Abstract
Background and Aims
Rock outcrop vegetation is distributed worldwide and hosts a diverse and unique flora that evolved under harsh environmental conditions. Unfortunately, seed ecology in such ecosystems has received little attention, especially regarding seed traits, germination responses to abiotic factors and the potential role of phylogenetic relatedness in shaping such features. Here, we provide the first quantitative and phylogenetically informed synthesis of the seed functional ecology of Brazilian rock outcrop vegetation, with a particular focus on quartzitic and ironstone campo rupestre.
Methods
Using a database of functional trait data, we calculated the phylogenetic signal for seven seed traits for 371 taxa and tested whether they varied among growth forms, geographic distribution and microhabitats. We also conducted meta-analyses that included 4252 germination records for 102 taxa to assess the effects of light, temperature and fire-related cues on the germination of campo rupestre species and explored how the aforementioned ecological groups and seed traits modulate germination responses.
Key Results
All traits and germination responses showed a moderate to strong phylogenetic signal. Campo rupestre species responded positively to light and had maximum germination between 20 and 25 °C. The effect of temperatures beyond this range was moderated by growth form, species geographic distribution and microhabitat. Seeds exposed to heat shocks above 80 °C lost viability, but smoke accelerated germination. We found a moderating effect of seed mass for responses to light and heat shocks, with larger, dormant seeds tolerating heat better but being less sensitive to light. Species from xeric habitats evolved phenological strategies to synchronize germination during periods of increased soil water availability.
Conclusions
Phylogenetic relatedness plays a major role in shaping the seed ecology of Brazilian rock outcrop vegetation. Nevertheless, seed traits and germination responses varied significantly between growth forms, species geographic distribution and microhabitats, providing support to the regeneration niche hypothesis and the role of functional traits in shaping germination in these ecosystems.
Keywords: Campo de altitude, campo rupestre, canga, germination requirements, inselberg, regeneration niche, seed dispersal, seed dormancy, seed mass, seed viability
INTRODUCTION
Rock outcrops are outstanding geological features where the bedrock protrudes above the land’s surface due to the erosion of softer parts of the landscape (Fitzsimons and Michael, 2017). They offer a unique habitat that drastically contrasts with the neighbouring vegetation (Porembski, 2007). Notably, they experience severe surface temperatures and have shallow, poorly developed soils with low water retention capacity, a combination of abiotic factors that has driven the evolution of distinctive traits that allow plants to establish and survive in such harsh environments (Kluge and Brulfert, 2000; Escudero et al., 2015; Oliveira et al., 2016; Bondi et al., 2023). Most studies, however, have focused on the ecophysiology of adult plants, and few comprehensive reviews on germination ecology have been produced to date (e.g. Wyatt, 1997; Biedinger et al., 2000). As a result, the potential role of regeneration traits (Donohue et al., 2010; Larson and Funk, 2016) and regeneration niche (Grubb, 1977) in shaping ecological processes in rock outcrops has been largely overlooked.
In Brazil, there are four main vegetation types associated with rock outcrops: campo rupestre, canga, campo de altitude and inselbergs (Martinelli, 2007). Campo rupestre occurs on quartzite, sandstone and ironstone outcrops throughout the country, but it is most common at high elevations in the Espinhaço Range, eastern Brazil (Miola et al., 2021). This vegetation is characterized by sandy and rocky grasslands dominated by grasses, sedges and forbs (mostly Xyridaceae and Eriocaulaceae) with isolated patches of shrubs (mainly Melastomataceae, Fabaceae, Asteraceae, Myrtaceae, Vochysiaceae and Clusiaceae) and desiccation-tolerant and epilithic species establishing directly in the rock (Silveira et al., 2016). The campo rupestre that develops on ironstone outcrops receives the name canga and is functionally analogous to the quartzitic outcrops (Jacobi et al., 2007). The campo de altitude is found on granite and gneissic outcrops within the Atlantic Forest and consists of a mosaic of shrubs (mostly Asteraceae, Melastomataceae and Myrtaceae) and small patches of dwarf forests amidst a matrix of grasses (Safford, 1999). In contrast, inselbergs are isolated, dome-shaped granitic outcrops present across the country. In such geological formations, the mat-like vegetation – dominated mostly by Bromeliaceae, Cactaceae, Orchidaceae, Velloziaceae and shrubs from several families – is notably patchy, occurring only in depressions in the rock surface that accumulate soil (Safford and Martinelli, 2000). All these open, grassy–shrubby, fire-prone and highly heterogeneous vegetation types are centres of species richness, phylogenetic diversity and endemism (Porembski, 2007; Silveira et al., 2016; Campos et al., 2018).
The germination ecology of Brazilian rock outcrop vegetation has been reviewed previously (Garcia and Oliveira, 2007; Nunes et al., 2016; Garcia et al., 2020), but these syntheses were restricted to the campo rupestre and focused on the differences in germination responses in a few emblematic families (e.g. Asteraceae, Bromeliaceae, Cactaceae, Eriocaulaceae, Melastomataceae, Velloziaceae and Xyridaceae). Still, based on seminal studies on seed functional ecology, these syntheses put forward various promising hypotheses on the role of seed and germination traits in the ecological dynamics of plant communities. A first hypothesis poses that germination responses are shaped by seed traits, especially seed mass. For example, Nunes et al. (2016) pointed out that campo rupestre species with lighter seeds were more dependent on light for germination than larger ones, as expected under the trade-off between light requirements and seed mass (Milberg et al., 2000; Pearson et al., 2003). Still, such an association between seed traits and germination responses remains to be formally tested for this ecosystem. The second hypothesis is that contrasting microhabitat preferences and geographical ranges derive from distinct regeneration traits, as proposed by the regeneration niche hypothesis (Grubb, 1977; Saatkamp et al., 2019). For instance, species from xeric microhabitats in the campo rupestre have been shown to germinate at higher temperatures than those from mesic environments (Oliveira and Garcia, 2011), and broadly distributed species have broader germination niches than those restricted to a single substrate (Marques et al., 2014). Species with a broader distribution range have also been shown to have a wider germination temperature range (Ranieri et al., 2012). Similar studies, however, have failed to find such a significant association between microhabitat preferences or geographic distributions in the campo rupestre (Oliveira and Garcia, 2011; Silveira et al., 2012a; Giorni et al., 2018), suggesting the patterns might be more complicated than previously thought. Moreover, these studies have been limited to a handful of species within single families, making it impossible to draw broad conclusions for the entire rock outcrop community. A new opportunity to address these questions at the community level has emerged owing to a comprehensive database recently published (Ordóñez‐Parra et al., 2023). Another limitation of previous reviews is that they did not explicitly address the potential role of shared evolutionary history in seed ecology. Local and global scale studies have highlighted that several seed traits, such as seed mass (Moles et al., 2005; Zanetti et al., 2020), dormancy (Willis et al., 2014; Dayrell et al., 2017) and germination requirements (Arène et al., 2017), are phylogenetically clustered, potentially due to a combination of adaptive evolution and phylogenetic conservatism (Vandelook et al., 2012; Carta et al., 2022b). As a result, a proper vegetation-wide assessment of ecological hypotheses regarding seed ecology must incorporate the relative importance of phylogenetic relatedness in seed functional traits and germination responses.
Here, we conducted the first integrative synthesis of seed functional ecology of Brazilian rock outcrop vegetation to address the two previously described long-standing hypotheses and the potential role of phylogenetic relatedness in the seed ecology of these ecosystems. First, we assessed whether seed functional traits associated with different dimensions of the seed ecological spectrum exhibit a significant phylogenetic signal and evaluated whether they differ between growth forms, species geographic distribution and microhabitats. Second, we assessed the effect of light, temperature and fire-related cues on the germination of plant species in these communities through phylogenetically informed meta-analyses. Such abiotic factors are established germination cues in these ecosystems and are the most studied in Brazilian rock outcrop vegetation, allowing us to provide more robust conclusions about their effects on germination (Supplementary Data Fig. S1). We then contrasted germination responses between growth forms, species geographic distribution and microhabitats to test the regeneration niche hypothesis (Grubb, 1977). Finally, we tested if such responses were moderated by seed mass and dormancy – central traits associated with multiple seed functions (Saatkamp et al., 2019). Because rock outcrops occur in all continents and biomes and their associated vegetation is quite similar in structure and function (Barthlott and Porembski, 2000), we hope this study provides a solid starting point towards a global synthesis of the germination ecology of rock outcrop vegetation.
MATERIALS AND METHODS
Data sources
Seed functional traits and germination experiments.
We retrieved seed trait and germination records from Rock n’ Seeds (Ordóñez‐Parra et al., 2023), a database of seed functional traits and germination experiments of plant species from Brazilian rock outcrop vegetation (Fig. 1). This database contains information on 16 functional traits in 383 taxa and 10 187 germination records for 281 taxa. We focused on seven functional traits associated with regeneration from seeds and major seed functions (Grubb, 1977; Saatkamp et al., 2019): seed dry mass (mg), seed water content (%), percentage of embryoless seeds, percentage of viable seeds, seed dispersal syndrome (anemochory, autochory and zoochory), seed dispersal season (early dry, late dry, early rain and late rainy season) and primary dormancy [dormant (D) and non-dormant (ND)]. This subset of traits included data from 103 studies on 371 species. We also retrieved information from experiments assessing the effects of light availability, constant and alternating temperatures and fire-related cues (i.e. heat shocks and smoke) from 22 studies and 102 species, totalling 4252 germination records. Beyond seed and germination traits, the database also provides species-level data on growth form, microhabitat (‘xeric’ substrates experiencing pronounced seasonal drought, otherwise ‘mesic’), and geographic distribution (‘restricted’ to rock outcrop vegetation, and ‘widespread’ if found in other vegetation types in addition to rock outcrops) (Ordóñez‐Parra et al., 2023).
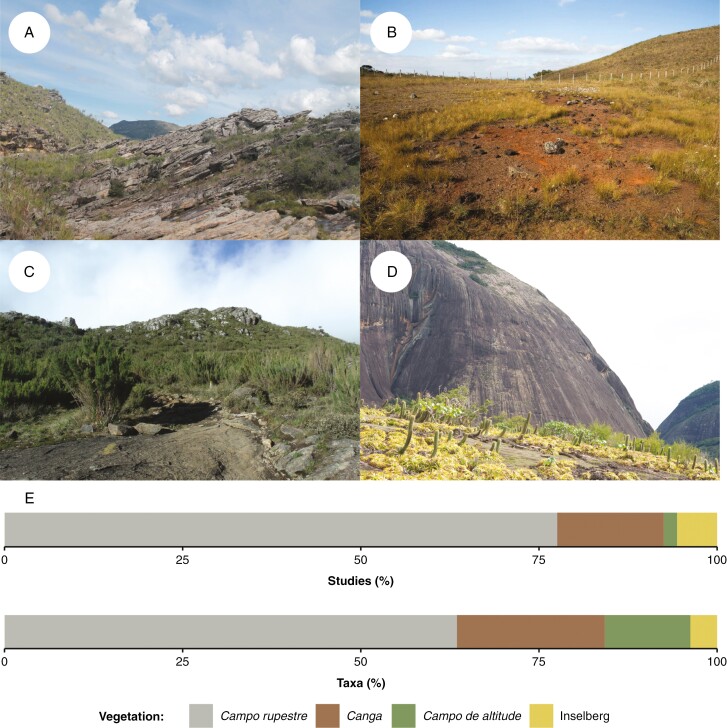
Fig. 1.
The four main vegetation types associated with rock outcrops in Brazil and their representation in the Rock n’ Seeds database (Ordóñez‐Parra et al., 2023). (A) A typical campo rupestre landscape at Serra do Cipó. (B) Canga vegetation at Parque Estadual da Serra do Rola Moça. (C) Campo de altitude at Parque Nacional do Caparaó. (D) Inselberg vegetation at Teófilo Otoni municipality. (E) Percentage of studies and taxa evaluated on each vegetation type. All locations are in the State of Minas Gerais. Photographs by Carlos A. Ordóñez-Parra (A), Roberta L. C. Dayrell (B), Daniela Calaça (C) and Fernando A. O. Silveira (D).
Phylogenetic tree.
We reconstructed the phylogenetic tree of the studied species using the package V.PhyloMaker2 (Jin and Qian, 2022). First, species names were checked and updated following The Leipzig Catalogue of Vascular Plants using the R package lcvplants (Freiberg et al., 2020). We kept taxa identified at the species level, and all subspecies and varieties (18 cases) were upgraded to the species level. A phylogenetic tree for the resulting 370 species was generated, based on the GBOTB phylogeny (Smith and Brown, 2018), which was updated and standardized following Freiberg et al. (2020). Taxa absent from this phylogeny were bound to their designated relatives using the bind.relative function of V.PhyloMaker2 based on different sources (Almeda et al., 2016; Rivera et al., 2016). Species with no known relatives in the phylogeny (Austrocritonia velutina, Cavalcantia glomerata, Cavalcantia percymosa and Parapiqueria cavalcantei) were added using the at.node function from the ape package (Paradis and Schliep, 2019). All these species belong to the Eupatorieae (Asteraceae), a tribe where infrageneric relationships remain unresolved (Rivera et al., 2016). Therefore, they were added to the base of the tribe as polytomies. Most infrageneric relationships in the phylogeny remained unresolved, appearing as polytomies, partially because the relationships within some genera of the highly diverse families in our database have low support (Alcantara et al., 2018; Guimarães et al., 2019). Nevertheless, phylogenetic metrics estimated from trees resolved to the genus level have been shown to be highly correlated with those derived from fully resolved trees, suggesting that these polytomies do not weaken the results (Qian and Jin, 2021).
Statistical analyses
All analyses were made using R v. 4.3.2 (R Core Team, 2023), and is available on GitHub (https://github.com/caordonezparra/outcrop_synthesis) (see Open Data).
Variation and phylogenetic signal of seed functional traits.
To test for the phylogenetic signal in the quantitative seed traits (i.e. dry mass, water content, percentage of viable and embryoless seeds), we calculated Pagel’s λ (Pagel, 1999) using the phylosig function from the package phytools (Revell, 2012). Values of λ range from 0 to 1, with λ = 0 indicating that there is no phylogenetic signal; thus, related taxa are not more similar than expected by chance, and λ = 1 implies a strong phylogenetic signal, so the traits of related taxa are more similar than expected by chance (Pagel, 1999). Pagel’s λ was selected among the available phylogenetic signal indices given its robustness in measuring phylogenetic signals based on incompletely resolved phylogenies (Molina-Venegas and Rodríguez, 2017). We also calculated Moran’s I coefficient to assess the phylogenetic autocorrelation of traits at the genus, family and order levels using the correlogram.formula function from ape (Paradis and Schliep, 2019). Values of Moran’s I range from −1 to 1, with I = 0 indicating that species resemble each other as expected by the Brownian motion model. Values above and below zero imply that related species are more or less similar than expected by such a model, respectively (Moran, 1950; Gittleman and Kot, 1990). All these tests were carried out using log-transformed seed mass values and logit-transformed water content, and percentage of embryoless and viable seed values to reduce the skewness of the data. For species with more than one record (25 species), we carried out the analyses using the average species value.
For the qualitative seed traits (i.e. seed dormancy, seed dispersal syndrome and seed dispersal season), the phylogenetic signal was tested by implementing the rtestdeciv function from the adiv package using 9999 permutations (Pavoine, 2020). This method decomposes trait diversity among the nodes of the phylogenetic tree and assesses whether it is skewed towards the tree’s root or tips, with significant root skewness implying the presence of a significant phylogenetic signal. Seed dormancy and dispersal season were treated as multichoice variables, as 11 species had records of both D and ND seeds, and 18 species reported more than one dispersal season.
To test for differences in quantitative traits between ecological groups (growth form, microhabitat and geographic distribution), we used phylogenetic generalized least squares models as implemented in the package caper (Orme et al., 2018). Likewise, qualitative trait variation was assessed using a phylogenetic logistic regression (Ives and Garland, 2010) implemented in the phylolm package (Ho and Ané, 2014). Because dispersal season and syndrome had more than two possible states, individual models were run to assess the probability of each season and syndrome. Species with records for more than one distribution class were classified as ‘widespread’, and species with occurrence in both microhabitats were coded as ‘mesic/xeric’. For this analysis, species with records from more than one combination of qualitative traits were included as different populations, using the add.taxa.phylo function from the daee package (Debastiani, 2021) and keeping the tree ultrametric.
Preliminary tests showed that the interaction between our qualitative predictors did not significantly affect any of the tested variables. Moreover, the models that included such interactions were outperformed by simple additive models based on Akaike information criteria (see code provided). As a result, our final models did not include these interactions. Growth form comparisons were made between herbs and shrubs only due to the low sample sizes of trees (26 species), succulents (9 species) and lianas (6 species).
Meta-analyses of germination responses to abiotic factors.
We estimated the overall effects of (1) light, (2) constant and alternating temperatures, and (3) fire-related cues on germination through meta-analyses. We achieved this by fitting phylogenetic generalized linear mixed models with Bayesian estimates using the Markov chain Monte Carlo (MCMC) method, an approach that has been previously used in meta-analyses of primary germination data (Vandelook et al., 2018; Fernández‐Pascual et al., 2021, 2022; Carta et al., 2022a). This analysis was restricted to campo rupestre and canga species, given the lack of data from campo de altitude and the small sample size of independent observations for inselbergs (two studies, three species) for these abiotic factors. Germination records under complete darkness were used as controls to test the effects of light. Also, given the overall positive effect of light on germination (see Results), only experiments carried out under light conditions were used to assess the effects of different temperatures and fire-related cues on seed germination. We used 25 °C as a control for constant and alternating temperatures because it is considered the optimum temperature for most species in this ecosystem (Garcia and Oliveira, 2007; Nunes et al., 2016; Garcia et al., 2020). Finally, we used untreated seeds (i.e. seeds not exposed to either heat shock or smoke) as controls for fire-related cues.
We assessed the effects of these abiotic factors on the proportion of germinated seeds and median germination time (t50). The numbers of germinated and ungerminated seeds were taken from the raw germination data deposited in Rock n’ Seeds. To avoid confounding effects from treatments with extremely low germination, we only included observations where either the control or treatment had ≥10 % germination. The t50 (Coolbear et al., 1984 as modified by Farooq et al., 2005) was calculated for the experiments that included raw daily germination data using the germinationmetrics package (Aravind et al., 2022). We calculated this index only for observations where both the control and treatment conditions had ≥10 % germination. Moreover, we assessed the effect of light, constant and alternating temperatures as germination cues in ND seeds or in seeds where dormancy had been experimentally alleviated. Alternating temperatures are known to break physical dormancy (Baskin and Baskin, 2014), but none of the studies in the dataset have tested this. In contrast, we included D species in the meta-analyses of fire-related cues because these are known to break the seed dormancy in some species in fire-prone ecosystems (Baskin and Baskin, 2014). Species with non-conclusive dormancy records were excluded.
We evaluated the effect of each abiotic factor (i.e. light, temperature and fire) on germination proportions and t50 on the whole dataset and separately for each growth form (herbs and shrubs), geographic distribution (widespread and restricted) and microhabitat (mesic, xeric and mesic/xeric). We also tested the interaction of seed mass and seed dormancy on germination responses in the whole dataset, and the interaction effect of seed dormancy only for responses to fire-related cues. Phylogeny, species identity, study and observations were included as random variables. Because seed mass exhibited a significant, strong phylogenetic signal (see Results), the missing seed mass values for 54 species were input using average values for the genera from Rock n’ Seeds (Ordóñez‐Parra et al., 2023) and the Seed Information Database (https://ser-sid.org/). The analyses were performed with the MCMCglmm package (Hadfield, 2010). All models were run with weakly informative priors, with parameter-expanded priors for the random effects. Each model was run for 500 000 MCMC steps, with an initial burn-in phase of 50 000 and a thinning interval of 50 (de Villemereuil and Nakagawa, 2014), resulting in an average of 9000 posterior distributions. We calculated mean parameter estimates and 95 % credible intervals from the resulting posterior distributions. Credible intervals overlapping zero were considered non-significant. To estimate the phylogenetic signal of germination responses, we also estimated the Pagel’s λ of each model, as described by de Villemereuil and Nakagawa (2014).
RESULTS
Phylogenetic signal and variation of seed functional traits
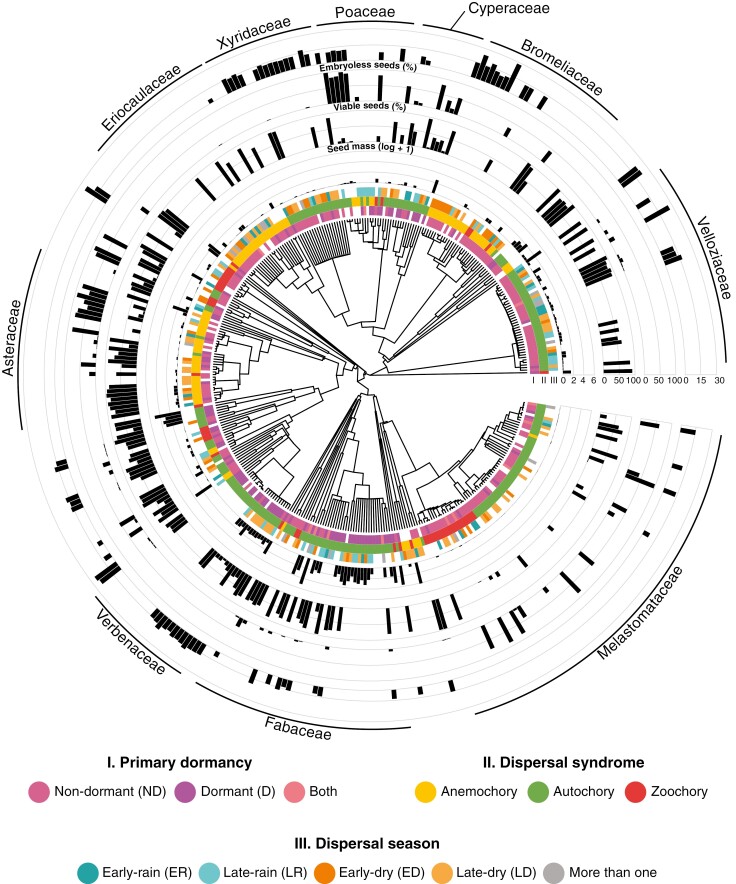
All examined seed traits exhibited a significant phylogenetic signal (Fig. 2, Table 1, P < 0.001). For all quantitative traits (i.e. seed mass, water content, percentage of embryoless seeds and percentage of viable seeds), the phylogenetic signal was moderate to strong (λ between 0.57 and 0.90), meaning that closely related taxa strongly resembled each other at both the genus and family level (Supplementary Data Table S1). At the order level, we only found a significant and negative phylogenetic autocorrelation for seed mass (I = −0.39, P < 0.001, Supplementary Data Table S1). Our data did not suggest the presence of phylogenetic autocorrelation for seed water content and the percentage of embryoless and viable seeds at the order level (P > 0.05). A summary of family-level variation in quantitative and qualitative traits is presented in Supplementary Data Fig. S2.
Fig. 2.
Phylogeny of studied species with available information on seed functional traits. Bars represent trait values for each species. The ten families with the largest number of species in the dataset are labelled. The figure was elaborated with the R packages ggtree (Yu et al., 2017), ggtreeExtra (Xu et al., 2021) and ggnewscale (Campitelli, 2022).
Table 1.
Results of phylogenetic signal test for seed traits in Brazilian rocky outcrop vegetation. P-values for quantitative traits come from the likelihood ratio test performed by the phylosig function, while for qualitative traits they correspond to the root-to-tip skewness test performed by the rtestdecdiv function.
| Quantitative traits | ||
|---|---|---|
| λ | P-value | |
| Seed mass | 0.90 | < 0.001 |
| Water content | 0.72 | < 0.001 |
| Percentage of embryoless seeds | 0.75 | < 0.001 |
| Percentage of viable seeds | 0.57 | < 0.001 |
| Qualitative traits | ||
| P-value | ||
| Primary dormancy | <0.001 | |
| Dispersal syndrome | <0.001 | |
| Dispersal season | <0.001 | |
Regarding the variation of quantitative traits, seed mass variation differed between growth forms and microhabitats, with shrubs (t = 2.19, P = 0.030) and species inhabiting both mesic and xeric microhabitats (t = 2.01, P = 0.045) producing heavier seeds than herbs and species restricted to xeric microhabitats, respectively. Seed water content and percentages of embryoless and viable seeds did not vary between any of the groups, and seed mass did not differ between species with distinct geographic distributions (Table 2).
Table 2.
Differences in seed functional traits between growth forms (herbs versus shrubs), and species distribution (restricted versus widespread) and microhabitat (mesic versus mesic/xeric versus xeric) in Brazilian rocky outcrop vegetation. Bold values indicate P-values <0.05. t and z correspond to the values obtained in the t- and z-tests that are part of the implementation of phylogenetic least squares models and phylogenetic logistic regressions, respectively.
| Growth form: shrub | Distribution: widespread | Microhabitat: mesic/xeric | Microhabitat: xeric | |||||
|---|---|---|---|---|---|---|---|---|
| Quantitative traits | t | P-value | t | P-value | t | P-value | t | P-value |
| Seed mass | 2.1872 | 0.0298 | −0.5986 | 0.5501 | 2.0141 | 0.0453 | 1.6115 | 0.1086 |
| Water content | 0.2789 | 0.7811 | −0.0770 | 0.9388 | 0.6075 | 0.5453 | 0.7834 | 0.4358 |
| Embryoless seeds | 1.9274 | 0.0572 | −0.2368 | 0.8134 | 0.9752 | 0.3322 | 1.2967 | 0.1982 |
| Viable seeds | −1.0221 | 0.3085 | 0.2381 | 0.8122 | −0.3040 | 0.7616 | 0.7006 | 0.4847 |
| Qualitative traits | z | P-value | z | P-value | z | P-value | z | P-value |
|---|---|---|---|---|---|---|---|---|
| Dormancy | 1.4125 | 0.1578 | 1.5052 | 0.1323 | 1.5266 | 0.1268 | 2.5218 | 0.0117 |
| Anemochory | −1.2154 | 0.2242 | 1.3943 | 0.1632 | 1.0047 | 0.3150 | 1.1497 | 0.2503 |
| Autochory | 0.0387 | 0.9691 | −0.0710 | 0.9434 | −0.0792 | 0.9368 | −0.0998 | 0.9205 |
| Zoochory | 0.3290 | 0.7422 | 0.0048 | 0.9962 | 0.6844 | 0.4937 | −0.0040 | 0.9968 |
| Early dry | −0.9086 | 0.3636 | −0.1266 | 0.8993 | −1.0427 | 0.2971 | 0.4504 | 0.6524 |
| Late dry | 3.4323 | 0.0006 | −0.7443 | 0.4567 | 0.7421 | 0.4580 | −1.3673 | 0.1713 |
| Early rain | 0.1036 | 0.9175 | −0.7541 | 0.4508 | −0.4839 | 0.6284 | 0.0445 | 0.9645 |
| Late rain | −2.0137 | 0.0440 | −0.1826 | 0.8551 | 2.0319 | 0.0422 | 2.1507 | 0.0315 |
For qualitative traits, we found that shrubs had a higher probability of dispersing seeds during the late dry season (z = 3.43, P < 0.001), while dispersal during the late rainy season was more likely in herbs (z = −2.01, P = 0.044). Primary dormancy and dispersal season varied significantly between microhabitats, with species from xeric environments having higher probabilities of producing dormant seeds (z = 2.52, P = 0.012) and dispersing their seeds during the late rainy season than species from mesic microhabitats (z = 2.15, P = 0.031). Similarly, species from both mesic and xeric microhabitats also had a higher probability of dispersing seeds during the late rainy season (z = 2.03, P = 0.042). We found no significant differences in the probability of any of the dispersal syndromes or the other dispersal seasons (Table 2).
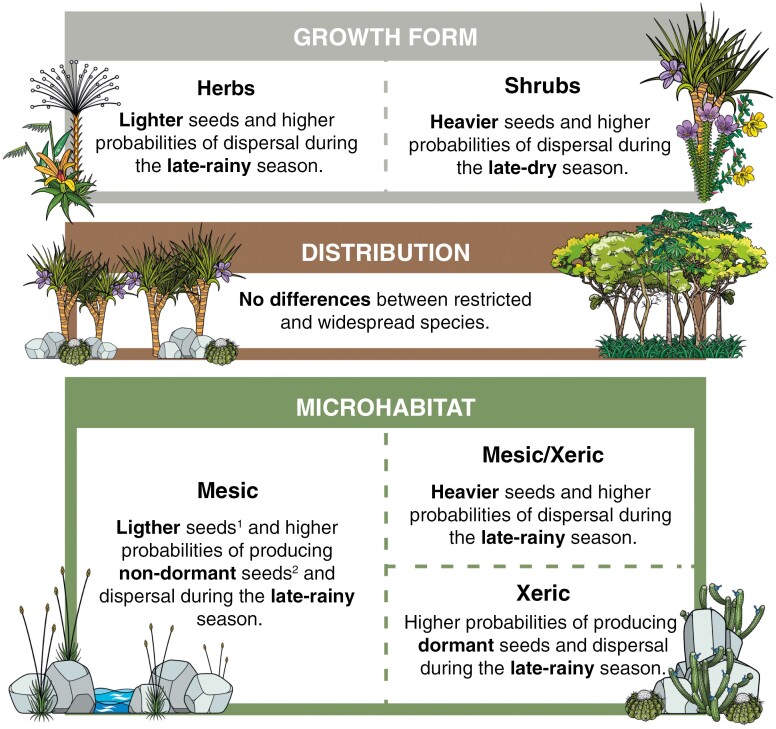
In summary, shrubs tended to produce heavier seeds and to have higher probabilities of dispersal during the late dry season. In contrast, herbs produced relatively smaller seeds with higher probabilities of dispersal during the late rainy season. Species from mesic/xeric microhabitats tended to produce heavier seeds and disperse them during the late rainy season compared with those exclusively from mesic microhabitats. Moreover, species restricted to xeric environments had higher probabilities of producing dormant seeds during late rainy dispersal than those from mesic environments. Finally, species restricted to outcrop vegetation and widespread ones did not differ in any seed trait (Fig. 3).
Fig. 3.
Summary of seed trait differences among growth forms, species geographic distribution and microhabitat, based on results from Table 2. Superscripts in Mesic panel: 1When compared with species from mesic/xeric microhabitats; 2When compared with species from xeric microhabitats.
Meta-analyses of germination responses
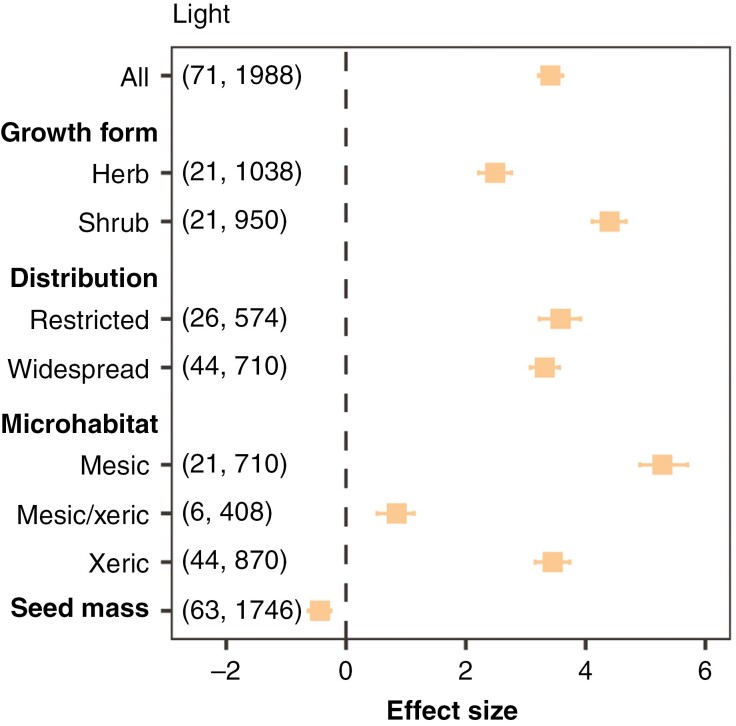
Light positively and strongly affected the final germination proportion regardless of growth form, distribution or microhabitat. Still, light effects were higher for shrubs and species from mesic environments compared with herbs and species from other microhabitats, respectively. Seed responses to light did not vary between species with different geographic distributions. Seed mass had a negative interactive effect with responses to light (Fig. 4).
Fig. 4.
Effect of light availability on germination proportion in campo rupestre species (control: total darkness). Squares indicate the posterior means of the effect for each moderator (growth form, distribution and microhabitat) and whiskers the 95 % credible intervals of the effect size. Numbers in parentheses indicate the numbers of species and observations (each observation is a replicate of a given germination experiment) employed for each model.
Regarding the overall effect of constant temperatures on germination, temperatures below 20 °C had no significant effect on germination proportions when compared with the control. However, temperatures below and above 20 °C had a consistent negative effect on germination proportion and a positive effect on t50. Herbs and shrubs exhibited differential responses to temperature regimes: germination proportion decreased more with decreasing temperature for shrubs, whereas the final germination proportion of herbs increased at 20 °C and remained relatively constant at 30 °C. For instance, the t50 of herbs was not significantly affected by 30 °C, contrasting with shrubs, for which germination was slowed at this temperature. Differential responses were also found for species with contrasting geographic distributions, with species with widespread distribution showing a more severe reduction in the germination proportion at 10 °C as well as a significant negative reduction at 30 °C. Similarly, the germination proportion of species from mesic microhabitats was less negatively affected by 15 °C conditions but was more inhibited at 35 and 40 °C than species from xeric microhabitats. Finally, seed mass exhibited a significant interaction with germination responses to temperature, being positive for germination proportion at 10 and 35 °C and negative at 20 and 40 °C. Still, we did not find a significant interaction between seed mass and germination responses at 15 and 30 °C (Fig. 5A).
Fig. 5.
Germination responses to (A) constant and (B) alternating temperatures in campo rupestre species (control: 25 °C). Squares indicate the standardized mean effect size for each moderator (growth form, distribution and microhabitat) and whiskers the 95 % confidence interval of the effect size. Coloured estimates indicate significant effects (i.e. where confidence intervals do not overlap zero). Numbers in parentheses indicate the numbers of species and observations (each observation is a replicate of a given germination experiment) employed for each model. The empty panel for germination time at 10 and 40 °C refers to a lack of data.
When evaluating the effects of alternating temperatures, we found that 25/15 °C and 30/20 °C regimes had an overall negative and a non-significant effect on germination proportion, respectively. Moreover, both treatments significantly increased t50. The 30/20 °C regime also had a negative effect on the germination proportion in shrubs and increased t50 in herbs. Seed mass had a negative interaction with germination proportion at 25/15 °C and a positive interaction with t50 at both treatments (Fig. 5B).
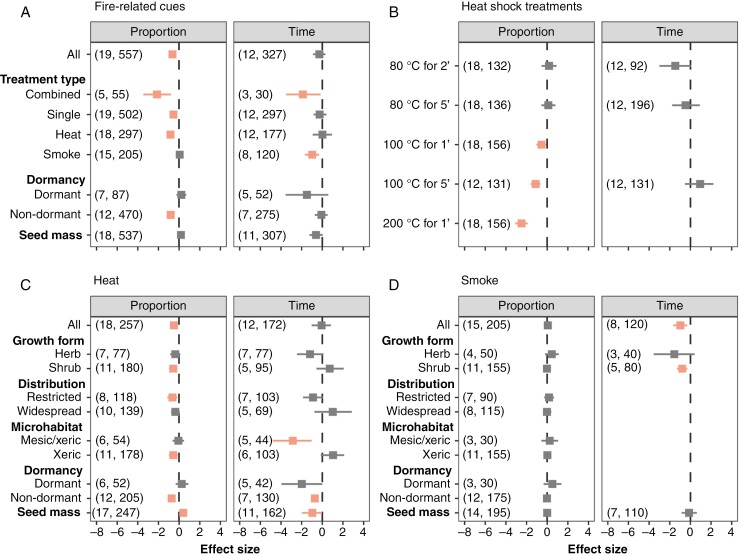
Finally, we found that fire-related cues reduced germination proportion while also affecting t50. The effect varied according to the treatment applied, with heat shocks, alone or in combination with smoke, reducing germination proportion but not germination time. Instead, smoke alone or in combination with heat did not significantly affect germination proportion but significantly reduced t50 (Fig. 6A). When comparing heat shock treatments, we found that treatments heat shocks of 100 °C (both of 1 and 5 min) and 200 °C for 1 min significantly reduced germination proportion, although they did not affect t50 (Fig. 6B). This negative effect of heat shocks on germination proportion varied between growth forms, species distribution and microhabitats, with only shrubs, restricted species and species from xeric microhabitats being negatively affected by heat shocks. Instead, heat shocks did not significantly affect herbs, widespread species, and species from mesic microhabitats (Fig. 6C). The negative effect of smoke on t50 remained significant for shrubs but was non-significant in herbs. Our analysis did not indicate that smoke’s effect was modulated by seed mass (Fig. 6D). Contrastingly, primary dormancy and seed mass altered germination responses to heat shocks, with ND seeds exhibiting a reduction in germination proportion and seed mass holding a positive effect (Fig. 6C).
Fig. 6.
Germination responses to fire-related cues in campo rupestre species (control: untreated seeds). (A) Overall effects. (B) Heat shock temperatures. (C) Heat shocks without 200 °C for 1 min. (D) Smoke. Squares indicate the standardized mean effect size for each moderator (growth form, distribution and microhabitat) and whiskers the 95 % confidence interval of the effect size. Coloured estimates indicate significant effects (i.e. where confidence intervals do not overlap zero). Numbers in parentheses indicate the number of species and observations (each observation is a replicate of a given germination experiment) employed for each model.
Overall, phylogeny played a major role in germination responses to all these abiotic factors, with most of our models having a λ significantly different from zero. Moreover, in most cases species identity had a stronger effect on germination response variability than study or observations within studies (Supplementary Data Tables S2 and S3).
DISCUSSION
Seed mass shapes germination responses in campo rupestre
Our analysis points out a significant interaction between seed mass and germination responses to light, constant temperature and heat shocks, supporting the long-standing first hypothesis that seed mass modulates germination responses. Regarding light, we found a significant and negative interaction between seed mass and germination responses to light, with larger seeds being less responsive to light availability. Such results support the hypothesis raised by Nunes et al. (2016) on the existence of this trade-off in the campo rupestre (Milberg et al., 2000; Pearson et al., 2003).
We also found that seed mass had a significant interaction with response to some temperatures. Nevertheless, there was no overall pattern between germination responses to temperature and seed mass, with a positive interaction with changes in germination proportion at 10 and 30 °C, a negative one at 20 and 40 °C and non-significant interactions at 15 and 35 °C. Despite being significant, the effect of such interactions is rather small (Supplementary Data Tables S2 and S3), which agrees with the results of Arène et al. (2017), who found a weak association between cardinal temperatures and seed mass that disappeared when accounting for phylogenetic relatedness. Therefore, it seems reasonable to conclude that variation in germination responses in campo rupestre is probably better explained by phylogeny rather than by seed mass, an observation supported by the high λ values found in our models. Moreover, the small seed mass variation in our dataset (86 % of records <1 mg) could prevent us from finding a more consistent pattern for the role of seed mass in this ecosystem.
We also found a significant interaction between seed mass and germination responses to heat shocks that indicate that campo rupestre species with larger seeds tend to have increased and faster germination after a heat shock, a result that is consistent with a positive correlation between seed mass and heat tolerance reported in the Cerrado (Ribeiro et al., 2015; Daibes et al., 2019). Such association can be explained by decreasing surface-to-volume ratio with increasing seed mass, indicating that larger, heavier seeds have higher insulation against higher, lethal temperatures (Ruprecht et al., 2015).
Finally, the lack of a significant effect of alternating temperatures or an interaction of such responses with seed mass could be explained by the fact that most campo rupestre species produce small, lighter seeds, which are not expected to benefit from alternating temperature regimes (Pearson et al., 2003).
Seed functional traits are phylogenetically clustered in Brazilian rock outcrop vegetation
Our results indicate that all seed traits showed moderate to strong phylogenetic signals, which is consistent with global studies showing phylogenetic clustering for multiple seed traits, such as seed mass (Moles et al., 2005) and dormancy (Willis et al., 2014). However, our results partially contrast with local studies in similar ecosystems. First, Zanetti et al. (2020) did not find a phylogenetic signal for seed water content in their study of 48 species from the cangas of Carajás (Eastern Brazilian Amazon). This discrepancy between this and our study might arise from differences in sample size and geographic extent or the distinct phylogenetic structure of each rock outcrop vegetation type (Massante et al., 2023). Second, we found a significant phylogenetic signal for seed dispersal season, while seed dispersal season in the Cerrado (Escobar et al., 2021) and other phenological events in the campo rupestre (Zanetti et al., 2020; Oliveira et al., 2021) have not shown such signal. As a result, seed dispersal season is the only phenophase that has shown a significant phylogenetic signal in the Brazilian rock outcrop vegetation, potentially because of an intense evolutionary pressure towards germination timing in these harsh ecosystems, where water is even scarcer than in the Cerrado (Oliveira et al., 2021), and thus may create stronger selective pressures for establishment.
Seed mass and seed dispersal season differed between herbs and shrubs, with shrubs tending to produce relatively larger seeds during the late dry season and herbs producing lighter seeds during the late rain season. Differences in seed mass between herbs and shrubs have been attributed to the distinct strategies employed by such growth forms: while shrubs invest in fewer, larger seeds that cope better with environmental hazards, herbs invest in numerous, lighter seeds to increase their establishment opportunities (Westoby et al., 2002; Moles et al., 2005). This relationship is also supported by the global spectrum of plant form and function (Díaz et al., 2016), suggesting that this global pattern also holds at small scales, such as for Brazilian rock outcrop vegetation. On the other hand, differences in seed dispersal season suggest differences in phenological strategies to cope with precipitation seasonality (see below).
Species from distinct geographic distributions and microhabitats exhibit different germination responses (and seed traits)
Rock outcrops and the surrounding vegetation differ in several aspects that should impact germination, such as nutrient and water availability, irradiance and daily temperature fluctuations (Oliveira et al., 2016). While we did not find significant differences in seed traits between restricted and widespread species, their germination responses to temperature and heat shocks differed. First, the germination proportion of widespread species was reduced or delayed at relatively low and high temperatures, implying they are more susceptible to these conditions than species restricted to outcrop vegetation. Second, species restricted to outcrop vegetation were more sensitive to heat shocks than widespread ones. The rocky substrate of the campo rupestre does not favour fire spread (Conceição et al., 2016), so natural fire events in these ecosystems are unlikely to reach the vegetation establishing directly on rock outcrops, reducing evolutionary pressures towards heat tolerance. In contrast, widespread species present in our dataset include species from the Cerrado, where fire events are relatively more frequent, and seeds exhibit relatively higher heat tolerance (Daibes et al., 2022).
Contrastingly, species from mesic and xeric microhabitats were found to differ both in terms of seed traits and germination responses, agreeing with local studies showing a correspondence between seed traits and species microhabitats (Oliveira and Garcia, 2011; Ranieri et al., 2012; Marques et al., 2014). These results support our second hypothesis that predicts distinct germination requirements expected between species with contrasting microhabitat preferences and geographical ranges, as expected under the regeneration niche hypothesis (Grubb, 1977). Interestingly, these results contrast with large-scale studies carried out in temperate ecosystems where an association between ecological preferences and germination responses has been found (Fernández‐Pascual et al., 2021, 2022), suggesting that the extreme abiotic conditions of both the campo rupestre and rock outcrop vegetation as a whole impose a stronger selective pressure on germination traits. However, seed traits did not differ in species with distinct geographic ranges, suggesting that multiple pressures operate on the selection of seed traits at larger scales.
Regardless of microhabitat, most species in our dataset dispersed their seeds during the late dry or the early dry season. However, species from xeric habitats had higher probabilities of producing dormant seeds and dispersing their seeds during the late rain season – two strategies presumably arising due to evolutionary pressures towards strategies to synchronize germination with optimum conditions for seedling establishment, i.e. under higher soil water availability (Jurado and Flores, 2005; Silveira et al., 2012b; Garcia et al., 2020). Nevertheless, only 36 % of our species produce dormant seeds, and campo rupestre is known to have the highest ND:D ratio globally (Dayrell et al., 2017), implying that seed dormancy might not be the main driver of seedling establishment in our study system. Additional strategies to control germination timing include germination requirements and the acquisition of secondary dormancy. A mismatch between temperature germination requirements and environmental conditions at the moment of dispersal might provide an alternative strategy to prevent germination in the absence of dormancy (Escobar et al., 2021); for example, seeds dispersed during the dry season have evolved to germinate under the relatively higher temperatures of the rainy season. Furthermore, secondary dormancy, as reported in a few Eriocaulaceae and Xyridaceae species from campo rupestre (Garcia et al., 2020), can contribute to fine-tuning seedling establishment with optimum conditions. All these species disperse their ND seeds between the early dry and the early rain season and become increasingly dormant as the rainy season advances. When the dry season starts, dormancy is progressively alleviated by reduced temperatures and low water availability, allowing germination at the onset of the rainy season (Duarte and Garcia, 2015).
Species from mesic microhabitats also exhibit distinct responses to light and temperature, with stronger positive responses to light and being more tolerant to low temperatures (<20 °C) but more sensitive to high temperatures (>30 °C). As a result, a stronger response to light could ensure that germination under light conditions occurs as quickly as possible. Moreover, high soil humidity in mesic habitats buffers these habitats from wide temperature fluctuations and contributes to maintaining relatively low soil temperatures (Oliveira and Garcia, 2011).
Campo rupestre species depend on light for germination
Our meta-analysis showed that light positively affects seed germination across all ecological groups, supporting previous assessments about its importance in campo rupestre germination ecology. Interestingly, we also found that germination responses to light were stronger in shrubs, a result that could be attributed to the fact that species with the lightest seeds in our dataset are shrubs from the Melastomataceae (Supplementary Data Fig. S2), which is both the best-represented family in our dataset (Ordóñez‐Parra et al., 2023) and a family characterized by an absolute light requirement for germination (Ordóñez-Parra et al., 2022).
Small-seeded species from our study are expected to have narrowly defined microsite requirements due to their limited internal resources, so further aspects of the light environment, such as spectral quality and the interaction between light and temperature, are expected to control their germination (Pearson et al., 2003; Pons, 2014). Still, studies assessing such aspects in our study system are scarce and have only involved very few species (Pereira et al., 2009; Hmeljevski et al., 2014; Vieira et al., 2018; Garcia et al., 2020), preventing robust inferences about the functional relevance of these germination cues.
Herbs and shrubs respond differently to low and high temperatures
We found that the germination of campo rupestre species was maximized between 20 and 25 °C, supporting previous qualitative reviews (Nunes et al., 2016; Garcia et al., 2020). Decreased germination proportion below this range could be a putative mechanism to avoid germination during the dry season when temperatures decrease and soil water potential does not support seedling establishment (Garcia et al., 2020). The negative effect of low temperatures was stronger in shrubs, suggesting that these require higher temperatures to germinate. In contrast, herbs had increased germination at 20 °C but were unaffected by 30 °C conditions. These results suggest that herbs dominate microsites experiencing lower soil temperatures.
Alternating temperature regimes had either a negative or null effect on germination. The reduction of germination proportion and the increase in germination time in the 25/15 °C regime is probably due to one of these temperatures being suboptimal. Conversely, studies in the campo de altitude have shown that several species benefit from alternating temperature regimes, suggesting this cue could be more important in this particular vegetation, which occurs on higher elevation belts (Andrade et al., 2021). Therefore, additional studies are required to elucidate the functional relevance of alternating temperatures and whether this varies between vegetation types.
Heat kills small, non-dormant seeds, but smoke accelerates germination
Seeds exposed to 100 or 200 °C heat shocks had their germination proportion reduced, while milder heat shocks (≤80 °C) did not affect germination proportion or time, supporting studies showing heat tolerance in the Cerrado (Daibes et al., 2022). In addition to seed mass, responses to heat shocks were also moderated by seed dormancy, with ND seeds being relatively less tolerant to heat shocks, as previously described for Cerrado species (Ramos et al., 2016; Daibes et al., 2019). Dormant species were mostly unaffected by heat shocks, supporting the notion that fire-mediated dormancy alleviation is not expected in rocky areas or savannas (Pausas and Lamont, 2022). The lack of detrimental effects on D species also implies that traits associated with seed dormancy promote heat tolerance, such as the accumulation of heat shock proteins or the presence of hard and water-impermeable coats (Tweddle et al., 2003; Ramos et al., 2016).
Smoke did not affect germination proportion but accelerated germination, supporting similar results found for Cerrado vegetation (Motta et al., 2024) and the notion that smoke-derived compounds stimulate germination of ND seeds or those where dormancy has been alleviated (Mackenzie et al., 2021). Smoke-stimulated germination in campo rupestre is thought to promote the germination of species resprouting and shedding seeds after fire (Fernandes et al., 2021) – a usual phenological syndrome in this vegetation (Figueira et al., 2016) – allowing recently dispersed seeds to exploit the post-fire environment where competition is relaxed (Le Stradic et al., 2015; Fernandes et al., 2021). While our meta-analysis supports this hypothesis, we found that the hastening effect of smoke is minor and only significant for shrubs. Moreover, such an effect has only been tested on a handful of species, and further studies are needed to rigorously test it.
Conclusions
Seed germination ecology in rock outcrop vegetation in Brazil is strongly shaped by species phylogenetic relatedness, as illustrated by the moderate to strong phylogenetic signal exhibited across seed traits and germination responses. As a result, knowledge about one species can provide highly relevant information about the germination ecology of other closely related species. Despite that, our analyses also point out that seed mass can shape germination responses that may differ between species with different growth form, species geographic distribution and microhabitats. Our integrative synthesis provides robust support for long-standing hypotheses in the seed ecology of Brazilian rock outcrop vegetation. We hope that our synthesis paves the way for future research about the functional role of seed traits in these megadiverse ecosystems and a solid baseline for using seeds in ecological restoration and biological conservation programs, such as direct seeding and seed banking.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Figure S1: state of the art of the germination ecology of Brazilian rock outcrop vegetation. Figure S2: variation in seven functional seed traits among the most represented families in the database. Table S1: Moran’s I value for the quantitative seed traits across different taxonomic levels. Table S2: summary of phylogenetic mixed models with Bayesian estimation (MCMCglmms) examining the effect of the different abiotic factors, seed mass and dormancy on the final seed germination proportions. Table S3: summary of phylogenetic mixed models with Bayesian estimation (MCMCglmms) examining the effect of the different abiotic factors, seed mass and dormancy on median germination time (t50).
ACKNOWLEDGEMENTS
The first author dedicates this article to the memory of Martha Galeano (1964–2022), whose fascination with tepuis and inspiring Vegetation Studies lectures led her to pursue a career in plant ecology. This study is part of the first author’s Master Dissertation at the Plant Biology Program at Universidade Federal de Minas Gerais. We also thank James Dalling and Sergey Rosbakh, who provided valuable comments on an earlier version of the manuscript, and Rohit Goswami, who helped in elaboration of the figures and the organization of the GitHub repository. The authors declare no conflict of interest.
FUNDING INFORMATION
C.A.O.-P and N.F.M. were supported by a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), respectively. F.A.O.S acknowledges support from Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
Contributor Information
Carlos A Ordóñez-Parra, Programa de Pós-Graduação em Biologia Vegetal, Departamento de Botânica, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, 31270-901, Brazil; Centro de Síntese Ecológica e Conservação, Departamento de Genética, Ecologia e Evolução, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, 31270-901, Brazil.
Natália F Medeiros, Centro de Síntese Ecológica e Conservação, Departamento de Genética, Ecologia e Evolução, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, 31270-901, Brazil; Programa de Pós-Graduação em Ecologia, Conservação e Manejo da Vida Silvestre, Departamento de Genética, Ecologia e Evolução, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, 31270-901, Brazil.
Roberta L C Dayrell, Royal Botanic Gardens, Kew, Wakehurst, Ardingly, Haywards Heath, West Sussex, RH17 6TN, UK.
Soizig Le Stradic, UMR BIOGECO, INRAE and Université de Bordeaux, Pessac, 33615, France.
Daniel Negreiros, Laboratório de Ecologia Evolutiva e Biodiversidade, Departamento de Genética, Ecologia e Evolução, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, 31270-901, Brazil.
Tatiana Cornelissen, Centro de Síntese Ecológica e Conservação, Departamento de Genética, Ecologia e Evolução, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, 31270-901, Brazil.
Fernando A O Silveira, Centro de Síntese Ecológica e Conservação, Departamento de Genética, Ecologia e Evolução, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, 31270-901, Brazil.
OPEN DATA
The R code for the analysis and creation of the figures is available on GitHub: https://github.com/caordonezparra/outcrop_synthesis.
LITERATURE CITED
- Alcantara S, Ree RH, Mello-Silva R. 2018. Accelerated diversification and functional trait evolution in Velloziaceae reveal new insights into the origins of the campos rupestres’ exceptional floristic richness. Annals of Botany 122: 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeda F, Michelangeli FA, Viana PL. 2016. Brasilianthus (Melastomataceae), a new monotypic genus endemic to ironstone outcrops in the Brazilian Amazon. Phytotaxa 273: 269–282. [Google Scholar]
- Andrade LG, Sánchez‐Tapia A, Andrade ACS. 2021. Germination, viability and dormancy of 47 species from threatened tropical montane grassland in southeast Brazil: Implications forex situconservation. Plant Biology 23: 735–742. [DOI] [PubMed] [Google Scholar]
- Aravind J, Vimala Devi S, Radhamani J, Jacob SR, Srinivasan K. 2022. germinationmetrics: seed germination indices and curve fitting. R package version 0.1.8.9. https://cran.r-project.org/web/packages/germinationmetrics/ (25 April 2024, date last accessed). [Google Scholar]
- Arène F, Affre L, Doxa A, Saatkamp A. 2017. Temperature but not moisture response of germination shows phylogenetic constraints while both interact with seed mass and lifespan. Seed Science Research 27: 110–120. [Google Scholar]
- Barthlott W, Porembski S. 2000. Why study inselbergs? In: Porembski S, Barthlott W. eds. Inselbergs. Berlin: Springer, 1–6. [Google Scholar]
- Baskin CC, Baskin JM. 2014. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Elsevier. [Google Scholar]
- Biedinger N, Porembski S, Barthlott W. 2000. Vascular plants on inselbergs: vegetative and reproductive strategies In: Porembski S, Barthlott W, eds. Inselbergs. Berlin: Springer, 117–142. [Google Scholar]
- Bondi L, de Paula LFA, Rosado BHP, Porembski S. 2023. Demystifying the convergent ecological specialization of desiccation-tolerant vascular plants for water deficit. Annals of Botany 131: 521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campitelli E. 2022. ggnewscale: multiple fill and colour scales in ‘ggplot2’. R package version 0.4.10. https://cran.r-project.org/package=ggnewscale/ (2 April 2024, date last accessed). [Google Scholar]
- Campos PV, Villa PM, Nunes JA, Schaefer CEGR, Porembski S, Neri AV. 2018. Plant diversity and community structure of Brazilian Páramos. Journal of Mountain Science 15: 1186–1198. [Google Scholar]
- Carta A, Fernández-Pascual E, Gioria M, et al. 2022a. Climate shapes the seed germination niche of temperate flowering plants: a meta-analysis of European seed conservation data. Annals of Botany 129: 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta A, Mattana E, Dickie J, Vandelook F. 2022b. Correlated evolution of seed mass and genome size varies among life forms in flowering plants. Seed Science Research 32: 46–52. [Google Scholar]
- Conceição AA, Rapini A, do Carmo FF, et al. 2016. Rupestrian grassland vegetation, diversity, and origin. In: Fernandes GW. ed. Ecology and conservation of mountaintop grasslands in Brazil. Cham: Springer, 105–127. [Google Scholar]
- Coolbear P, Francis A, Grierson D. 1984. The effect of low temperature pre-sowing treatment on the germination performance and membrane integrity of artificially aged tomato seeds. Journal of Experimental Botany 35: 1609–1617. [Google Scholar]
- Daibes LF, Pausas JG, Bonani N, Nunes J, Silveira FAO, Fidelis A. 2019. Fire and legume germination in a tropical savanna: ecological and historical factors. Annals of Botany 123: 1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daibes LF, Ordóñez-Parra CA, Dayrell RLC, Silveira FAO. 2022. Regeneration from seeds in South American savannas, in particular the Brazilian Cerrado. In: Baskin CC, Baskin JM. eds. Plant regeneration from seeds: a global warming perspective. Oxford: Elsevier, 183–197. [Google Scholar]
- Dayrell RLC, Garcia QS, Negreiros D, Baskin CC, Baskin JM, Silveira FAO. 2017. Phylogeny strongly drives seed dormancy and quality in a climatically buffered hotspot for plant endemism. Annals of Botany 119: 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debastiani VJ. 2021. Data analysis for ecology and evolution. R package version 0.1.7. https://github.com/vanderleidebastiani/daee (November 2021, date last accessed). [Google Scholar]
- de Villemereuil P, Nakagawa S. 2014. General quantitative genetic methods for comparative biology. In: Garamszegi L. ed. Modern phylogenetic comparative methods and their application in evolutionary biology. Berlin: Springer, 287–303. [Google Scholar]
- Díaz S, Kattge J, Cornelissen JHC, et al. 2016. The global spectrum of plant form and function. Nature 529: 167–171. [DOI] [PubMed] [Google Scholar]
- Donohue K, Rubio De Casas R, Burghardt L, Kovach K, Willis CG. 2010. Germination, postgermination adaptation, and species ecological ranges. Annual Review of Ecology, Evolution, and Systematics 41: 293–319. [Google Scholar]
- Duarte DM, Garcia QS. 2015. Interactions between substrate temperature and humidity in signalling cyclical dormancy in seeds of two perennial tropical species. Seed Science Research 25: 170–178. [Google Scholar]
- Escobar DFE, Rubio de Casas R, Morellato LPC. 2021. Many roads to success: different combinations of life-history traits provide accurate germination timing in seasonally dry environments. Oikos 130: 1865–1879. [Google Scholar]
- Escudero A, Palacio S, Maestre FT, Luzuriaga AL. 2015. Plant life on gypsum: a review of its multiple facets. Biological Reviews of the Cambridge Philosophical Society 90: 1–18. [DOI] [PubMed] [Google Scholar]
- Farooq M, Basra SMA, Ahmad N, Hafeez K. 2005. Thermal hardening: a new seed vigor enhancement tool in rice. Journal of Integrative Plant Biology 47: 187–193. [Google Scholar]
- Fernandes AF, Oki Y, Fernandes GW, Moreira B. 2021. The effect of fire on seed germination of campo rupestre species in the South American Cerrado. Plant Ecology 222: 45–55. [Google Scholar]
- Fernández‐Pascual E, Carta A, Mondoni A, et al. 2021. The seed germination spectrum of alpine plants: a global meta‐analysis. New Phytologist 229: 3573–3586. [DOI] [PubMed] [Google Scholar]
- Fernández-Pascual E, Vaz M, Morais B, et al. 2022. Seed ecology of European mesic meadows. Annals of Botany 129: 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueira JEC, Ribeiro KT, Ribeiro MC, et al. 2016. Fire in rupestrian grasslands: plant response and management. In: Fernandes GW. ed. Ecology and conservation of mountaintop grasslands in Brazil. Cham: Springer, 415–448. [Google Scholar]
- Fitzsimons JA, Michael DR. 2017. Rocky outcrops: a hard road in the conservation of critical habitats. Biological Conservation 211: 36–44. [Google Scholar]
- Freiberg M, Winter M, Gentile A, et al. 2020. LCVP, the Leipzig Catalogue of Vascular Plants, a new taxonomic reference list for all known vascular plants. Scientific Data 7: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia QS, Oliveira PG. 2007. Germination patterns and seed longevity of monocotyledons from the Brazilian campos rupestres. Seed Science and Biotechnology 1: 35–41. [Google Scholar]
- Garcia QS, Barreto LC, Bicalho EM. 2020. Environmental factors driving seed dormancy and germination in tropical ecosystems: a perspective from campo rupestre species. Environmental and Experimental Botany 178: 104164. [Google Scholar]
- Giorni VT, Bicalho EM, Garcia QS. 2018. Seed germination of Xyris spp. from Brazilian campo rupestre is not associated to geographic distribution and microhabitat. Flora 238: 102–109. [Google Scholar]
- Gittleman JL, Kot M. 1990. Adaptation: statistics and a null model for estimating phylogenetic effects. Systematic Zoology 39: 227. [Google Scholar]
- Grubb PJ. 1977. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Reviews 52: 107–145. [Google Scholar]
- Guimarães PJF, Michelangeli FA, Sosa K, Santiago Gómez JR. 2019. Systematics of Tibouchina and allies (Melastomataceae: Melastomateae): a new taxonomic classification. Taxon 68: 937–1002. [Google Scholar]
- Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. Journal of Statistical Software 33: 1–22. [PMC free article] [PubMed] [Google Scholar]
- Hmeljevski KV, Freitas L, Domingues R, et al. 2014. Conservation assessment of an extremely restricted bromeliad highlights the need for population-based conservation on granitic inselbergs of the Brazilian Atlantic Forest. Flora 209: 250–259. [Google Scholar]
- Ho LST, Ané C. 2014. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Systematic Biology 63: 397–408. [DOI] [PubMed] [Google Scholar]
- Ives AR, Garland T. 2010. Phylogenetic logistic regression for binary dependent variables. Systematic Biology 59: 9–26. [DOI] [PubMed] [Google Scholar]
- Jacobi CM, Carmo FF, Vincent RC, Stehmann JR. 2007. Plant communities on ironstone outcrops: a diverse and endangered Brazilian ecosystem. Biodiversity and Conservation 16: 2185–2200. [Google Scholar]
- Jin Y, Qian H. 2022. V.PhyloMaker2: an updated and enlarged R package that can generate very large phylogenies for vascular plants. Plant Diversity 44: 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado E, Flores J. 2005. Is seed dormancy under environmental control or bound to plant traits? Journal of Vegetation Science 16: 559–564. [Google Scholar]
- Kluge M, Brulfert J. 2000. Ecophysiology of vascular plants on inselbergs. In: Porembski S, Barthlott W. eds. Inselbergs. Berlin: Springer, 143–174. [Google Scholar]
- Larson JE, Funk JL. 2016. Regeneration: an overlooked aspect of trait-based plant community assembly models. Journal of Ecology 104: 1284–1298. [Google Scholar]
- Le Stradic S, Silveira FAO, Buisson E, Cazelles K, Carvalho V, Fernandes GW. 2015. Diversity of germination strategies and seed dormancy in herbaceous species of campo rupestre grasslands. Austral Ecology 40: 537–546. [Google Scholar]
- Mackenzie BDE, Auld TD, Keith DA, Ooi MKJ. 2021. Fire seasonality, seasonal temperature cues, dormancy cycling, and moisture availability mediate post-fire germination of species with physiological dormancy. Frontiers in Plant Science 12: 795711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AR, Atman APF, Silveira FAO, Lemos-Filho JP. 2014. Are seed germination and ecological breadth associated? Testing the regeneration niche hypothesis with bromeliads in a heterogeneous neotropical montane vegetation. Plant Ecology 215: 517–529. [Google Scholar]
- Martinelli G. 2007. Mountain biodiversity in Brazil. Revista Brasileira de Botânica 30: 587–597. [Google Scholar]
- Massante JC, Neri AV, Villa PM, et al. 2023. Looking similar but all different: phylogenetic signature of Brazilian rocky outcrops and the influence of temperature variability on their phylogenetic structure. Journal of Ecology 111: 1905–1920. [Google Scholar]
- Milberg P, Andersson L, Thompson K. 2000. Large-seeded species are less dependent on light for germination than small-seeded ones. Seed Science Research 10: 99–104. [Google Scholar]
- Miola DTB, Ramos VDV, Silveira FAO. 2021. A brief history of research in campo rupestre: identifying research priorities and revisiting the geographical distribution of an ancient, widespread Neotropical biome. Biological Journal of the Linnean Society 133: 464–480. [Google Scholar]
- Moles AT, Ackerly DD, Webb CO, et al. 2005. Factors that shape seed mass evolution. Proceedings of the National Academy of Sciences of the USA 102: 10540–10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Venegas R, Rodríguez MA. 2017. Revisiting phylogenetic signal; strong or negligible impacts of polytomies and branch length information? BMC Evolutionary Biology 17: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran PAP. 1950. Notes on continuous stochastic phenomena. Biometrika 37: 17–23. [PubMed] [Google Scholar]
- Motta GST, Pilon N, Fidelis A, Kolb RM. 2024. Smoke effects on the germination of Cerrado species. Plant Ecology 225: 685–693. [Google Scholar]
- Nunes FP, Dayrell RLC, Silveira FAO, et al. 2016. Seed germination ecology in rupestrian grasslands In: Fernandes GW. ed. Ecology and conservation of mountaintop grasslands in Brazil. Cham, Switzerland: Springer, 207–225. [Google Scholar]
- Oliveira PG, Garcia QS. 2011. Germination characteristics of Syngonanthus seeds (Eriocaulaceae) in campos rupestres vegetation in south-eastern Brazil. Seed Science Research 21: 39–45. [Google Scholar]
- Oliveira RS, Abrahão A, Pereira C, et al. 2016. Ecophysiology of campos rupestres plants. In: Fernandes GW. ed. Ecology and conservation of mountaintop grasslands in Brazil. Cham: Springer, 227–272. [Google Scholar]
- Oliveira CS, Messeder JVS, Teixido AL, Arantes MRR, Silveira FAO. 2021. Vegetative and reproductive phenology in a tropical grassland–savanna–forest gradient. Journal of Vegetation Science 32: 1–16. [Google Scholar]
- Ordóñez-Parra CA, Messeder JVS, Mancipe-Murillo C, Calderón-Hernández M, Silveira FAO. 2022. Seed germination ecology in Neotropical Melastomataceae: past, present, and future. In: Goldenberg R, Michelangeli FA, Almeda F. eds. Systematics, evolution, and ecology of Melastomataceae. Cham: Springer, 707–733. [Google Scholar]
- Ordóñez‐Parra CA, Dayrell RLC, Negreiros D, et al. 2023. Rock n’ Seeds: a database of seed functional traits and germination experiments from Brazilian rock outcrop vegetation. Ecology 104: e3852. [DOI] [PubMed] [Google Scholar]
- Orme D, Freckleton R, Thomas G, et al. 2018. Comparative analyses of phylogenetics and evolution in R. R package version 1.0.3. https://cran.r-project.org/web/packages/caper/index.html (29 February 2024, date last accessed). [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- Paradis E, Schliep K. 2019. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35: 526–528. [DOI] [PubMed] [Google Scholar]
- Pausas JG, Lamont BB. 2022. Fire-released seed dormancy – a global synthesis. Biological Reviews of the Cambridge Philosophical Society 97: 1612–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavoine S. 2020. adiv: an R package to analyse biodiversity in ecology. Methods in Ecology and Evolution 11: 1106–1112. [Google Scholar]
- Pearson TRH, Burslem DFRP, Mullins CE, Dalling JW. 2003. Functional significance of photoblastic germination in neotropical pioneer trees: a seed’s eye view. Functional Ecology 17: 394–402. [Google Scholar]
- Pereira AR, Andrade ACS, Pereira TS, Forzza RC, Rodrigues AS. 2009. Comportamento germinativo de espécies epífitas e rupícolas de Bromeliaceae do Parque Estadual do Ibitipoca, Minas Gerais, Brasil. Revista Brasileira de Botanica 32: 827–838. [Google Scholar]
- Pons TL. 2014. Light-mediated germination. In: Gallagher RS. ed. Seeds: the ecology of regeneration in plant communities. Wallingord, UK: CABI, 111–134. [Google Scholar]
- Porembski S. 2007. Tropical inselbergs: habitat types, adaptive strategies and diversity patterns. Revista Brasileira de Botânica 30: 579–586. [Google Scholar]
- Qian H, Jin Y. 2021. Are phylogenies resolved at the genus level appropriate for studies on phylogenetic structure of species assemblages? Plant Diversity 43: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2023. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- Ramos DM, Liaffa ABS, Diniz P, et al. 2016. Seed tolerance to heating is better predicted by seed dormancy than by habitat type in Neotropical savanna grasses. International Journal of Wildland Fire 25: 1273–1280. [Google Scholar]
- Ranieri BD, Pezzini FF, Garcia QS, Chautems A. 2012. Testing the regeneration niche hypothesis with Gesneriaceae (tribe Sinningiae) in Brazil: implications for the conservation of rare species. Austral Ecology 37: 125–133. [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Ribeiro LC, Barbosa ERM, van Langevelde F, Borghetti F. 2015. The importance of seed mass for the tolerance to heat shocks of savanna and forest tree species. Journal of Vegetation Science 26: 1102–1111. [Google Scholar]
- Rivera VL, Panero JL, Schilling EE, Crozier BS, Moraes MD. 2016. Origins and recent radiation of Brazilian Eupatorieae (Asteraceae) in the eastern Cerrado and Atlantic Forest. Molecular Phylogenetics and Evolution 97: 90–100. [DOI] [PubMed] [Google Scholar]
- Ruprecht E, Fenesi A, Fodor EI, Kuhn T, Tökölyi J. 2015. Shape determines fire tolerance of seeds in temperate grasslands that are not prone to fire. Perspectives in Plant Ecology, Evolution and Systematics 17: 397–404. [Google Scholar]
- Saatkamp A, Cochrane A, Commander L, et al. 2019. A research agenda for seed-trait functional ecology. New Phytologist 221: 1764–1775. [DOI] [PubMed] [Google Scholar]
- Safford HD. 1999. Brazilian Páramos I. An introduction to the physical environment and vegetation of the campos de altitude. Journal of Biogeography 26: 693–712. [Google Scholar]
- Safford HD, Martinelli G. 2000. Southeast Brazil. In: Porembski S, Barthlott W. eds. Inselbergs: Biotic Diversity of Isolated Rock Outcrops in Tropical and Temperate Regions. Berlin: Springer, 339–389. [Google Scholar]
- Silveira FAO, Negreiros D, Araújo LM, Fernandes GW. 2012a. Does seed germination contribute to ecological breadth and geographic range? A test with sympatric Diplusodon (Lythraceae) species from rupestrian fields. Plant Species Biology 27: 170–173. [Google Scholar]
- Silveira FAO, Ribeiro RC, Oliveira DMT, Fernandes GW, Lemos-Filho JP. 2012b. Evolution of physiological dormancy multiple times in Melastomataceae from neotropical montane vegetation. Seed Science Research 22: 37–44. [Google Scholar]
- Silveira FAO, Negreiros D, Barbosa NPU, et al. 2016. Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant and Soil 403: 129–152. [Google Scholar]
- Smith SA, Brown JW. 2018. Constructing a broadly inclusive seed plant phylogeny. American Journal of Botany 105: 302–314. [DOI] [PubMed] [Google Scholar]
- Tweddle JC, Dickie JB, Baskin CC, Baskin JM. 2003. Ecological aspects of seed desiccation sensitivity. Journal of Ecology 91: 294–304. [Google Scholar]
- Vandelook F, Janssens SB, Probert RJ. 2012. Relative embryo length as an adaptation to habitat and life cycle in Apiaceae. New Phytologist 195: 479–487. [DOI] [PubMed] [Google Scholar]
- Vandelook F, Newton RJ, Carta A. 2018. Photophobia in lilioid monocots: photoinhibition of seed germination explained by seed traits, habitat adaptation and phylogenetic inertia. Annals of Botany 121: 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira BC, Rodrigues BMA, Garcia QS. 2018. Light exposure time and light quality on seed germination of Vellozia species (Velloziaceae) from Brazilian campo rupestre. Flora 238: 94–101. [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. 2002. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33: 125–159. [Google Scholar]
- Willis CG, Baskin CC, Baskin JM, et al. ; NESCent Germination Working Group. 2014. The evolution of seed dormancy: environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytologist 203: 300–309. [DOI] [PubMed] [Google Scholar]
- Wyatt R. 1997. Reproductive ecology of granite outcrop plants from the south-eastern United States. Journal of the Royal Society of Western Australia 80: 123–129. [Google Scholar]
- Xu S, Dai Z, Guo P, et al. 2021. ggtreeExtra: compact visualization of richly annotated phylogenetic data. Molecular Biology and Evolution 38: 4039–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Smith DK, Zhu H, Guan Y, Lam TT. 2017. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods in Ecology and Evolution 8: 28–36. [Google Scholar]
- Zanetti M, Dayrell RLC, Wardil MV, et al. 2020. Seed functional traits provide support for ecological restoration and ex situ conservation in the threatened Amazon ironstone outcrop flora. Frontiers in Plant Science 11: 599496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.