Abstract
1. The whole-cell and outside-out patch configurations of the patch-clamp technique were used to study the mechanisms of block produced by external tetraethylammonium ions (TEA+) and quinine on delayed rectifying K+ channels in mouse pancreatic beta-cells. 2. In whole-cell recordings, TEA+ blocks the delayed outward current (which reflects the activity of delayed rectifying K+ channels by greater than 85%) in a concentration-dependent manner. The block appeared to be 1:1 with a Kd of approximately 1.4 mM at a membrane potential of 0 mV. The value of Kd varied with the membrane potential and there was an e-fold increase for a 70 mV depolarization. 3. Single-channel recordings revealed that delayed rectifying K+ channels have a unitary conductance of 8.5 pS ([K+]1 = 155 mM; [K+]o = 5.6 mM) and a single-channel K+ permeability of 2.8 X 10(-14) cm3 s-1. 4. First latency histograms of channel openings during voltage pulses from -70 to 0 mV peaked after 4 ms. A reaction scheme involving two closed states adequately but not perfectly described the distribution of the first latencies. The openings of the channels were grouped in bursts and the distribution of the closed times required two exponentials with time constants of 2.0 and 13 ms, respectively. The distribution of the open times could be described by a single exponential with a time constant of 25 ms. 5. Channel block produced by TEA+ (1 mM) was associated with a 40% decrease of the single-channel current amplitudes and a reduction in single-channel K+ permeability to 1.9 X 10(-14) cm3 s-1 but did not measurably affect the single-channel kinetics suggesting that the blocking reaction is very rapid. 6. Quinine blocked the whole-cell delayed outward current in a concentration-dependent manner. Half-maximal inhibition was attained at approximately 4 microM and the binding appeared to be 2:1. 7. Single-channel recordings indicated that the inhibition produced by quinine (10 microM) resulted from a decrease in the duration of the openings to a mean value of 6.7 ms. The time constants for the distribution of the closures were increased by approximately 30%. Quinine did not affect the amplitude of the openings. The rate constant of the blocking reaction (kB) was 15 mM-1 ms-1 at 0 mV.
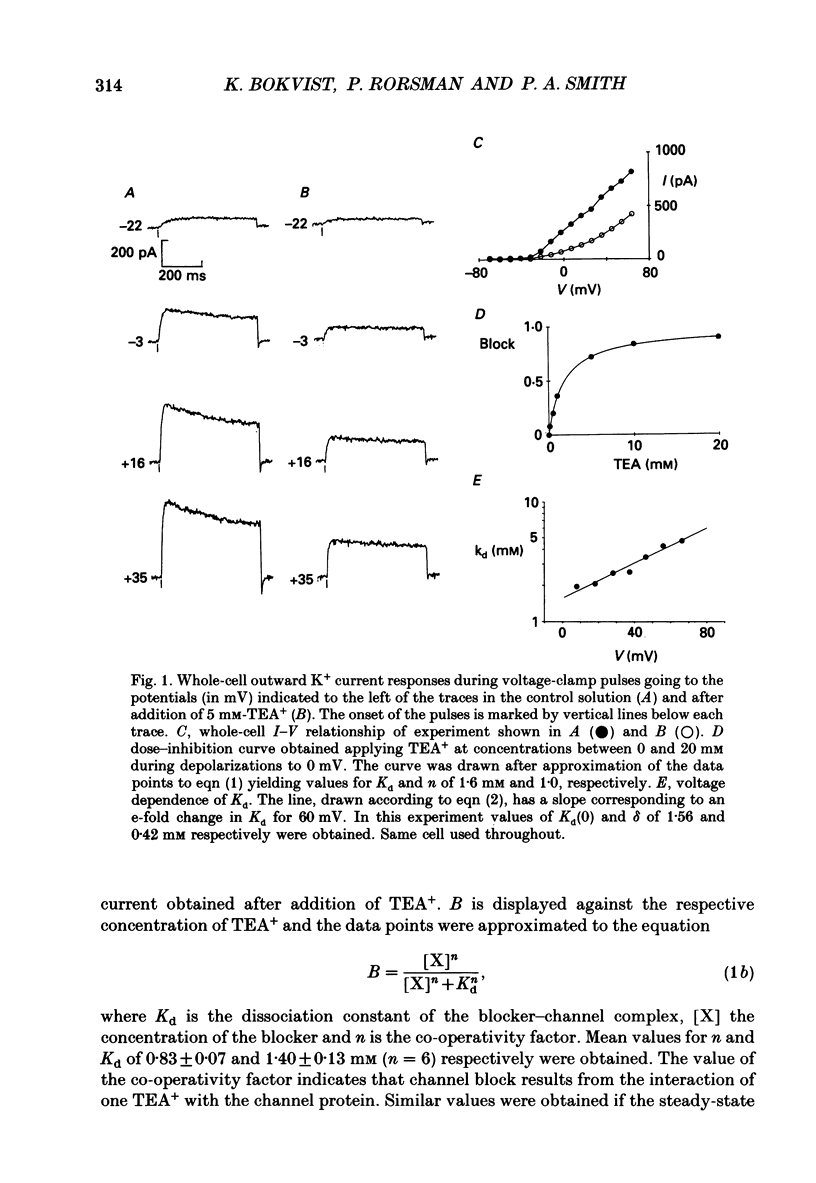
Full text
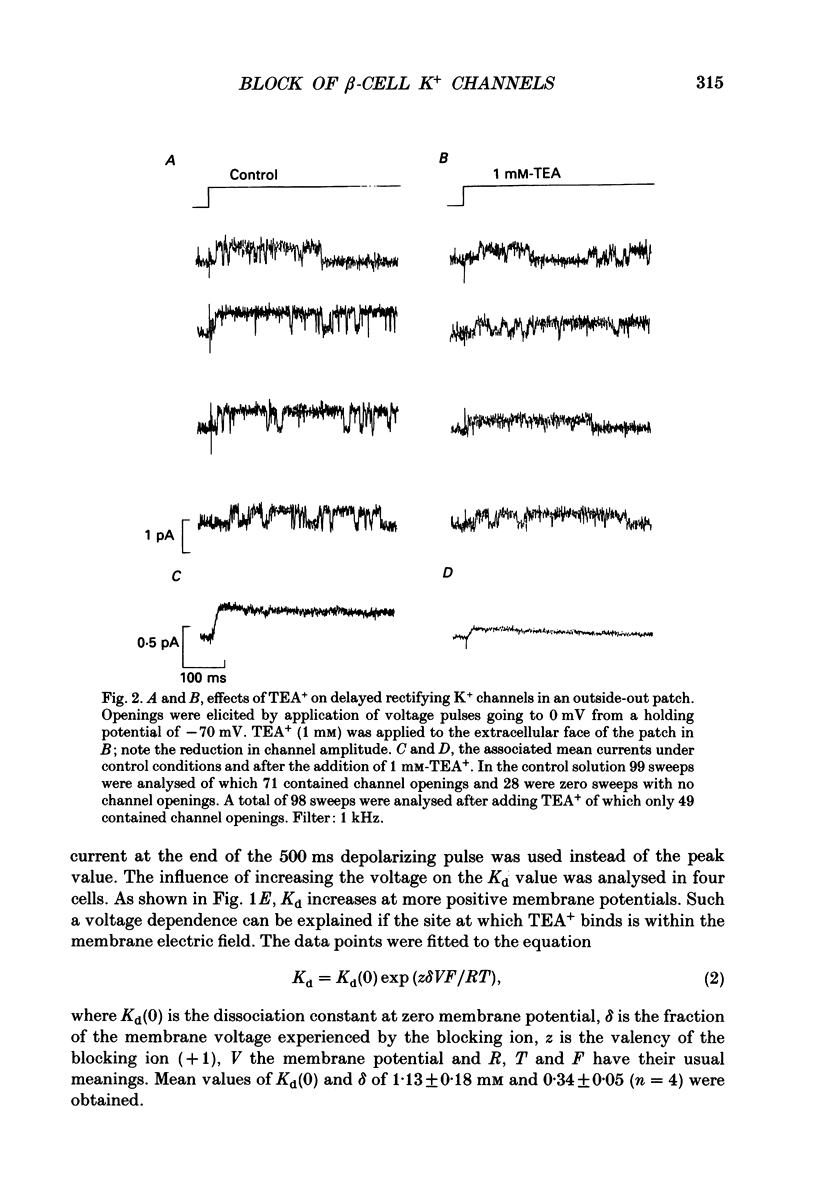
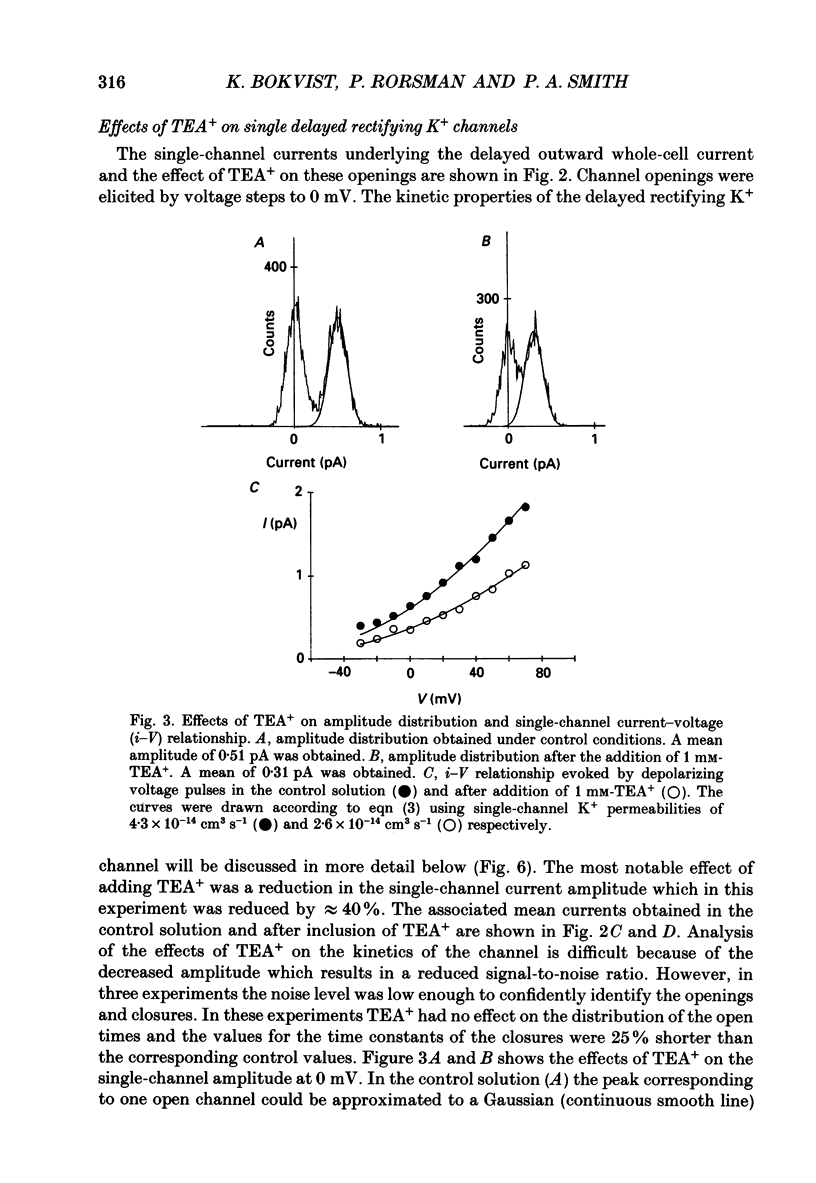
PDF



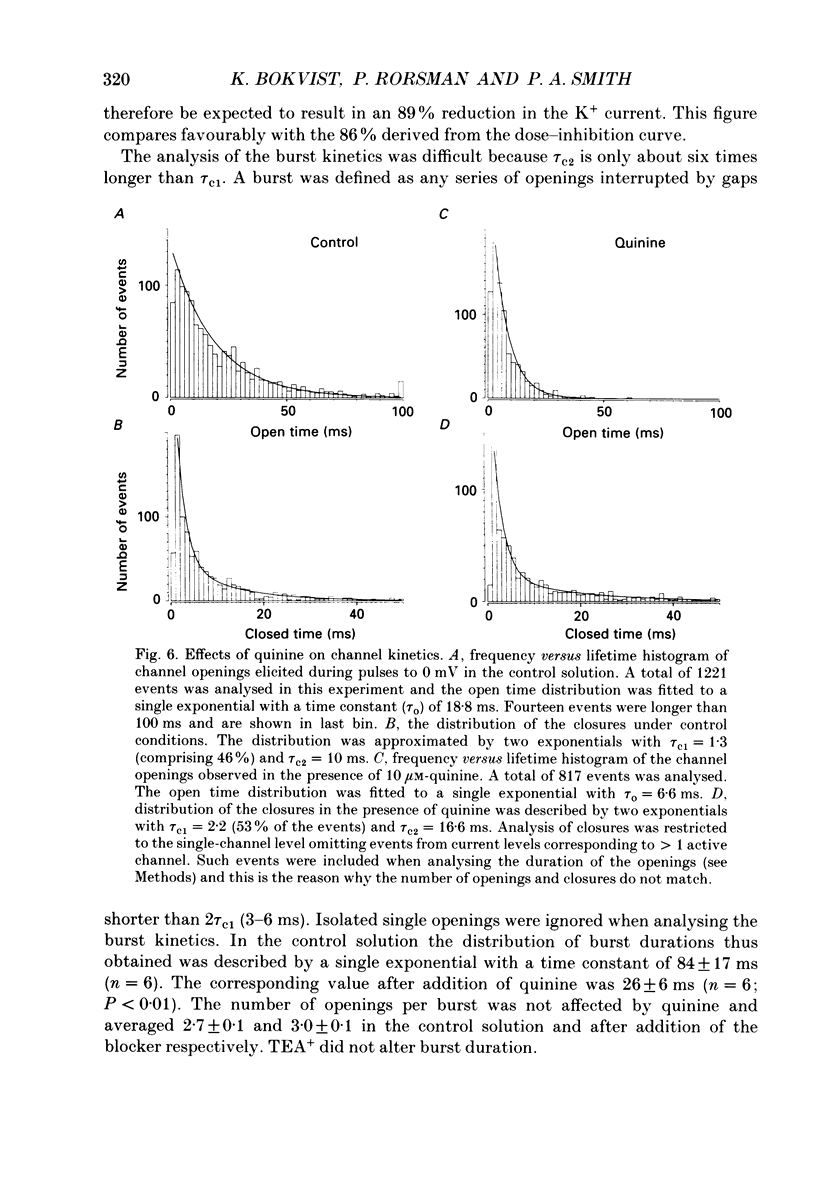
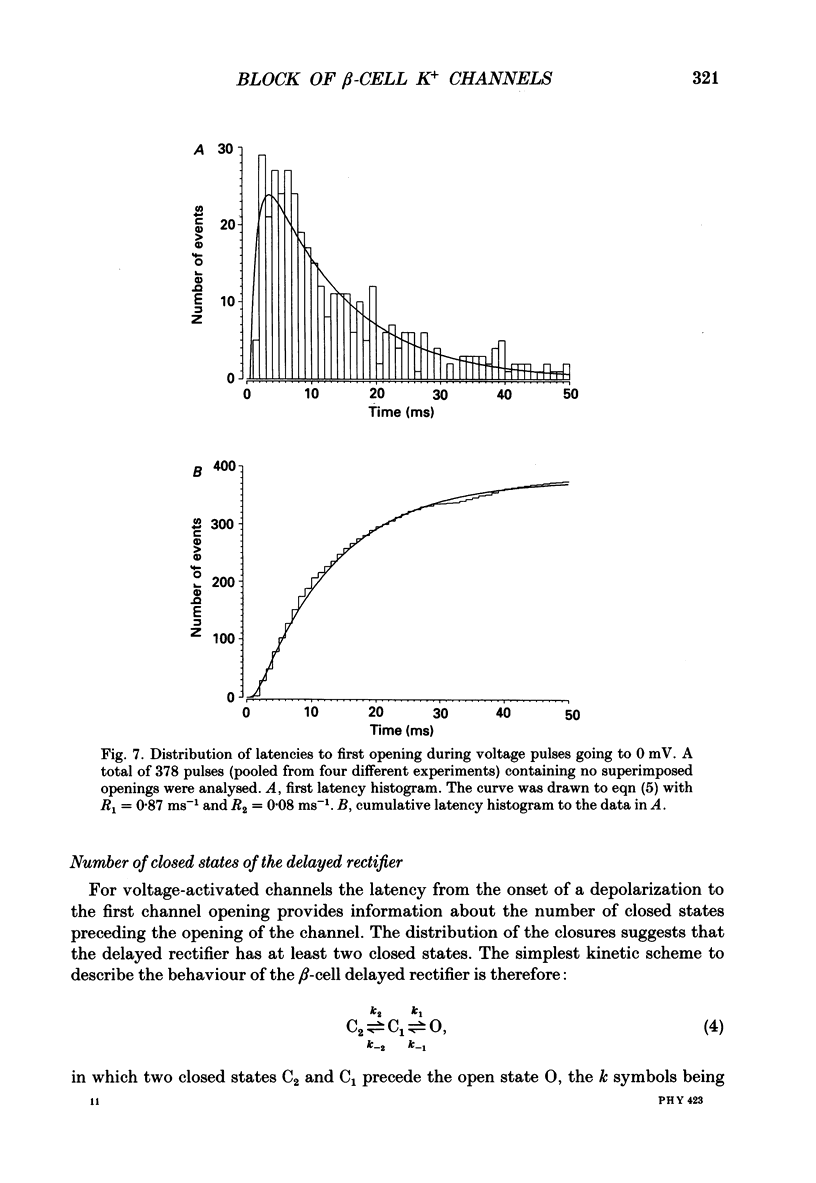




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
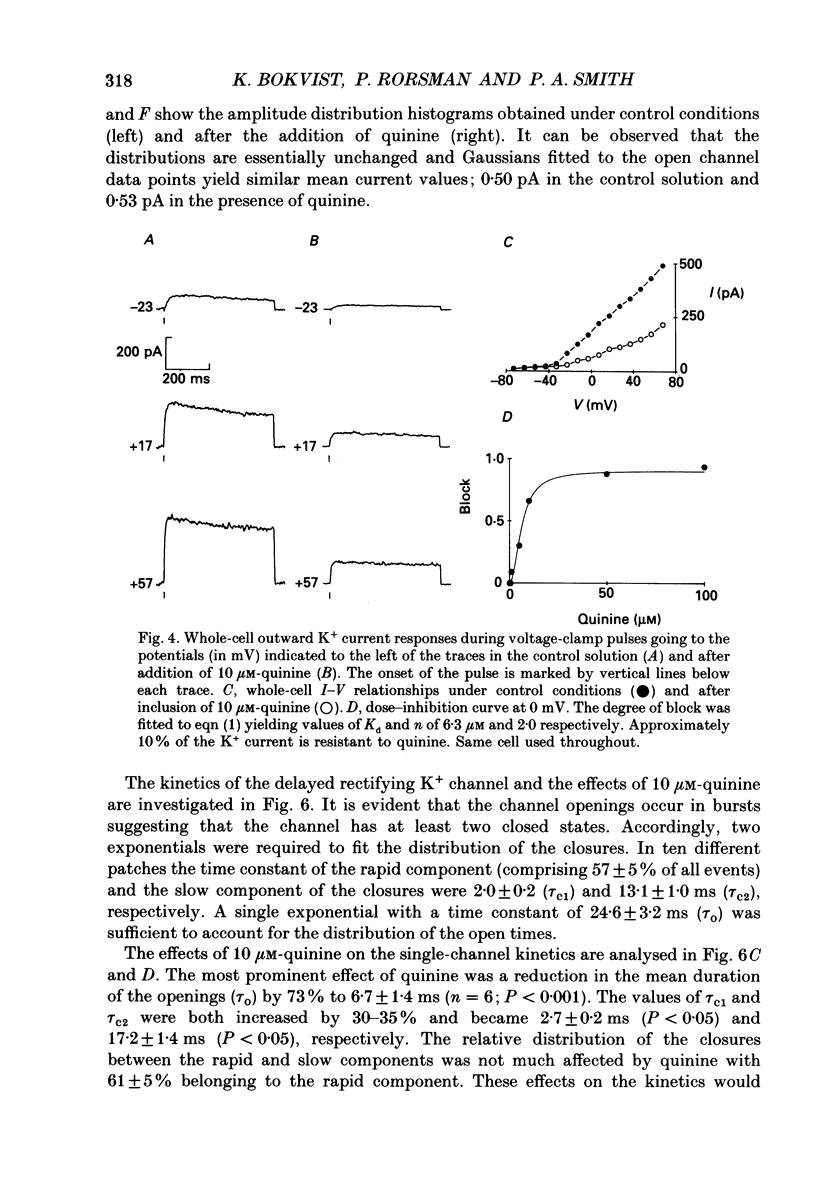
- Atwater I., Dawson C. M., Ribalet B., Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979 Mar;288:575–588. [PMC free article] [PubMed] [Google Scholar]
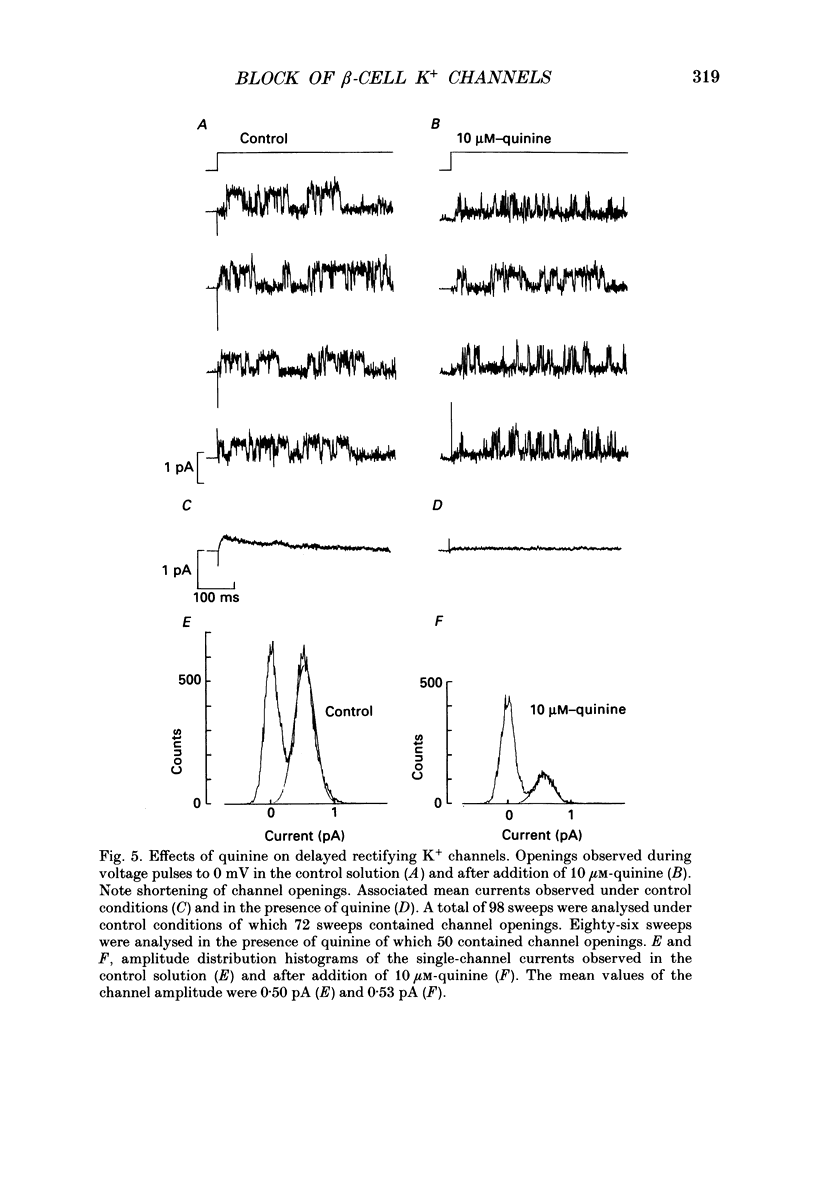
- Atwater I., Ribalet B., Rojas E. Mouse pancreatic beta-cells: tetraethylammonium blockage of the potassium permeability increase induced by depolarization. J Physiol. 1979 Mar;288:561–574. [PMC free article] [PubMed] [Google Scholar]
- Bokvist K., Rorsman P., Smith P. A. Block of ATP-regulated and Ca2(+)-activated K+ channels in mouse pancreatic beta-cells by external tetraethylammonium and quinine. J Physiol. 1990 Apr;423:327–342. doi: 10.1113/jphysiol.1990.sp018025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle N. A., Haylett D. G., Jenkinson D. H. Toxins in the characterization of potassium channels. Trends Neurosci. 1989 Feb;12(2):59–65. doi: 10.1016/0166-2236(89)90137-9. [DOI] [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Glucose-induced electrical activity in pancreatic islet cells. J Physiol. 1970 Sep;210(2):255–264. doi: 10.1113/jphysiol.1970.sp009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Meissner H. P., Schmelz H. Membrane potential of beta-cells in pancreatic islets. Pflugers Arch. 1974;351(3):195–206. doi: 10.1007/BF00586918. [DOI] [PubMed] [Google Scholar]
- Patlak J., Horn R. Effect of N-bromoacetamide on single sodium channel currents in excised membrane patches. J Gen Physiol. 1982 Mar;79(3):333–351. doi: 10.1085/jgp.79.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Findlay I. Electrophysiology of the pancreas. Physiol Rev. 1987 Jul;67(3):1054–1116. doi: 10.1152/physrev.1987.67.3.1054. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986 May;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. The action of external tetraethylammonium ions on unitary delayed rectifier potassium channels of frog skeletal muscle. J Physiol. 1987 Dec;393:467–478. doi: 10.1113/jphysiol.1987.sp016833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R., Ward T. A. Properties of single potassium channels in vesicles formed from the sarcolemma of frog skeletal muscle. J Physiol. 1985 Jul;364:339–358. doi: 10.1113/jphysiol.1985.sp015749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Stocker M., Sakmann B., Seeburg P., Baumann A., Grupe A., Pongs O. Potassium channels expressed from rat brain cDNA have delayed rectifier properties. FEBS Lett. 1988 Dec 19;242(1):199–206. doi: 10.1016/0014-5793(88)81015-9. [DOI] [PubMed] [Google Scholar]
- Zünkler B. J., Trube G., Ohno-Shosaku T. Forskolin-induced block of delayed rectifying K+ channels in pancreatic beta-cells is not mediated by cAMP. Pflugers Arch. 1988 Jun;411(6):613–619. doi: 10.1007/BF00580856. [DOI] [PubMed] [Google Scholar]


