientation and surgical approach. By incorporating a comprehensive understanding of the intracanal vessel anatomy, surgeons can effectively minimize bleeding, optimize visualization, and streamline the operative flow. Additionally, these anatomical landmarks serve as valuable cues in complex surgical scenarios, contributing to improved surgical outcomes.
Moreover, in contrast to the uniportal endoscopic spinal surgery, where the right-side approach mirrors the left side approach, the biportal endoscopic spinal surgery offers a slightly altered perspective due to variations in the craniocaudal trajectory between left and right. Specifically, during the transforaminal approach, upon entering Kambin's triangle and adopting a more caudal viewpoint, the interarticular artery, spinal artery, and exiting root can share the same running direction, albeit at distinct horizontal levels (Figure 14, Supplement 11). Notably, the interarticular artery may appear as a vessel running from the base of the screen to the right, leading to potential confusion with the spinal artery. Mistaking the interarticular artery for the spinal artery can impede further dissection and result in failure to locate the target root, potentially misidentifying a protruded disc as an exiting root.
Figure 14.
During the right side transforaminal approach, the interarticular artery, spinal artery, and exiting root can share the same running direction.
The surgical view during endoscopic surgery differs from the vertical orientation typically seen in open surgery. It is often under an oblique angle and can vary depending on the lens used. As a result, the actual appearance of vascular structures in the surgical field may differ from illustrations or anatomical descriptions based on cadaveric studies or radiographic images in anteroposterior or lateral views. This article aims to bridge the gap between the clinical view observed during endoscopy and the anatomical knowledge reported in the literature. By gaining a better understanding of the microvascular geometry, surgeons can effectively control bleeding during endoscopic surgery without excessive soft tissue damage or prolonged anesthesia time.
Conclusions
This study combines the existing angiography, phlebography and cadaveric studies with in vivo endoscopic findings to delineate the microvascular geometry in detail. Through the complete understanding of the vascular distribution and the common bleeding foci under endoscope, not only can we effectively control the bleeding but also prevent devastating complications. Based on the current study, surgical technique could be modified as well to avoid the bleeding foci and improve the surgical efficiency.
Supplemental Material
Video 1. Supplement 1
Video 2. Supplement 2
Video 3. Supplement 3
Video 4. Supplement 4
Video 5. Supplement 5
Video 6. Supplement 6
Video 7. Supplement 7
Video 8. Supplement 8
Video 9. Supplement 9
Video 10. Supplement 10
Video 11. Supplement 11
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Tsung-Mu Wu https://orcid.org/0000-0002-0540-6283
Dae-Jung Choi https://orcid.org/0000-0002-4047-0088
Wen-Shuo Chang https://orcid.org/0000-0002-9934-6970
References
- 1.Mayer HM, Brock M. Percutaneous endoscopic lumbar discectomy (PELD). Neurosurg Rev. 1993;16:115-120. [DOI] [PubMed] [Google Scholar]
- 2.Nam HGW, Kim HS, Lee DK, Park CK, Lim KT. Percutaneous stenoscopic lumbar decompression with paramedian approach for foraminal/extraforaminal lesions. Asian Spine J. 2019;13:672-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heo DH, Son SK, Eum JH, Park CK. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: technical note and preliminary clinical results. Neurosurg Focus. 2017;43:E8. [DOI] [PubMed] [Google Scholar]
- 4.Kim JE, Choi DJ. Biportal endoscopic transforaminal lumbar interbody fusion with arthroscopy. Clin Orthop Surg. 2018;10:248-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sairyo K, Morimoto M, Yamashita K, et al. Full-endoscopic trans-Kambin’s triangle lumbar interbody fusion: technique and review of literature. J Minim Invasive Spine Surg Tech. 2021;6:S123-S129. [Google Scholar]
- 6.Kim JE, Choi DJ, Jin JP, et al. Biportal Endoscopic Spinal Surgery for Lumbar Spinal Stenosis. Asian Spine J 2019;13(2):334-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi DJ, Kim JE, Jung JT, et al. Biportal Endoscopic Spine Surgery for Various Foraminal Lesions at the Lumbosacral Lesion. Asian Spine J 2018;12(3):569-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi CM, Chung JT, Lee SJ, Choi DJ. How I do it? Biportal endoscopic spinal surgery (BESS) for treatment of lumbar spinal stenosis. Acta Neurochir. 2016;158:459-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn JS, Lee HJ, Choi DJ, Lee KY, Hwang SJ. Extraforaminal approach of biportal endoscopic spinal surgery: a new endoscopic technique for transforaminal decompression and discectomy. J Neurosurg Spine. 2018;28:492-498. [DOI] [PubMed] [Google Scholar]
- 10.Arslan M, Comert A, Acar HI, et al. Surgical view of the lumbar arteries and their branches: an anatomical study. Neurosurg. 2011;68:16-22. [DOI] [PubMed] [Google Scholar]
- 11.Caglar S, Dolgun H, Ugur HC, et al. Extraforaminal lumbar arterial anatomy. Surg Neurol. 2004;61:29-33. [DOI] [PubMed] [Google Scholar]
- 12.Ratcliffe JF. The arterial anatomy of the adult human lumbar vertebral body: a microarteriographic study. J Anat. 1980;131:57-79. [PMC free article] [PubMed] [Google Scholar]
- 13.Tatara Y, Nasu H, Tsutsumi M, Akita K. Origins, courses, and distributions of the lumbar arterial branches in relation to the spinal nerves: an anatomical study. Spine (Phila Pa 1976). 2019;44:E808-E814. [DOI] [PubMed] [Google Scholar]
- 14.Steinmetz Mecb MP. Benzel’s spine surgery 4th. 2017;79:7. [Google Scholar]
- 15.Nava-Dimaano H-D. Put it into practice: the unilateral biportal endoscopic surgery. In: Quillo-Olvera J, Quillo-Olvera D, Quillo-Reséndiz J, Mayer M, eds. Unilateral Biportal Endoscopy of the Spine: An Atlas of Surgical Techniques. Cham: Springer International Publishing; 2022:149-181. [Google Scholar]
- 16.Oh YM, Choi HY, Eun JP. Delayed retroperitoneal hemorrhage due to lumbar artery pseudoaneurysm after lumbar posterolateral fusion. J Korean Neurosurg Soc. 2013;54:344-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crock HV, Yoshizawa H. The blood supply of the lumbar vertebral column. Clin Orthop Relat Res. 1976:6-21. [PubMed] [Google Scholar]
- 18.Roland J, Treil J, Larde D, Picard L, Manelfe C. Lumbar phlebography in the diagnosis of disc herniations. J Neurosurg. 1978;49:544-550. [DOI] [PubMed] [Google Scholar]
- 19.Kim JE, Choi DJ, Park EJ. Evaluation of postoperative spinal epidural hematoma after biportal endoscopic spine surgery for single-level lumbar spinal stenosis: clinical and magnetic resonance imaging study. World Neurosurg. 2019;126:e786-e792. [DOI] [PubMed] [Google Scholar]
- 20.Kim JE, Choi DJ, Kim MC, Park EJ. Risk factors of postoperative spinal epidural hematoma after biportal endoscopic spinal surgery. World Neurosurg. 2019;129:e324-e329. [DOI] [PubMed] [Google Scholar]
- 21.Ahn Y, Kim JU, Lee BH, et al. Postoperative retroperitoneal hematoma following transforaminal percutaneous endoscopic lumbar discectomy: clinical article. J Neurosurg Spine. 2009;10:595-602. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Ju CI, Kim SW, Kim JG. Huge psoas muscle hematoma due to lumbar segmental vessel injury following percutaneous endoscopic lumbar discectomy. J Korean Neurosurg Soc. 2009;45:192-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn Y. Transforaminal percutaneous endoscopic lumbar discectomy: technical tips to prevent complications. Expert Rev Med Devices. 2012;9:361-366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Supplement 1
Video 2. Supplement 2
Video 3. Supplement 3
Video 4. Supplement 4
Video 5. Supplement 5
Video 6. Supplement 6
Video 7. Supplement 7
Video 8. Supplement 8
Video 9. Supplement 9
Video 10. Supplement 10
Video 11. Supplement 11