Abstract
Background and Aims
Invasive species usually demonstrate remarkable adaptability across diverse environments, successfully inhabiting a wide variety of regions. This adaptability often links to genetic differentiation and phenotypic plasticity, leading to latitudinal trends in phenotypic traits. In this study, we collected seeds of the invasive plant Phytolacca americana from different latitudes and planted them in homogeneous gardens to investigate the latitudinal variation of P. americana phenotypic traits and to evaluate the effects of herbivory and heavy metals on plant growth, defence and reproductive characteristics.
Methods
Phytolacca americana seeds from different latitudes were planted in a homogeneous garden. For the experimental treatment, the seeds were divided into four groups: a heavy metal treatment group and its corresponding control group, and a cover treatment group with its corresponding control group. After the fruits matured, their growth, reproduction and defence indicators were measured.
Key Results
Significant latitudinal trends were observed in P. americana’s growth and defence characteristics, including changes in branch number, underground biomass, total biomass and leaf tannin content. Compared with previous field surveys on P. americana, our study found that the latitude trends in growth structure and defence traits were consistent, but the latitudinal trend of reproductive structure was different. Moreover, heavy metals and herbivory substantially influenced the plant’s growth, reproduction and defence mechanisms, further shaping its latitudinal patterns.
Conclusions
The observed phenotypic variations in P. americana across latitudes can be largely attributed to the synergistic effects of phenotypic plasticity and genetic variation. At a broader geographical scale, adaptations to heavy metal stress and herbivory pressure among different P. americana populations involve distinct trade-offs related to growth, reproduction and defence strategies.
Keywords: Plant invasion, Phytolacca americana, heavy metal, herbivore, latitudinal gradient, phenotypic plasticity, adaptive evolution
INTRODUCTION
Understanding the distribution patterns and causative factors of invasive alien plants at a regional scale is essential in predicting the ecological impact of invasions and formulating management strategies for these species (Richardson and Pyšek, 2006; Colautti et al., 2010). Invasive plants typically occupy a diverse array of habitats in their introduced regions, exhibiting remarkable adaptability across significantly varying climatic conditions and biotic stresses (Hejda et al., 2009; Richardson and Pyšek, 2012). Therefore, studying how invasive species adapt to these varying habitats provides new insights into ecological and evolutionary biology (Funk, 2008; Davidson et al., 2011). Geographical gradients in species traits are influenced by a range of biotic and abiotic factors (Moreira et al., 2014; Sakata et al., 2017). Biotic factors such as herbivore and pathogen pressure may play a determining role in latitudinal trends in plant traits (Schemske et al., 2009), Meanwhile, abiotic factors like temperature, precipitation and growing season duration also affect plant traits (Kollmann and Bañuelos, 2004; Montague et al., 2007; Leiblein-Wild and Tackenberg, 2014). Invasive plant growth, reproduction and defence traits often display latitudinal trends that may relate to resource allocation strategies influencing the colonization, establishment and spread of invasive plants (Theoharides and Dukes, 2007; Cronin et al., 2015).
Multiple studies have shown that invasive plants can rapidly adapt to new habitats through phenotypic plasticity and genetic differentiation upon introduction to these environments (Lee, 2002; Geng et al., 2006; Dlugosch and Parker, 2008). Phenotypic plasticity specifically refers to the ability of genetically identical plants to modify their phenotypic traits in response to diverse environmental conditions. This plasticity allows them to expand their ecological niche, increase their resilience to environmental and selective pressures, maintain a high level of genetic diversity within populations, and facilitate invasion into new regions (Pigliucci, 2006; Yulong et al., 2009). For example, Geng et al. (2006) found that Alternanthera philoxeroides populations at various invasion sites exhibited phenotypic plasticity in adapting to habitat changes from terrestrial to aquatic environments, without undergoing genetic differentiation. However, phenotypic plasticity, while enabling quick environmental adaptation, is distinct from adaptive evolution, which represents a longer-term process. Adaptive evolution involves genetic differentiation that enables invasive species to colonize and disseminate in new environments. This process allows invasive alien species to undergo genetic alterations that promote their adaptation to novel environments, ultimately resulting in successful colonization and dissemination (Maron et al., 2004; Bossdorf et al., 2005). For instance, high-latitude populations of Lythrum salicaria from invaded sites exhibited earlier flowering, with a negative correlation between plant size at flowering and latitude. This latitudinal variation persisted when seeds from diverse L. salicaria populations were planted in controlled gardens, reflecting trends observed in their natural habitats and indicating the occurrence of adaptive evolution in this species (Montague et al., 2007).
Based on previous research, we have selected P. americana as the primary focus of our study. While it is native to North America, it has been introduced to South America, Europe, Africa and Asia. In recent years, P. americana has emerged as a notable invasive species in China (Huang and Ding, 2015). Previous field studies conducted by Xiao et al. (2019) did not find significant latitudinal differences in plant size or defence levels in growth tissues. However, plants at higher latitudes exhibited a tendency towards increased branching and thinner stems. Furthermore, at higher latitudes, P. americana produced fewer fruits per raceme but displayed a greater number of racemes per plant. There was also a marked increase in the defence level of reproductive tissues with increasing latitude. Nevertheless, it is important to note that Xiao et al.’s research was limited to field observations and did not include homogeneous garden experiments. Consequently, a definitive determination of whether the observed latitudinal trends in the growth, reproduction and defence of P. americana represent phenotypic plasticity or adaptive evolution could not be made. To address this research gap, we planted P. americana seeds from various latitudes in homogeneous gardens and compared the experimental outcomes with the field survey findings of Xiao et al. Our aim was to determine whether the latitudinal variation patterns observed in P. americana during field surveys can be attributed to phenotypic plasticity or adaptive evolution.
Furthermore, plants located at lower latitudes often experience a higher frequency of herbivore attacks compared with those at higher latitudes, resulting in the development of enhanced herbivory resistance among plants at lower latitudes (Cronin et al., 2015; Bhattarai et al., 2017). This trend has been observed in studies comparing temperate and tropical species (Schemske et al., 2009; Marquis et al., 2012). Our investigation aimed to further explore this phenomenon by examining whether P. americana from distinct latitudes exhibit varying defence mechanisms against herbivory in controlled garden settings.
In addition to alterations in the biotic environment, differences between source and invasive sites, especially factors such as heavy metal pollution, can significantly affect plant invasion success and biotic interactions (Li et al., 2021). In recent decades, environmental pollution has escalated profoundly due to the relentless expansion of human activities and industries, with soil heavy metal pollution emerging as a pressing global environmental concern (Ha et al., 2014; Zhao et al., 2015). Heavy metals have been shown to have detrimental effects on plant germination, growth, nutrient absorption, and both primary and secondary metabolism (DalCorso et al., 2013; Shahid et al., 2016; Dubey et al., 2018). Research indicates that heavy metals can influence the activity of enzymes involved in secondary metabolic pathways, thereby altering the composition and quantity of secondary metabolites (Maksymiec et al., 2005; Foroughi et al., 2014). For instance, plants under heavy metal stress exhibit an increased content of phenolic compounds, and the species Noccaea praecox specifically produces higher quantities of secondary metabolites such as flavonoids, tannins and phenolic acids (Llugany et al., 2013). Numerous studies have revealed that invasive species often demonstrate superior tolerance to heavy metals compared with their native counterparts in invaded regions (Wang et al., 2020, 2021a). Notably, certain highly invasive plants can withstand substantial concentrations of heavy metal pollution, thriving even in severely polluted areas (Fu et al., 2017). For instance, A. philoxeroides exhibits remarkable tolerance and accumulation capacity for various heavy metals (Wang et al., 2021b). Given that invasive plants typically occupy a broad spectrum of habitats during their invasion process, it remains crucial to investigate whether invasive plants originating from different latitudes respond variably to heavy metal stress. In our study, we exposed P. americana plants from diverse latitudes to herbivory and heavy metal stress in controlled garden environments. Our objective was to investigate whether these plants exhibit differential trade-offs in growth, defence and reproduction as a response to these stressors. This approach allowed us to gain a deeper understanding of the adaptive mechanisms employed by plants from different geographical regions in coping with environmental challenges.
MATERIALS AND METHODS
Study area and sampling of seeds
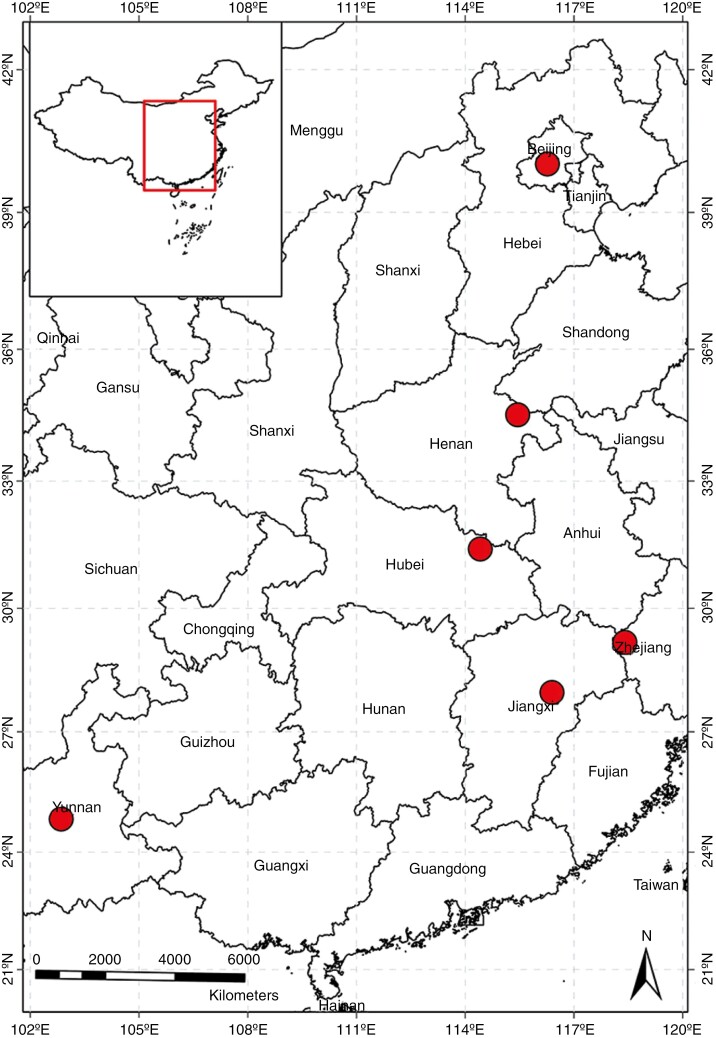
Phytolacca americana flowers annually from June to August and is renowned for its prolific fruit production and seeds’ longevity (Zhou et al., 2013). During the period from July to September 2019 we collected mature fruits from six geographically diverse populations of P. americana throughout China, as depicted in Fig. 1. These sampling sites encompassed a latitudinal range from Yunnan (24°48ʹ N) to Beijing (40°03ʹ N). We obtained a dataset of mean annual temperature and mean annual precipitation from 1980 to 2010 for different sampling sites from https://chelsa-climate.org (Supplementary Data Table S1). The dataset was downscaled by the ERA-Interim Climate Reanalysis model to bring temperature and precipitation estimates to a high resolution of 30 arcseconds (Karger et al., 2017). Within each sampling location, we designated two separate patches, situated ~2–3 km apart, from which five to ten plants were randomly selected. Subsequently, ten mature and undamaged racemes were randomly collected from each plant, ensuring they exhibited no signs of herbivory or seed fragmentation. Following the removal of the pulp, the seeds were air-dried for preservation.
Fig. 1.
Detailed information on the six sampling locations for P. americana that we used in this experiment.
Germination and growth conditions
In May 2020, we soaked the seeds in concentrated sulfuric acid to break dormancy and then washed them with pure water (Liu et al., 2022). Subsequently, the seeds were placed in a germination medium consisting of 1 % agar, and after 3 d the germinated seeds were transplanted into sealed plastic boxes containing soil. The germination process was conducted within the controlled environment of an incubator, maintained at a constant temperature of 27 °C and a photoperiod of 12 h of light alternating with 12 h of darkness. Once the seedlings had developed two leaves, those exhibiting uniform growth were randomly selected and transplanted into pots (17 cm in diameter and 14 cm in height) within a standardized garden located in Kunming, China (24°50ʹ N, 102°52ʹ E). This greenhouse environment provided optimal conditions for plant growth, maintaining an average daytime temperature of 30 °C, a night-time temperature of 18 °C and a relative humidity ranging from 50 to 80 %.
Experimental treatments
We designed an experiment to investigate the trait variation patterns and differential responses of species P. americana to herbivory pressure and heavy metal stress across various latitudes. Seeds of P. americana originating from diverse latitudinal regions were planted in standardized garden conditions to facilitate a controlled analysis. The experiment consisted of four treatments. The heavy metal control group did not receive any addition of heavy metal Mn solution. In the heavy metal treatment group, after the seedlings had grown in pots for 2 months, a solution of 100 mL of 10 000 μm MnCl2·4H2O was applied to the soil. In the cover control group no protective covering was provided to prevent herbivory in this group. In the cover treatment group, following transplantation into pots, one seedling from each of six geographical locations was randomly selected and placed within a 1 m × 1 m × 1 m insect-resistant net cover. The net cover exhibits excellent light and water permeability while segregating herbivores, which can significantly mitigate the impact on plant growth. Each treatment group was replicated five times, resulting in a total of 120 plants (6 geographical locations × 4 treatment variables × 5 replicates per variable). Plants were watered every 3 d with 500 mL water each time to ensure growth. Both the treatment group and the control group were housed in a semi-open greenhouse environment, designed to permit the natural ingress of herbivorous insects while effectively excluding larger herbivores. Throughout the plant growth period, unprotected plants within the greenhouse were subjected to predation by generalist herbivorous insects, specifically Spodoptera exigua and Spodoptera litura. Conversely, plants covered with protective netting remained unaffected by herbivorous insects, as confirmed through regular inspections conducted every 3 d. To uphold the experimental design’s integrity and mitigate potential location effects, the positions of the treatment and control groups were randomly interchanged on a monthly basis.
Measurement of P. americana growth and reproduction
Phytolacca americana is a perennial plant, so we measured its growth, defence and reproductive indicators over 2 years in the experiment. In November 2020, we harvested all above-ground plant parts and a minimal section of the root system for heavy metal analysis, ensuring minimal disruption to the plant’s potential for germination and subsequent growth in the subsequent year. Following the harvest, regular irrigation of the soil was maintained to sustain the healthy development of the remaining underground plant components. In March 2021, seedlings had sprouted from the existing roots, and 100 mL of 10 000 µm MnCl2·4H2O was added to the heavy metal treatment group. In July 2021, all the above-ground and underground parts of the plants were harvested. We measured the growth and reproduction indexes of P. americana, including plant height, basal diameter, the number of branches, the number of racemes and the number of fruits. All plants were divided into five parts: roots, stems, leaves, racemes and fruits. They were dried at 60 °C for 5 d until they reached a constant weight, and then the dry weight of each part was measured using an analytical balance (BSA223S, Olabo, Shandong, China) with an accuracy of 0.0001 g. We calculated the biomass distribution pattern of each plant. These included: above-ground biomass, which was equal to flower biomass + fruit biomass + leaf biomass + stem biomass; below-ground biomass, which was equal to root biomass; total biomass, which was equal to above-ground biomass + below-ground biomass; root mass/shoot mass, which was equal to below-ground biomass/above-ground biomass; leaf mass ratio, which was equal to leaf biomass/total biomass; stem mass ratio, which was equal to stem biomass/total biomass; root mass ratio, which was equal to root biomass/total biomass; and reproductive mass ratio, which was equal to (flower biomass + fruit biomass)/total biomass.
Secondary metabolite, nutrient and heavy metal contents of P. americana
We selected fresh P. americana leaves, dried them at 60 °C for 72 h until they maintained a constant weight, and then ground the dried leaves into a powder using a spherical grinder (MM400, Retsch, Daichi, Germany). The leaf powder was passed through a 100-mesh sieve and stored at room temperature. We measured the P. americana secondary metabolite indicators: tannins, total phenols, total flavonoids, total saponins, and the nutrient levels of plant starch and soluble sugars. One plant represented one sample (6 geographical locations × 4 variables × 5 replicates = 120 samples). All indicators were measured by spectrophotometry using purchased kits. Kits for tannins, total phenols, total flavonoids, starch and soluble sugars were purchased from Grace Biotechnology Suzhou (http://www.geruisi-bio.com), and kits for saponins were purchased from Comin Suzhou (http://www.cominbio.com). Using a spectrophotometric system, we conducted a systematic analysis to determine the content of various secondary metabolites.
Tannin content.
In alkaline conditions, tannin compounds reduce phosphomolybdate to yield blue compounds. By quantifying the absorbance of these blue compounds at 650 nm, we accurately determined the tannin content, which is directly proportional to the measured absorbance.
Total flavonoids.
Employing the NaNO2-Al(NO3)3-NaOH colorimetric system, flavonoid compounds formed a red complex with aluminium ions under alkaline conditions, exhibiting a distinct absorption peak at 510 nm. The total flavonoid content in the samples was subsequently calculated based on the absorbance measurements at this wavelength.
Total alkaloids.
Alkaloids reacted with bromocresol green to produce a yellow substance, featuring a characteristic absorption peak at 415 nm. By monitoring the increase in absorbance at this wavelength, we precisely quantified the total alkaloid content within the samples.
Total saponins.
Initially, saponins were extracted from the samples using ultrasonic techniques. Subsequently, the vanillal–perchloric acid colorimetric system was applied to measure the absorbance of reaction products at 589 nm, allowing accurate calculation of total saponin content.
Total phenols.
Adhering to the Folin–Ciocalteu method, phenolic compounds reduced tungstate molybdic acid to form a blue compound under alkaline conditions, which displayed a prominent absorption peak at 710 nm. By recording the absorbance value at this wavelength, we precisely assessed the total phenol content in the samples.
Soluble sugars.
Soluble sugars underwent dehydration by concentrated sulfuric acid, converting them into furfural or hydroxymethyl furfural. These intermediates then reacted with anthrone, generating blue–green derivatives that exhibited maximum absorption peaks at 620 nm. By precisely measuring the absorbance at this wavelength, we quantitatively analysed the soluble sugar content, which demonstrated a linear relationship with the absorbance values.
Starch.
To quantify starch content, we first converted starch into glucose through acid hydrolysis. Subsequently, we employed the anthrone colorimetric method to determine the glucose content at 620 nm wavelength. Based on this measurement, we calculated the original starch content in the samples.
Heavy metal measurements included roots and leaves. Sample preparation was as above, and measurements were done by Shiyanjia Lab (http://www.shiyanjia.com).
Statistical analysis
In order to examine latitudinal patterns in growth, reproduction and defence indices, we employed plant height, basal diameter, biomass, fruit count, tannin content and saponin contents as dependent variables in a linear regression analysis. Subsequently, we utilized the derived P-values to ascertain the existence of a statistically significant latitudinal trend. Furthermore, to assess the impact of heavy metal stress, herbivory pressure and latitude on the phenotypic variation of P. americana, we conducted a mixed-model ANOVA, incorporating various indicators of growth, reproduction and defence, such as plant height, basal diameter, fruit count and the contents of resistance compounds. To reduce the dimensionality and explore the possible relationship between variables, principal component analyses (PCAs) were conducted on the variables related to plant growth. All statistical analyses were executed utilizing R software version 4.1.0, while all graphical representations were generated with Origin 2021.
RESULTS
Growth of P. americana
The cover treatment had a statistically significant impact (P < 0.0001) on the first-year growth parameters (plant height, basal diameter and above-ground biomass) and reproduction of P. americana, as detailed in Supplementary Data Table S2. Additionally, latitude exhibited a significant influence on the number of branches, underground biomass and total biomass in the second year (Table 1), demonstrating a significant positive correlation (Fig. 2A, D, G). However, no significant latitudinal variation was observed in the root/shoot ratio (Table 1, Fig. 2J, K, L). Furthermore, neither the cover treatment nor the heavy metal treatment significantly altered the latitudinal differences in the number of branches in P. americana (Fig. 2B, C). Both the cover treatment and the heavy metal treatment influenced the latitudinal differences in the biomass and total biomass of P. americana (Fig. 2E, F, H, I).
Table 1.
Effects of latitude, cover treatment and heavy metal on growth traits in the second year of P. americana. To make the data conform to a normal distribution before analysis, the following conversions were performed on the non-normally distributed data: Box‒Cox transformation: number of branches, underground biomass and stem mass ratio; log10 conversion: root/shoot ratio. Bold type means P<0.05.
| Plant height | Basal diameter | Number of branches | Overground biomass | Underground biomass | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | Degrees of freedom | F | P | F | P | F | P | F | P | F | P |
| Latitude | 5105 | 0.2780 | 0.5992 | 0.0207 | 0.8860 | 13.0833 | 0.0005 | 0.1742 | 0.6773 | 7.6359 | 0.0068 |
| Heavy metal | 1105 | 12.2293 | 0.0007 | 2.6195 | 0.1087 | 4.4078 | 0.0383 | 0.0327 | 0.8568 | 0.0974 | 0.7556 |
| Cover | 1105 | 99.8201 | <0.0001 | 0.0215 | 0.8838 | 2.2035 | 0.1408 | 4.6719 | 0.0330 | 0.4174 | 0.5197 |
| Latitude: heavy metal | 1105 | 2.9771 | 0.0875 | 2.2643 | 0.1355 | 0.1034 | 0.7484 | 0.6134 | 0.4354 | 0.3544 | 0.5530 |
| Latitude: cover | 1105 | 0.0332 | 0.8558 | 0.3654 | 0.5469 | 0.2974 | 0.5866 | 0.0706 | 0.7910 | 0.3439 | 0.5589 |
| Heavy metal: cover | 1105 | 1.9899 | 0.1614 | 0.0000 | 0.9959 | 0.1660 | 0.6845 | 0.1392 | 0.7098 | 5.4703 | 0.0213 |
| Latitude: heavy metal: cover | 1105 | 0.5389 | 0.4646 | 0.2883 | 0.5925 | 0.2697 | 0.6074 | 1.1801 | 0.2799 | 0.6959 | 0.4061 |
| Total biomass | Root/shoot ratio | Leaf mass fraction | Stem mass fraction | Root mass fraction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | F | P | F | P | F | P | F | P | F | P | |
| Latitude | 6.7458 | 0.0108 | 2.3582 | 0.1278 | 0.5952 | 0.4422 | 0.5421 | 0.4633 | 2.7738 | 0.0989 | |
| Heavy metal | 0.1165 | 0.7336 | 0.1025 | 0.7496 | 0.5657 | 0.4537 | 0.8901 | 0.3470 | 0.0464 | 0.8299 | |
| Cover | 4.1731 | 0.0437 | 0.3727 | 0.5429 | 0.0076 | 0.9307 | 0.2095 | 0.6482 | 0.4306 | 0.5132 | |
| Latitude: heavy metal | 1.2721 | 0.2621 | 0.0024 | 0.9609 | 0.6487 | 0.4225 | 0.1018 | 0.7504 | 0.0026 | 0.9591 | |
| Latitude: cover | 0.4084 | 0.5242 | 0.6790 | 0.7792 | 0.0708 | 0.7907 | 0.1163 | 0.7338 | 0.0424 | 0.8373 | |
| Heavy metal: cover | 1.7857 | 0.1845 | 6.3994 | 0.0130 | 4.1139 | 0.0452 | 4.9611 | 0.0281 | 5.5843 | 0.0200 | |
| Latitude: heavy metal: cover | 0.0445 | 0.8333 | 1.8027 | 0.1824 | 0.4911 | 0.4850 | 0.9628 | 0.3288 | 2.1579 | 0.1450 | |
Fig. 2.
Growth traits in the second year of P. americana in relation to latitude. (A, B, C) Number of branches. (D, E, F) Underground biomass. (G, H, I) Total biomass. (J, K, L) Root/shoot ratio. Panels (A, D, G and J) show the relationship of mean values with latitude across treatments. P < 0.05 indicates that the trait has a significant linear relationship with latitude.
In the PCA of growth traits, PC1 and PC2 explained 37.21 and 27.60 % of the total variation, respectively (Fig. 3A). The first principal component is positively correlated with plant height and basal diameter and negatively correlated with the root/shoot ratio; therefore we define it as a composite measure of plant size. Conversely, the second principal component is positively correlated with the root/shoot ratio, plant height and basal diameter and negatively correlated with branch number; thus we define it as a composite measure of growth architecture (Xiao et al., 2019). There was no significant latitudinal difference in plant size (Supplementary Data Fig. S1C), but growth architecture was significantly and negatively correlated with latitude (Fig. 3B). The higher the latitude, the greater the root/shoot ratio (Fig. 2J), and the higher the number of branches (Fig. 2A), the smaller the basal diameter (Supplementary Fig. S1B) and the shorter the plant (Supplementary Data Fig. S1A).
Fig. 3.
The results of the principal component analysis (PCA) on the growth variables of P. americana in the second year (A). Higher values of growth architecture indicate a smaller root/shoot ratio, a lower number of branches, a higher plant height and a thicker basal diameter. Relationship between the composite variable of growth architecture and latitude in the second year of P. americana (B,C,D). Fig. 3 (B) Relationship of mean values with latitude across treatments. P < 0.05 indicates a significant linear relationship between this trait and latitude.
Reproduction of P. americana
There were no significant latitudinal differences between the first and second years of P. americana for reproductive traits, including fruit number and raceme number (Table 2, Supplementary Data Table S3). However, there was an interactive effect of heavy metal and latitude on the number of fruits in the second year (Table 2). In the heavy metal treatment group the number of fruits in P. americana increased significantly with increasing latitude, while in the heavy metal control group the number of fruits decreased with increasing latitude (Fig. 4B). There was no significant difference in the number of fruits of P. americana under the cover treatment (Fig. 4C). Interestingly, we noted that the reproductive mass ratio of P. americana was significantly negatively correlated with latitude (Fig. 4D).
Table 2.
Effects of latitude, cover treatment and heavy metal on reproductive traits of P. americana in the second year. To make the data conform to the normal distribution before analysis, the following conversion was performed on the non-normally distributed data: log10 conversion: number of racemes. Bold type means P<0.05.
| Number of fruits | Number of racemes | Reproductive mass fraction | ||||
|---|---|---|---|---|---|---|
| Effect | F | P | F | P | F | P |
| Latitude | 0.0657 | 0.7983 | 0.0266 | 0.8708 | 5.2034 | 0.0246 |
| Heavy metal | 0.5693 | 0.4523 | 0.2448 | 0.6218 | 1.3040 | 0.2562 |
| Cover | 0.2148 | 0.6441 | 0.6362 | 0.4270 | 0.6560 | 0.4199 |
| Latitude: heavy metal | 7.5198 | 0.0072 | 0.7273 | 0.3958 | 0.0877 | 0.7677 |
| Latitude: cover | 0.3942 | 0.5315 | 0.1527 | 0.6968 | 0.4929 | 0.4843 |
| Heavy metal: cover | 0.1260 | 0.7234 | 2.7230 | 0.1020 | 1.8020 | 0.1825 |
| Latitude: heavy metal: cover | 1.4455 | 0.2321 | 0.0368 | 0.8483 | 1.5703 | 0.2131 |
Fig. 4.
Relationship between second-year reproductive traits of P. americana and latitude: number of fruits (A, B, C) and reproductive mass ratio (D). (A, D) Relationship of mean values with latitude across treatments. P < 0.05 indicates a significant linear relationship between this trait and latitude.
Phytolacca americana’s investment in defence
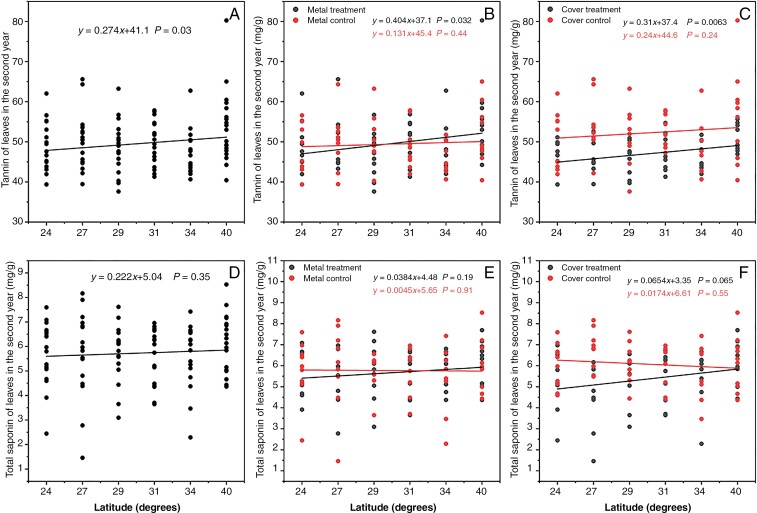
In the first year of the study, the relative content of defences and nutrients in P. americana did not show significant latitudinal differences (Supplementary Data Table S4). In the second year, latitude significantly influenced the tannin content in P. americana leaves, whereas it had no significant impact on the other resistance substances (viz. total phenols, total flavonoids and total saponins) or nutrients (viz. starch and soluble sugars), as evident from Table 3. The tannin content in P. americana leaves displayed a significant and positive correlation with latitude, regardless of whether the plants were subjected to heavy metal or cover treatments (Fig. 5A). Both heavy metal and cover treatments individually led to a significant positive correlation between tannin content and latitude. However, in the absence of either of these treatments latitudinal differences were not significant (Fig. 5B, C). No significant latitudinal variations were observed in the saponin content of P. americana leaves (Fig. 5D). Nevertheless, heavy metal and cover treatments emerged as critical factors influencing leaf saponin content. In the absence of cover treatment or heavy metal treatment, saponin content negatively correlated with latitude, while the opposite was true when either of these treatments was applied (Fig. 5E, F).
Table 3.
Effects of latitude, cover treatment and heavy metal on the content of resistance substances and nutrients in the second year of P. americana leaves. To make the data conform to a normal distribution before analysis, the following conversions were performed on data that were not normally distributed: log10 conversion: total flavonoids, starch, soluble sugar; Box–Cox conversion: total saponins. Bold type means P<0.05.
| Tannin | Total phenols | Total flavonoids | Total saponins | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | Degrees of freedom | F | P | F | P | F | P | F | P |
| Latitude | 5105 | 5.3906 | 0.0223 | 3.4909 | 0.0646 | 0.4369 | 0.5101 | 0.9086 | 0.3428 |
| Heavy metal | 1105 | 0.0116 | 0.9145 | 0.1508 | 0.6986 | 1.1299 | 0.2903 | 0.6713 | 0.4145 |
| Cover | 1105 | 16.1877 | 0.0001 | 28.4431 | <0.0001 | 70.3434 | <0.0001 | 9.0724 | 0.0033 |
| Latitude: heavy metal | 1105 | 1.3069 | 0.2577 | 0.0090 | 0.9246 | 0.330 | 0.8563 | 0.7045 | 0.4032 |
| Latitude: cover | 1105 | 0.0576 | 0.8108 | 0.0764 | 0.7828 | 2.4820 | 0.1183 | 2.9110 | 0.0911 |
| Heavy metal: cover | 1105 | 0.6624 | 0.4176 | 0.2681 | 0.6057 | 0.4526 | 0.5027 | 1.9933 | 0.1611 |
| Latitude: Heavy metal: cover | 1105 | 0.3910 | 0.5332 | 1.5836 | 0.2112 | 0.0461 | 0.8303 | 4.6536 | 0.0344 |
| Starch | Soluble sugar | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | F | P | F | P | |||||
| Latitude | 0.4167 | 0.5201 | 0.4114 | 0.5227 | |||||
| Heavy metal | 11.5427 | 0.0010 | 11.5833 | 0.0010 | |||||
| Cover | 19.6654 | 0.0002 | 19.6416 | 0.0002 | |||||
| Latitude: Heavy metal | 1.1422 | 0.2877 | 1.1447 | 0.2872 | |||||
| Latitude: cover | 1.6369 | 0.2037 | 1.6494 | 0.2020 | |||||
| Heavy metal: cover | 0.5292 | 0.4686 | 0.5401 | 0.4641 | |||||
| Latitude: heavy metal: cover | 0.0161 | 0.8992 | 0.0204 | 0.8868 |
Fig. 5.
Resistance compound and nutrient contents in the leaves of P. americana in the second year in relation to latitude: tannins (A, B, C) and total saponins (D, E, F). (A, D) Relationship of mean values with latitude across treatments. P < 0.05 indicates a significant linear relationship between this trait and latitude.
Root and leaf heavy metal content of P. americana
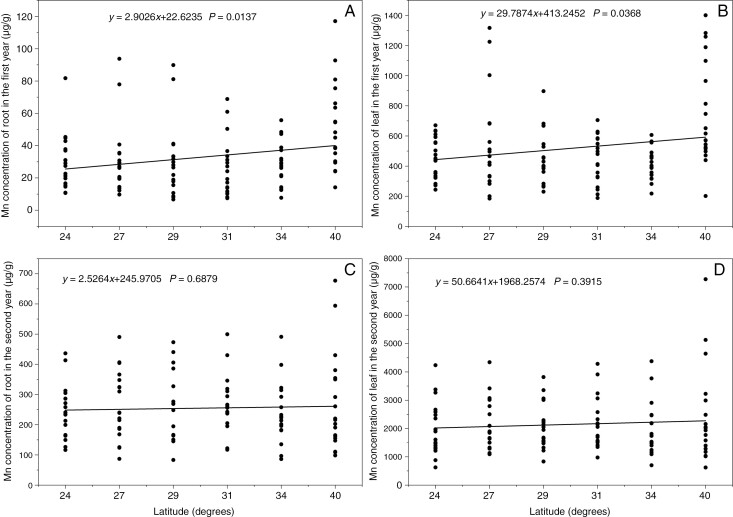
In the first year of our study, we observed a positive correlation between the concentration of the heavy metal Mn in both the roots and leaves of P. americana and latitude (Fig. 6A, B). This suggests that, as latitude increased, the content of Mn in the roots and leaves of P. americana also increased. However, in the second year of our study we did not find any significant differences in the levels of Mn in the roots and leaves of P. americana across different latitudes (Fig. 6C, D).
Fig. 6.
Heavy metal (Mn) content in the roots and leaves of P. americana in the first (A, B) and second (C, D) year. P < 0.05 indicates a significant linear relationship between Mn content and latitude.
DISCUSSION
Invasive species are frequently introduced into various novel ecosystems, posing significant challenges to ecological balance. To succeed, these species must cope with different environmental conditions. However, our understanding of the specific mechanisms by which exotic species cope with and overcome these new environmental challenges within their expanding ranges remains limited. In our experiment, we collected seeds of P. americana from various latitudes and subsequently planted them in homogeneous gardens. Our primary objective was to compare the differences in P. americana traits between these controlled gardens and natural field conditions, with the aim of elucidating the roles of rapid adaptive evolution and phenotypic plasticity in influencing the colonization ability of this widespread invasive species. Additionally, we investigated the variations in P. americana’s response to heavy metals and herbivory across different latitudes.
Plant growth
Plant growth is an important aspect of plant adaptation and is influenced by a wide range of environmental conditions. Previous studies usually assumed that plants growing at higher latitudes will show smaller sizes than plants growing at lower latitudes due to unfavourable environmental conditions (Li et al., 1998; Zhu et al., 2022). However, our study revealed that the growth characteristics of P. americana did not conform to the anticipated latitudinal trend in our controlled garden experiment (Supplementary Data Fig. S1C), aligning with the findings of a field investigation conducted by Xiao et al. (2019). One explanation for this latitudinal trend is that reductions in precipitation at higher latitudes potentially contribute to enhanced growth, as P. americana grows well in soil with moderate moisture (USDA Natural Resources Conservation Service, http://plants.usda.gov/java/charProfile?symbol=PHAM4). Furthermore, Xiao et al. (2019) observed that P. americana at high latitudes exhibited an increased number of branches and a reduced stem diameter in their field survey. Similarly, our controlled garden experiment demonstrated a significant positive correlation between branch number and latitude, along with a negative correlation between stem diameter and latitude (Fig. 2A, Supplementary Data Fig. S1B). This pattern suggests an adaptive evolution in the growth structure of P. americana to accommodate the high-latitude environment and enhance light capture. Comparable findings have been reported in other studies, such as the notable increase in the number of Stipa baicalensis needlegrass branches with increasing latitude (Zhang et al., 2015). This trend may be attributed to the stimulatory effect of lower temperatures on plant tillering (Friend, 1965; Chaturvedi et al., 1981).
Plant reproduction
Reproduction is another important aspect of plant adaptability, especially for invasive species. Successful invasive species usually have high reproductive capacity and can produce a large number of seeds quickly (Seneviratne et al., 2016; Vedder et al., 2021). In the field study of Xiao et al. (2019) there was no significant effect of latitude on reproductive output, while reproductive architecture was strongly and negatively related to latitude. With increasing latitude, plants produced fewer fruits per raceme, but more racemes per plant (Xiao et al., 2019). However, we did not observe this trend in our homogeneous garden experiment; we found that there was no significant latitudinal difference in the number of fruits and number of racemes of P. americana (Table 2), but the reproductive mass ratio was significantly negatively correlated with latitude (Fig. 4D). This may be due to the fact that the length of the growing season typically shortens with latitude (at a constant altitude), and individuals from high-latitude regions typically start flowering earlier during their individual development to ensure successful seed maturation at the end of the growing season (Olsson and Ågren, 2002; Stinchcombe et al., 2004). Early flowering will lead to smaller seeds and less reproductive input (Hartnett, 1990; Helsen et al., 2020).
Plant defence
Plant defence is crucial for the survival and fitness of plants, especially in the face of herbivory. The latitudinal herbivory-defence hypothesis (Coley and Aide, 1991) suggests that because the diversity, density and activity of natural enemy insects are often thought to be higher in the tropics, plants at lower latitudes should invest more in defence (Pennings et al., 2001; Rasmann and Agrawal, 2011). This is contrary to our experimental results, where this study found a significant positive correlation between tannin content and latitude in P. americana leaves (Fig. 5A). This may be due to the fact that the relative distribution of tannins is sensitive to the abiotic factor temperature, but not to herbivory. Consistent with our research, Moreira et al. (2018) found that levels of herbivory on the leaves of Quercus robur increased significantly towards lower latitudes in their survey. However, investment in leaf chemical defence exhibited an inverse pattern, with the concentration of condensed tannins increasing towards higher latitudes. This phenomenon may be attributed to the strong influence of climatic and soil factors on the concentration of condensed tannins in plant leaves. Specifically, populations originating from cold and dry regions or grown on porous soils with poor water and nutrient retention, which are conditions often associated with limited plant resource availability, tend to exhibit higher investments in condensed tannins in their leaves (Moreira et al., 2018). These findings are in line with the resource availability hypothesis (Coley et al., 1985; Endara and Coley, 2011), which maintains that plants growing under resource-limited circumstances show slow growth, higher tissue construction costs, and consequently higher investment in defences, since tissues consumed by herbivores are costly to replace (Coley et al., 1985; Fine et al., 2004). In addition, precipitation was negatively associated with leaf phenolics across populations of the herb Ruellia nudiflora, suggesting that plants growing in sites with lower water availability (and presumably also lower resource availability) are more highly defended (Abdala-Roberts et al., 2016). In addition, in our homogeneous garden experiment there was no significant latitude difference in the saponin content in P. americana leaves (Fig. 5 D), and in the field investigation experiment conducted by Xiao et al. (2019) it was also found that the triterpenoid saponin content in P. americana nutrient tissues (such as roots, stems and leaves) was not correlated with latitude. This may be because invasive plants escaped natural enemies, leading to lack of selection for the latitudinal herbivory-defence hypothesis. Conversely, a latitudinal trend in plant defence may be driven by variation in abiotic factors with latitude.
Phenotypic plasticity and adaptive evolution
Trait variation can be based on adaptive evolution (genetics) or on plastic responses to environmental gradients (Maron et al., 2004). While phenotypic plasticity may be an important mechanism for enabling alien species to succeed in invaded areas, the same may be true of evolution. Early classical studies of plants emphasized the fact that alien species often have great evolutionary potential and that rapid genetic adaptation to new environments may be more important in invasion ecology than previously thought (Parker et al., 2003; Woods and Sultan, 2022). One potential criticism of our study is that we only conducted a homogeneous garden experiment and did not investigate the latitudinal trends in growth, defence and reproduction of P. americana in the field. However, when we compared our results with the field survey of P. americana by Xiao et al. (2019), we think our results were similar with the exception of the influence of latitude on reproductive traits. In the field investigation, P. americana showed an obvious latitude variation pattern in growth structure, which still existed in the homogeneous garden (Fig. 2A), indicating that P. americana might have undergone adaptive evolution. However, the latitudinal trend in reproductive structure exhibited by P. americana in the field survey disappeared in the homogeneous garden (Table 2). This suggests that the latitudinal trend in reproductive structure exhibited by P. americana may be a plastic response to the environment. Thus, both phenotypic plasticity and adaptive evolution may be associated with variation in P. americana traits. Phenotypic plasticity and genetic differentiation are two different strategies for plants to adapt to heterogeneous environments, but they are not contradictory or exclusive, and both are conducive to the adaptation of alien plants to new environments in invaded areas (Bossdorf et al., 2005). Studies have shown that the invasion of some alien species is the result of ecological adaptation and rapid evolution (Cordell et al., 1998; Sexton et al., 2002). The invasive plant Metrosideros polymorpha showed obvious morphological and physiological differences in different habitats in Hawaii Island. The differences in physiological and anatomical characters were found to be the result of phenotypic plasticity in field and homogeneous plantation experiments, whereas the differences in morphology (leaf blade size, petiole length, stem node length, etc.) had a genetic basis, which suggests that the invasion of this species is the result of phenotypic plasticity and genetic differentiation (Cordell et al., 1998). In a common garden experiment designed to assess population differentiation in plant traits through field-collected seeds, it is necessary to consider the potential confounding influence of maternal environmental effects on observed among-population differences (Maron et al., 2004). Although we cannot completely rule out the possibility that maternal effects influenced our results, this seems unlikely. We found no significant difference in seed mass based on region of origin, nor was there a significant relationship between latitude of origin and seed mass in different sampling areas (Supplementary Data Fig. S2A, B). Additionally, maternal effects are typically expressed early in the life cycle and decrease as plants age (Wolfe, 1993). In our study, P. americana, a perennial plant, was measured 1 year after it was planted, and we measured its traits for 2 years, a period sufficient to reduce any initial maternal effects.
Adaptation to heavy metal and herbivory
Heavy metal tolerance is a crucial factor for plant survival and reproduction in environments contaminated with heavy metals (Deng et al., 2006; Chowdhury and Maiti, 2016). These contaminants interact with plants in two primary ways: firstly, they compete with essential nutrients during absorption by roots from the soil, hindering normal plant growth (Ejaz et al., 2023). Secondly, once inside the plant, heavy metals disrupt metabolic processes and inflict toxic effects on both internal and external plant structures (Naz et al., 2022). Elevated concentrations of heavy metals, exceeding permissible limits, exert deleterious effects on plants, both directly and indirectly. Among the direct negative impacts, heavy metals can inhibit enzymatic activities by binding to sulfhydryl groups, or lead to deficiencies in essential metals within metalloproteins and metal–protein complexes, thereby disrupting their functionality (Van Assche and Clijsters, 2006). Additionally, oxidative stress induced by these heavy metals causes damage to vital cellular structures, notably chloroplasts and mitochondria, further exacerbating the adverse effects on plant health (Jadia and Fulekar, 2009). High concentrations of certain heavy metals further decelerate crucial physiological processes, including photosynthesis, transpiration, and overall growth rates across various plant species (Yadav, 2010). While heavy metals are not universally known as primary inducers of trait variation, there are documented cases where species like Scenedesmus incrassatulus (Chlorophyceae) (Peña-Castro et al., 2004) and Arachis hypogaea (Shi and Cai, 2009) undergo phenotypic alterations in response to heavy metal stress. Our study specifically found that P. americana originating from different latitudes, when planted in homogeneous gardens, invested significantly differently in growth, defence and reproduction strategies to adapt to heavy metal stress. This adaptation manifested in altered latitudinal trends in underground biomass, total biomass, fruit number, leaf tannins and total saponins of P. americana (Figs 2, 4 and 5). In the first year of our experiment, variations in heavy metal contents were observed in the roots and leaves of P. americana sourced from different latitudes (Fig. 6A, B), highlighting the influence of geographical origin on the plant’s capacity to accumulate and respond to these contaminants. Overall, our findings underscore the complex interplay between heavy metal stress, plant adaptation, and the potential for phenotypic plasticity in response to environmental challenges. Additionally, herbivory also influenced the latitudinal trends in underground biomass, total biomass and leaf saponin content of P. americana (Figs 2 and 5). This indicates that plants can reduce the effects of herbivory through trait variation, as observed in other studies (Oduor et al., 2011; Schiestl et al., 2014; Hawkins, 2018). Notably, the observed variability in this trait was found to be geographically distinct, with distinct patterns emerging across different latitudes. This latitudinal variation underscores the intricate diversity of plant adaptation strategies in response to environmental stresses, highlighting the intricate interplay between plant trait variation and environmental cues.
The study’s findings indicate that the invasive plant species P. americana has evolved a survival strategy in response to the less favourable conditions of reduced precipitation and lower temperatures typically found at higher latitudes. This strategy is characterized by an increased allocation of resources to growth and defence mechanisms, while reducing the investment in reproductive processes, evidenced by the observed increase in subsurface biomass, total biomass and leaf tannin content as latitude increases (Figs 2 and 5), as well as a decrease in the reproductive quality fraction (Fig. 4). These observations highlight the importance of intraspecific variability in physiological and ecological traits, which are crucial for the plant’s ability to adapt to both biotic and abiotic factors during the invasion process. In a broader ecological context, a reduced allocation to defence and reproduction may enhance growth and metabolite synthesis, which could improve tolerance to abiotic stressors and facilitate the plant’s successful invasion across a range of diverse environments.
Our study offers novel perspectives into the realms of ecology and evolutionary biology, elucidating that the phenotypic disparities observed in P. americana across various latitudes stem primarily from the concerted action of phenotypic plasticity and genetic diversity. The adaptive response of diverse P. americana populations to a broad spectrum of environmental stressors, notably heavy metal and herbivorous pressures, entails intricate trade-offs pertaining to growth, reproduction and defence mechanisms. These findings underscore the remarkable adaptability of P. americana, which is manifested through its dynamic modulation of growth, defence and reproductive strategies in the context of escalating environmental contamination, particularly heavy metal pollution. Consequently, the imperative for reinforcing measures aimed at the prevention, control and management of invasive species becomes even more pronounced.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following. Table S1: collection sites of P. americana seeds along with the mean annual temperature, mean annual precipitation and average seed mass information for each collection site. Table S2: effects of latitude, cover treatment and heavy metal on growth traits in the first year of P. americana. Table S3: effects of latitude, cover treatment and heavy metal on reproductive traits of P. americana in the first year. Table S4: effect of latitude, cover treatment, and heavy metal on the contents of resistance substances and nutrients in the first year of P. americana leaves. Figure S1: relationship between second-year growth traits and latitude of P. americana: plant height (A), basal diameter (B) and plant size (C). Figure S2: seed mass from different sampling sites.
ACKNOWLEDGEMENTS
We thank Wang Yi and Li Bo for their support in funding access, methods and resources, as well as Yuxin Lai, Yunshan Liu, Yulin Li and Yan Wang for their help in the homogeneous garden experiment.
Contributor Information
Zhisen Yan, Ministry of Education Key Laboratory for Transboundary Eco Security of Southwest China, Yunnan Key Laboratory of Plant Reproductive Adaptation and Evolutionary Ecology and Centre for Invasion Biology, Institute of Biodiversity, School of Ecology and Environmental Science, Yunnan University, Kunming, Yunnan, 650504, China.
Yue Zhou, Ministry of Education Key Laboratory for Transboundary Eco Security of Southwest China, Yunnan Key Laboratory of Plant Reproductive Adaptation and Evolutionary Ecology and Centre for Invasion Biology, Institute of Biodiversity, School of Ecology and Environmental Science, Yunnan University, Kunming, Yunnan, 650504, China.
Yuxin Lai, Ministry of Education Key Laboratory for Transboundary Eco Security of Southwest China, Yunnan Key Laboratory of Plant Reproductive Adaptation and Evolutionary Ecology and Centre for Invasion Biology, Institute of Biodiversity, School of Ecology and Environmental Science, Yunnan University, Kunming, Yunnan, 650504, China.
Yunshan Liu, Ministry of Education Key Laboratory for Transboundary Eco Security of Southwest China, Yunnan Key Laboratory of Plant Reproductive Adaptation and Evolutionary Ecology and Centre for Invasion Biology, Institute of Biodiversity, School of Ecology and Environmental Science, Yunnan University, Kunming, Yunnan, 650504, China.
Yulin Li, Ministry of Education Key Laboratory for Transboundary Eco Security of Southwest China, Yunnan Key Laboratory of Plant Reproductive Adaptation and Evolutionary Ecology and Centre for Invasion Biology, Institute of Biodiversity, School of Ecology and Environmental Science, Yunnan University, Kunming, Yunnan, 650504, China.
Yan Wang, Ministry of Education Key Laboratory for Transboundary Eco Security of Southwest China, Yunnan Key Laboratory of Plant Reproductive Adaptation and Evolutionary Ecology and Centre for Invasion Biology, Institute of Biodiversity, School of Ecology and Environmental Science, Yunnan University, Kunming, Yunnan, 650504, China.
Bo Li, Ministry of Education Key Laboratory for Transboundary Eco Security of Southwest China, Yunnan Key Laboratory of Plant Reproductive Adaptation and Evolutionary Ecology and Centre for Invasion Biology, Institute of Biodiversity, School of Ecology and Environmental Science, Yunnan University, Kunming, Yunnan, 650504, China.
Yi Wang, Ministry of Education Key Laboratory for Transboundary Eco Security of Southwest China, Yunnan Key Laboratory of Plant Reproductive Adaptation and Evolutionary Ecology and Centre for Invasion Biology, Institute of Biodiversity, School of Ecology and Environmental Science, Yunnan University, Kunming, Yunnan, 650504, China.
FUNDING
The study was supported by the National Key Research and Development Program of China (2022YFC2601100 and 2023YFC2604500), the National Natural Science Foundation of China (U2102218 and 32371751), the Scientific Research Fund project of Yunnan Education Department (2023Y0208) and the Postgraduate Research and Innovation Foundation of Yunnan University (KC-22221423).
DATA AVAILABILITY
All data included in this study are available upon request by contact with the corresponding author.
LITERATURE CITED
- Abdala-Roberts L, Moreira X, Rasmann S, Parra-Tabla V, Mooney KA. 2016. Test of biotic and abiotic correlates of latitudinal variation in defences in the perennial herb Ruellia nudiflora. Journal of Ecology 104: 580–590. [Google Scholar]
- Bhattarai GP, Meyerson LA, Anderson J, Cummings D, Allen WJ, Cronin JT. 2017. Biogeography of a plant invasion: genetic variation and plasticity in latitudinal clines for traits related to herbivory. Ecological Monographs 87: 57–75. [Google Scholar]
- Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D. 2005. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144: 1–11. [DOI] [PubMed] [Google Scholar]
- Chaturvedi GS, Aggarwal PK, Singh AK, Joshi MG, Sinha SK. 1981. Effect of irrigation on tillering in wheat, triticale and barley in a water-limited environment. Irrigation Science 2: 225–235. [Google Scholar]
- Chowdhury A, Maiti SK. 2016. Identification of metal tolerant plant species in mangrove ecosystem by using community study and multivariate analysis: a case study from Indian Sunderban. Environmental Earth Sciences 75: 1–21. [Google Scholar]
- Colautti RI, Eckert CG, Barrett SCH. 2010. Evolutionary constraints on adaptive evolution during range expansion in an invasive plant. Proceedings Biological Sciences 277: 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley PD, Aide TM. 1991. Comparison of herbivory and plant defenses in temperate and tropical broad-leaved forests. In: Price PW, Lewinsohn TM, Wilson Fernandes G, Benson WW. eds. Plant–animal interactions: evolutionary ecology in tropical temperate regions. New York: Wiley, pp. 25–49. [Google Scholar]
- Coley PD, Bryant JP, Chapin FS. 1985. Resource availability and plant antiherbivore defense. Science 230: 895–899. [DOI] [PubMed] [Google Scholar]
- Cordell S, Goldstein G, Mueller-Dombois D, Webb D, Vitousek PM. 1998. Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: the role of phenotypic plasticity. Oecologia 113: 188–196. [DOI] [PubMed] [Google Scholar]
- Cronin JT, Bhattarai GP, Allen WJ, Meyerson LA. 2015. Biogeography of a plant invasion: plant–herbivore interactions. Ecology 96: 1115–1127. [DOI] [PubMed] [Google Scholar]
- DalCorso G, Manara A, Furini A. 2013. An overview of heavy metal challenge in plants: from roots to shoots. Metallomics 5: 1117–1132. [DOI] [PubMed] [Google Scholar]
- Davidson AM, Jennions M, Nicotra AB. 2011. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecology Letters 14: 419–431. [DOI] [PubMed] [Google Scholar]
- Deng H, Ye ZH, WongWong MH. 2006. Lead and zinc accumulation and tolerance in populations of six wetland plants. Environmental Pollution 141: 69–80. [DOI] [PubMed] [Google Scholar]
- Dlugosch KM, Parker IM. 2008. Invading populations of an ornamental shrub show rapid life history evolution despite genetic bottlenecks. Ecology Letters 11: 701–709. [DOI] [PubMed] [Google Scholar]
- Dubey S, Shri M, Gupta A, Rani V, Chakrabarty D. 2018. Toxicity and detoxification of heavy metals during plant growth and metabolism. Environmental Chemistry Letters 16: 1169–1192. [Google Scholar]
- Ejaz U, Khan SM, Khalid N, et al. 2023. Detoxifying the heavy metals: a multipronged study of tolerance strategies against heavy metals toxicity in plants. Frontiers in Plant Science 14: 1154571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endara M-J, Coley PD. 2011. The resource availability hypothesis revisited: a meta-analysis. Functional Ecology 25: 389–398. [Google Scholar]
- Fine PVA, Mesones I, Coley PD. 2004. Herbivores promote habitat specialization by trees in Amazonian forests. Science 305: 663–665. [DOI] [PubMed] [Google Scholar]
- Foroughi S, Baker AJM, Roessner U, Johnson AAT, Bacic A, Callahan DL. 2014. Hyperaccumulation of zinc by Noccaea caerulescens results in a cascade of stress responses and changes in the elemental profile. Metallomics 6: 1671–1682. [DOI] [PubMed] [Google Scholar]
- Friend DJC. 1965. Tillering and leaf production in wheat as affected by temperature and light intensities. Canadian Journal of Botany 43: 1063–1076. [Google Scholar]
- Fu W, Huang K, Cai H-H, et al. 2017. Exploring the potential of naturalized plants for phytoremediation of heavy metal contamination. International Journal of Environmental Research 11: 515–521. [Google Scholar]
- Funk JL. 2008. Differences in plasticity between invasive and native plants from a low resource environment. Journal of Ecology 96: 1162–1173. [Google Scholar]
- Geng Y-P, Pan X-Y, Xu C-Y, et al. 2006. Phenotypic plasticity rather than locally adapted ecotypes allows the invasive alligator weed to colonize a wide range of habitats. Biological Invasions 9: 245–256. [Google Scholar]
- Ha H, Olson JR, Bian L, Rogerson PA. 2014. Analysis of heavy metal sources in soil using kriging interpolation on principal components. Environmental Science & Technology 48: 4999–5007. [DOI] [PubMed] [Google Scholar]
- Hartnett DC. 1990. Size-dependent allocation to sexual and vegetative reproduction in four clonal composites. Oecologia 84: 254–259. [DOI] [PubMed] [Google Scholar]
- Hawkins NJ. 2018. Digest: plants adapt under attack: genotypic selection and phenotypic plasticity under herbivore pressure. Evolution 72: 1184–1185. [DOI] [PubMed] [Google Scholar]
- Hejda M, Pyšek P, Pergl J, Sádlo J, Chytrý M, Jarošík V. 2009. Invasion success of alien plants: do habitat affinities in the native distribution range matter? Global Ecology and Biogeography 18: 372–382. [Google Scholar]
- Helsen K, Acharya KP, Graae BJ, et al. 2020. Earlier onset of flowering and increased reproductive allocation of an annual invasive plant in the north of its novel range. Annals of Botany 126: 1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Ding J. 2015. Effects of generalist herbivory on resistance and resource allocation by the invasive plant, Phytolacca americana. Insect Science 23: 191–199. [DOI] [PubMed] [Google Scholar]
- Jadia CD, Fulekar MH. 2009. Phytoremediation of heavy metals: recent techniques. African Journal of Biotechnology 8: 921–928. [Google Scholar]
- Karger DN, Conrad O, Boehner J, et al. 2017. Data descriptor: climatologies at high resolution for the earth’s land surface areas. Scientific Data 4: 170122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann J, Bañuelos MJ. 2004. Latitudinal trends in growth and phenology of the invasive alien plant Impatiens glandulifera (Balsaminaceae). Diversity and Distributions 10: 377–385. [Google Scholar]
- Lee CE. 2002. Evolutionary genetics of invasive species. Trends in Ecology & Evolution 17: 386–391. [Google Scholar]
- Leiblein-Wild MC, Tackenberg O. 2014. Phenotypic variation of 38 European Ambrosia artemisiifolia populations measured in a common garden experiment. Biological Invasions 16: 2003–2015. [Google Scholar]
- Li B, Suzuki JI, Toshihiko H. 1998. Latitudinal variation in plant size and relative growth rate in Arabidopsis thaliana. Oecologia 115: 293–301. [DOI] [PubMed] [Google Scholar]
- Li J, Leng Z, Wu Y, et al. 2021. Interactions between invasive plants and heavy metal stresses: a review. Journal of Plant Ecology 15: 429–436. [Google Scholar]
- Liu DF, Chen L, Chen C, et al. 2022. Effect of plant VOCs and light intensity on growth and reproduction performance of an invasive and a native Phytolacca species in China. Ecology and Evolution 12: e8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llugany M, Martin SR, Barceló J, Poschenrieder C. 2013. Endogenous jasmonic and salicylic acids levels in the Cd-hyperaccumulator Noccaea (Thlaspi) praecox exposed to fungal infection and/or mechanical stress. Plant Cell Reports 32: 1243–1249. [DOI] [PubMed] [Google Scholar]
- Maksymiec W, Wianowska D, Dawidowicz AL, Radkiewicz S, Mardarowicz M, Krupa Z. 2005. The level of jasmonic acid in Arabidopsis thaliana and Phaseolus coccineus plants under heavy metal stress. Journal of Plant Physiology 162: 1338–1346. [DOI] [PubMed] [Google Scholar]
- Maron JL, Vilà M, Bommarco R, Elmendorf S, Beardsley P. 2004. Rapid evolution of an invasive plant. Ecological Monographs 74: 261–280. [Google Scholar]
- Marquis RJ, Ricklefs RE, Abdala-Roberts L. 2012. Testing the low latitude/high defense hypothesis for broad-leaved tree species. Oecologia 169: 811–820. [DOI] [PubMed] [Google Scholar]
- Montague JL, Barrett SCH, Eckert CG. 2007. Re-establishment of clinal variation in flowering time among introduced populations of purple loosestrife (Lythrum salicaria, Lythraceae). Journal of Evolutionary Biology 21: 234–245. [DOI] [PubMed] [Google Scholar]
- Moreira X, Mooney KA, Rasmann S, et al. 2014. Trade-offs between constitutive and induced defences drive geographical and climatic clines in pine chemical defences. Ecology Letters 17: 537–546. [DOI] [PubMed] [Google Scholar]
- Moreira X, Castagneyrol B, Abdala-Roberts L, et al. 2018. Latitudinal variation in plant chemical defences drives latitudinal patterns of leaf herbivory. Ecography 41: 1124–1134. [Google Scholar]
- Naz M, Ghani MI, Sarraf M, Liu M, Fan X. 2022. Ecotoxicity of nickel and its possible remediation. Phytoremediation: 297–322. [Google Scholar]
- Oduor AMO, Lankau RA, Strauss SY, Gomez JM. 2011. Introduced Brassica nigra populations exhibit greater growth and herbivore resistance but less tolerance than native populations in the native range. New Phytologist 191: 536–544. [DOI] [PubMed] [Google Scholar]
- Olsson K, Ågren J. 2002. Latitudinal population differentiation in phenology, life history and flower morphology in the perennial herb Lythrum salicaria. Journal of Evolutionary Biology 15: 983–996. [Google Scholar]
- Parker IM, Rodriguez J, Loik ME. 2003. An evolutionary approach to understanding the biology of invasions: local adaptation and general-purpose genotypes in the weed Verbascum thapsus. Conservation Biology 17: 59–72. [Google Scholar]
- Peña-Castro JM, Martínez-Jerónimo F, Esparza-García F, Cañizares-Villanueva RO. 2004. Phenotypic plasticity in Scenedesmus incrassatulus (Chlorophyceae) in response to heavy metals stress. Chemosphere 57: 1629–1636. [DOI] [PubMed] [Google Scholar]
- Pennings SC, Siska EL, Bertness MD. 2001. Latitudinal differences in plant palatability in Atlantic coastal salt marshes. Ecology 82: 1344–1359. [Google Scholar]
- Pigliucci M. 2006. Evolution of phenotypic plasticity: where are we going now? Trends in Ecology Evolution 20: 481–486. [DOI] [PubMed] [Google Scholar]
- Rasmann S, Agrawal AA. 2011. Latitudinal patterns in plant defense: evolution of cardenolides, their toxicity and induction following herbivory. Ecology Letters 14: 476–483. [DOI] [PubMed] [Google Scholar]
- Richardson DM, Pyšek P. 2006. Plant invasions: merging the concepts of species invasiveness and community invasibility. Progress in Physical Geography: Earth and Environment 30: 409–431. [Google Scholar]
- Richardson DM, Pyšek P. 2012. Naturalization of introduced plants: ecological drivers of biogeographical patterns. New Phytologist 196: 383–396. [DOI] [PubMed] [Google Scholar]
- Sakata Y, Craig TP, Itami JK, Yamasaki M, Ohgushi T. 2017. Parallel environmental factors drive variation in insect density and plant resistance in the native and invaded ranges. Ecology 98: 2873–2884. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K. 2009. Is there a latitudinal gradient in the importance of biotic interactions? Annual Review of Ecology, Evolution, and Systematics 40: 245–269. [Google Scholar]
- Schiestl FP, Kirk H, Bigler L, Cozzolino S, Desurmont GA. 2014. Herbivory and floral signaling: phenotypic plasticity and tradeoffs between reproduction and indirect defense. New Phytologist 203: 257–266. [DOI] [PubMed] [Google Scholar]
- Seneviratne M, Gunaratne S, Bandara T, et al. 2016. Plant growth promotion by Bradyrhizobium japonicum under heavy metal stress. South African Journal of Botany 105: 19–24. [Google Scholar]
- Sexton JP, McKay JK, Sala A. 2002. Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecological Applications 12: 1652–1660. [Google Scholar]
- Shahid M, Dumat C, Khalid S, Schreck E, Xiong T, Niazi NK. 2016. Foliar heavy metal uptake, toxicity and detoxification in plants: a comparison of foliar and root metal uptake. Journal of Hazardous Materials 325: 36–58. [DOI] [PubMed] [Google Scholar]
- Shi G, Cai Q. 2009. Leaf plasticity in peanut (Arachis hypogaea L.) in response to heavy metal stress. Environmental and Experimental Botany 67: 112–117. [Google Scholar]
- Stinchcombe JR, Weinig C, Ungerer M, et al. 2004. A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proceedings of the National Academy of Sciences of the USA 101: 4712–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides KA, Dukes JS. 2007. Plant invasion across space and time: factors affecting nonindigenous species success during four stages of invasion. New Phytologist 176: 256–273. [DOI] [PubMed] [Google Scholar]
- Van Assche F, Clijsters H. 2006. Effects of metals on enzyme activity in plants. Plant, Cell and Environment 13: 195–206. [Google Scholar]
- Vedder D, Leidinger L, Sarmento Cabral J. 2021. Propagule pressure and an invasion syndrome determine invasion success in a plant community model. Ecology and Evolution 11: 17106–17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wei M, Cheng H, Wu B, Du D, Wang C. 2020. Indigenous plant species and invasive alien species tend to diverge functionally under heavy metal pollution and drought stress. Ecotoxicology and Environmental Safety 205: 111160. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen C, Xiong Y, Wang Y, Li Q. 2021a. Combination effects of heavy metal and inter-specific competition on the invasiveness of Alternanthera philoxeroides. Environmental and Experimental Botany 189: 104532. [Google Scholar]
- Wang Y, Xiong Y, Wang Y, Li Q. 2021b. Long period exposure to serious cadmium pollution benefits an invasive plant (Alternanthera philoxeroides) competing with its native congener (Alternanthera sessilis). Science of the Total Environment 786: 147456. [DOI] [PubMed] [Google Scholar]
- Wolfe LM. 1993. Inbreeding depression in Hydrophyllum appendiculatum: role of maternal effects, crowding, and parental mating history. Evolution 47: 374–386. [DOI] [PubMed] [Google Scholar]
- Woods EC, Sultan SE. 2022. Post-introduction evolution of a rapid life-history strategy in a newly invasive plant. Ecology 103: 3803–3803. [DOI] [PubMed] [Google Scholar]
- Xiao L, Herve MR, Carrillo J, Ding J, Huang W. 2019. Latitudinal trends in growth, reproduction and defense of an invasive plant. Biological Invasions 21: 189–201. [Google Scholar]
- Yadav SK. 2010. Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African Journal of Botany 76: 167–179. [Google Scholar]
- Yulong F, Zhiyong L, Ru Z, Yulong Z, Yanbao L. 2009. Adaptive evolution in response to environmental gradients and enemy release in invasive alien plant species. Biodiversity Science 17: 340. [Google Scholar]
- Zhang B, Chen H, Hou X, et al. 2015. Ecological response of reproductive performance of Stipa baicalensis in Xilingol steppe of Inner Mongolia. Journal of Gansu Agricultural University 50: 103–108. [Google Scholar]
- Zhao F, Ma Y, Zhu Y, Tang Z, McGrath SP. 2015. Soil contamination in China: current status and mitigation strategies. Environmental Science & Technology 49: 750–759. [DOI] [PubMed] [Google Scholar]
- Zhou B, Yan X, Xiao Y, Zhang Z, Li X, Yang J. 2013. Traits of reproductive biology associated with invasiveness in alien invasive plant Phytolacca americana. Ecology and Environmental Sciences 22: 567–574. [Google Scholar]
- Zhu B, Huang F, Fan Z, Huang Q. 2022. Responses to nutrient addition, shading and clipping in a range expanding invasive plant, Mikania micrantha. Weed Research 62: 372–379. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data included in this study are available upon request by contact with the corresponding author.