Abstract
Atezolizumab plus bevacizumab (Ate/Bev) and lenvatinib (Len) are first-line therapies for unresectable hepatocellular carcinoma (uHCC). However, Ate/Bev’s high cost limits its common use in real-life practice, while Len is usually covered by national health insurance (NHI). We conducted this study to compare their effectiveness and safety in real-world settings. We retrospectively evaluated 346 uHCC patients treated with first-line Ate/Bev (n=80) or Len (n=266) from December 2019 to December 2022, using 1:2 ratio propensity score matching (PSM) analyses. Compared to the Len group, the Ate/Bev group exhibited higher incidences of Child-Pugh class B (14.1% vs. 5.7%, P=0.014), larger main tumors (58.8% vs. 40.2%, P=0.003), and more main portal vein invasion (25% vs. 12.8%, P=0.008). Treatment-related adverse events were notably lower in the Ate/Bev group (56.3% vs. 72.3%, P=0.007). After PSM, no significant differences were observed in the objective response rate (21.9% vs. 21.6%, P=0.983), progression-free survival (5.1 vs. 6 months, P=0.783), and overall survival (13.3 vs. 14.1 months, P=0.945) between the Ate/Bev (n=73) and Len (n=142) groups. Patients in the Ate/Bev group received more sequential post-treatments compared to the Len group (45.2% vs. 24.6%, P=0.009). Len-based therapies (n=28, 84.8%) and mono- or combined-immunotherapy (n=19, 54.3%) were the most frequently administered sequential therapies following Ate/Bev and Len, respectively. Patients with uHCC who received first-line self-paid Ate/Bev seemed to have lower liver function reserve and more advanced tumor characteristics compared to those who underwent NHI-reimbursed Len. However, the treatment outcomes and safety profiles were similar between these two groups.
Keywords: Atezolizumab plus bevacizumab, lenvatinib, national health insurance, propensity score matching analysis, unresectable hepatocellular carcinoma
Introduction
In recent years, the advent of immune checkpoint inhibitors (ICIs) and targeted therapies has transformed the treatment landscape for advanced hepatocellular carcinoma (HCC) [1]. The IMbrave150 trial, a landmark phase III study, established atezolizumab plus bevacizumab (Ate/Bev) as a new standard of care for unresectable HCC, showing a superior overall survival (OS) of 19.2 months compared to 13.4 months in patients receiving sorafenib (hazard ratio (HR): 0.66, P=0.0009), the previous standard first-line therapy [2,3]. Lenvatinib (Len), a multitargeted tyrosine kinase inhibitor (TKI), has also emerged as a frontline treatment option for unresectable HCC [4]. The REFLECT trial demonstrated non-inferiority of Len compared to sorafenib in terms of OS (median 13.6 versus 12.3 months, respectively), with favorable objective response rates (ORR) and progression-free survival (PFS) [5]. Subsequent real-world studies have corroborated the efficacy and safety of Len in routine clinical practice, highlighting its role as a valuable therapeutic option for patients with advanced HCC [6-8].
However, randomized controlled trials comparing the effectiveness and safety of Ate/Bev versus Len are still lacking. Several real-world studies have evaluated the clinical outcomes of these two regimens in patients with unresectable HCC, but the results remain inconsistent [9,10]. For instance, a single-institution study from Taiwan found no significant differences in ORR, PFS, or OS between the Len and Ate/Bev groups [9]. Conversely, a multicenter retrospective study from Japan reported that the Ate/Bev group had better PFS (0.5-/1-/1.5-year rates: 56.6%/31.6%/non-estimable vs. 48.6%/20.4%/11.2%, P<0.0001) and OS rates (0.5-/1-/1.5-year rates: 89.6%/67.2%/58.1% vs. 77.8%/66.2%/52.7%, P=0.002) than the Len group [10]. Furthermore, a recent meta-analysis of eight real-world studies indicated that the Ate/Bev group had significantly longer PFS than the Len group, though no significant differences were observed in OS, ORR, or disease control rate (DCR) between the groups [11].
While both treatments have demonstrated efficacy in clinical trials, their comparative effectiveness in real-world settings shows variability. One factor contributing to these inconsistencies may be differences in healthcare systems and reimbursement policies. In Taiwan, Len has been covered by National Health Insurance (NHI) since January 2020 [12], making it more accessible to patients, whereas Ate/Bev may require higher out-of-pocket expenses, potentially limiting its use among the general population. The high cost of Ate/Bev has indeed become an economic barrier for patients with advanced HCC when choosing a first-line treatment in real-life settings.
To better understand the relative effectiveness and safety profiles of Ate/Bev and Len, particularly within Taiwan’s distinct reimbursement framework, this study was conducted. We aimed to investigate the comparative efficacy and safety of Ate/Bev versus Len as first-line treatments for patients with unresectable HCC under different NHI reimbursement statuses in real-world settings.
Patients and methods
Patients
We evaluated patients with unresectable HCC treated with Ate/Bev or Len between December 2019 and December 2022 in Kaohsiung Chang Gung Memorial Hospital. In cirrhotic patients, HCC diagnosis was based on non-invasive criteria or pathology, while in non-cirrhotic patients, diagnosis required histologic confirmation. Non-invasive criteria, applicable only to cirrhotic patients with liver nodules larger than 1 cm due to the high pre-test probability, are based on imaging techniques such as multiphasic computed tomography (CT) or dynamic contrast-enhanced magnetic resonance imaging (MRI) [13]. The typical hallmark is the combination of hypervascularity in the late arterial phase and washout in the portal venous and/or delayed phases, reflecting the vascular derangement that occurs during hepatocarcinogenesis [14]. Clinical data, including patient demographics, tumor characteristics, treatment details, and outcomes, were collected from electronic medical records. Clinical data, including age, gender, Child-Pugh class, viral etiology, albumin-bilirubin (ALBI) grade, and tumor characteristics (such as Barcelona Clinic Liver Cancer (BCLC) stage, extrahepatic metastasis (EHM), macrovascular invasion (MVI), and tumor size), as well as laboratory values (such as liver function and alpha-fetoprotein (AFP)), and treatment details (such as treatment agents, duration, dose reduction, early cessation rate, concurrent therapies, and post-treatment information for all enrolled patients), were reviewed from electronic medical charts and analyzed. Mortality data were collected through follow-up visits and medical records to ensure the most accurate and up-to-date information. The study inclusion criteria were as follows: patients had to have unresectable HCC classified as intermediate or advanced stage according to the Barcelona Clinic Liver Cancer (BCLC) system, be receiving Ate/Bev or Len as first-line systemic therapy, and be classified as Child-Pugh class A or B. Exclusion criteria included prior treatment with other systemic therapies, concurrent cancers, insufficient clinical data (such as baseline characteristics or follow-up data needed for prognostic analysis), classification as Child-Pugh class C, or loss to follow-up during treatment. The use of Ate/Bev or Len was determined by clinicians’ decisions and patient preferences. The major outcomes of the study were the assessment of PFS and OS between these two agents, while secondary outcomes included their treatment response rates and adverse events. Patients who received Len could be reimbursed if they met the criteria of Taiwan NHI including Child-Pugh class A liver function reserve, tumor in Barcelona BCLC stage C, or tumor in BCLC stage B with TACE refractory [12]. Concurrent use of Ate/Bev or Len with other treatments is permissible. Ate/Bev or Len treatment was discontinued upon tumor progression, deterioration of liver function reserve or performance status, occurrence of severe TRAEs, or at the patient’s request. The study protocol was approved by the Research Ethics Committee of Chang Gung Memorial Hospital (IRB No. 202001701A3).
Assessment of treatment outcome
Treatment response was assessed using CT or MRI based on the Response Evaluation Criteria in Solid Tumors version 1.1. (RECIST 1.1) [15]. The ORR was defined as patients achieving complete response (CR) or partial response (PR), while the disease control rate (DCR) was defined as patients achieving CR, PR, or stable disease status (SD). Progression disease (PD) was identified as tumors demonstrating obvious progression during assessment. Follow-up radiologic evaluations were conducted at approximately two- or three-month intervals during Ate/Bev or Len treatment, or upon clinical deterioration.
Assessment of adverse events
Following Ate/Bev or Len administration guidelines, dosage adjustments or temporary treatment pauses were implemented if a patient experienced any TRAE of grade 3 or higher severity, or if any unacceptable grade 2 TRAE occurred. TRAEs graded 3 or higher were deemed severe. In the event of a TRAE, dose reductions or temporary treatment pauses were maintained until the TRAE resolved to grade 1 or 2, in accordance with the manufacturer’s guidelines. Adverse events were assessed and recorded at each follow-up visit by clinicians and specialized nurses. Follow-up visits were scheduled every three weeks for Ate/Bev and every four weeks for Len, but intervals were typically shortened based on the severity of TRAEs.
Statistical analysis
Continuous variables were presented as mean ± standard deviation or median with interquartile range, while categorical variables were presented as frequencies and percentages. Differences between groups were analyzed using Student’s t-test, Mann-Whitney U test, chi-square test, or Fisher’s exact test, as appropriate. Survival outcomes, including PFS and OS, were analyzed using Kaplan-Meier curves and Cox regression models. Propensity-score matching (PSM) analysis was performed using Age, Sex, AFP, Child-Pugh class, Viral etiology, EHM, MVI, and Maximal tumor size with a 1:2 ratio to reduce the real-life baseline difference between Ate/Bev and Len groups. All enrolled patients were followed up till Dec 2023. All statistical analyses were performed using SPSS 26 software (SPSS Inc., Chicago, IL, USA), and a p-value <0.05 was considered statistically significant.
Results
The baseline clinical characteristics
The flowchart of enrollment in this study is shown in Figure 1. There were 430 patients with unresectable HCC who received Ate/Bev or Len between December 2019 and December 2022. Eighty-four patients were excluded due to receiving other systemic therapies before, having insufficient data, or being lost to follow-up. Therefore, a total of 346 patients including 80 (23.1%) with Ate/Bev and 266 (76.9%) with Len were further assigned to the Ate/Bev group (number, n=73) and the Len group (n=142) by using PSM analysis with a 1:2 ratio. Table 1 presents the baseline characteristics of all enrolled patients before and after PSM analysis. Before PSM, the Ate/Bev group were younger (61.6 vs. 65.4 years, P=0.012), showed more Child-Pugh class B (14.1 vs. 5.7%, P=0.014), larger main tumor (58.8 vs. 40.2%, P=0.003), more main portal vein invasion (Vp4) (25% vs. 12.8%, P=0.008), more treatment termination (93.8 vs. 83.8%, P=0.024) and fewer concurrent treatments (20 vs. 40.6%, P<0.001) compared with the Len group. After the performance of PSM, the baseline characteristics of the two groups were balanced, except that the proportion of receiving concurrent treatment (21.9 vs. 45.8%, P<0.001) remained lower in the Ate/Bev than in the Len group. In the PSM cohort, the leading four concurrent treatments with Len were mono-immunotherapy as pembrolizumab or nivolumab, radiotherapy, TACE, and proton beam radiotherapy. In the Ate/Bev group, the mostly concurrent treatment was proton beam radiotherapy, followed by TACE and radiotherapy.
Figure 1.

Flow chart of the study population.
Table 1.
Baseline characteristics of patients receiving Ate/Bev or Len before and after PSM
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Ate/Bev (n=80) | Len (n=266) | P-value | Ate/Bev (n=73) | Len (n=142) | P-value | |
| Male sex, n (%) | 61 (76.3) | 201 (75.6) | 0.9 | 55 (75.3) | 103 (72.5) | 0.659 |
| Age (years) | 61.6±11.6 | 65.4±11.4 | 0.012 | 63.2±10.5 | 63.2±11.4 | 0.987 |
| Child-Pugh class | ||||||
| A, n (%) | 67 (85.9) | 249 (94.3) | 0.014 | 64 (87.7) | 131 (92.3) | 0.273 |
| B, n (%) | 11 (14.1) | 15 (5.7) | 9 (12.3) | 11 (7.7) | ||
| Viral etiology, n (%) | 61 (76.3) | 199 (74.8) | 0.794 | 55 (75.3) | 106 (74.6) | 0.911 |
| HBV infection, n (%) | 51 (63.7) | 137 (51.5) | 45 (61.6) | 78 (54.9) | 0.346 | |
| HCV infection, n (%) | 14 (17.9) | 67 (25.2) | 14 (19.2) | 31 (21.8) | 0.651 | |
| ALBI grade | ||||||
| 1, n (%) | 36 (46.2) | 151 (57) | 0.178 | 35 (48.6) | 65 (45.8) | 0.676 |
| 2, n (%) | 38 (48.7) | 107 (40.4) | 33 (45.8) | 72 (50.7) | ||
| 3, n (%) | 4 (5.1) | 7 (2.6) | 4 (5.6) | 5 (3.5) | ||
| BCLC stage | ||||||
| B, n (%) | 10 (12.5) | 53 (19.9) | 0.131 | 10 (13.7) | 17 (12) | 0.717 |
| C, n (%) | 70 (87.5) | 213 (80.1) | 63 (86.3) | 125 (88) | ||
| EHM, n (%) | 40 (50) | 144 (45.9) | 0.516 | 35 (47.9) | 65 (45.8) | 0.763 |
| MVI, n (%) | 44 (55) | 117 (44) | 0.083 | 41 (56.2) | 74 (52.1) | 0.573 |
| Vp4, n (%) | 20 (25) | 34 (12.8) | 0.008 | 18 (24.7) | 24 (16.9) | 0.174 |
| Tumor size >6 cm, n (%) | 47 (58.8) | 107 (40.2) | 0.003 | 43 (58.9) | 76 (53.5) | 0.578 |
| BMI, kg/m2 | 24.5±3.2 | 24.7±4 | 0.506 | 24.4±3.2 | 24.3±3.6 | 0.812 |
| AST, IU/L | 76.5±48.6 | 63.6±51.9 | 0.045 | 73.4±47.6 | 73.7±61.6 | 0.973 |
| ALT, IU/L | 51.9±36 | 52.9±63 | 0.864 | 52.6±37.3 | 59.9±81 | 0.17 |
| AFP, ng/ml | 8802±20738 | 6753±18331 | 0.428 | 7553±21568 | 7281±18006 | 0.441 |
| AFP≥400, n (%) | 34 (43) | 91 (34.2) | 0.152 | 31 (42.5) | 56 (39.4) | 0.668 |
| NLR | 4.5±2.9 | 3.8±2.5 | 0.057 | 4.5±3.0 | 3.9±2.7 | 0.181 |
| NLR>3, N (%) | 52 (65) | 120 (52.9) | 0.06 | 46 (63) | 59 (50) | 0.079 |
| PLR | 4.6±3.5 | 3.9±2.6 | 0.228 | 189.5±119 | 169.9±99 | 0.243 |
| PLR>230, N (%) | 25 (45.5) | 42 (38.9) | 0.421 | 16 (21.9) | 26 (22.2) | 0.961 |
| Concurrent treatment, n (%) | 16 (20) | 108 (40.6) | 0.001 | 16 (21.9) | 65 (45.8) | 0.001 |
| Pembrolizumab/Nivolumab | 0 | 17/8 | 0 | 12/5 | ||
| Radiotherapy | 2 | 23 | 2 | 16 | ||
| TACE | 4 | 19 | 4 | 8 | ||
| Proton bean radiotherapy | 9 | 18 | 9 | 12 | ||
| Post treatment, n (%) | 43 (53.8) | 107 (46.9) | 0.294 | 40 (54.8) | 54 (43.9) | 0.14 |
| Treatment stop, n (%) | 75 (93.8) | 223 (83.8) | 0.024 | 68 (93.2) | 120 (84.5) | 0.07 |
Abbreviations: AFP, alpha-fetoprotein; ALBI grade, albumin-bilirubin grade; ALT, alanine aminotransferase; AST, aspartate transaminase; Ate/Bev, Atezolizumab plus Bevacizumab; BCLC stage, Barcelona Clinic Liver Cancer stage; BMI, body mass index; EHM, extra-hepatic metastasis; Len, Lenvatinib; NLR, neutrophil lymphocyte ratio; PLR, platelet lymphocyte ratio; PSM, propensity score matching; TACE, trans-arterial chemoembolization; Vp4, main portal vein invasion or bilateral portal vein invasion.
Treatment response of patients before and after PSM
Treatment response was assessed via those patients who received following CT or MRI imaging (Table 2). Before PSM, the ORR was compatible between the Ate/Bev and Len group (20% vs. 20.3%); however, patients in the Len group had a superior DCR (72 vs. 55.7%, P=0.004). After PSM, there were no statistically significant differences between the Ate/Bev and Len groups regarding CR, PR, SD, PD, DCR, and death. However, a trend was observed indicating a better DCR in the Len group.
Table 2.
Treatment response of patients receiving Ate/Bev or Len before and after PSM
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Ate/Bev (n=80) | Len (n=266) | P-value | Ate/Bev (n=73) | Len (n=142) | P-value | |
| Treatment response evaluation, n (%)† | 70 (82.1) | 225 (89.3) | 64 (87.7) | 116 (81.7) | ||
| Complete Response, n (%) | 2 (2.9) | 15 (6.7) | 0.069 | 2 (3.1) | 6 (5.2) | 0.245 |
| Partial Response, n (%) | 12 (17.1) | 31 (13.8) | 12 (18.8) | 19 (16.4) | ||
| Stable Disease, n (%) | 25 (35.7) | 116 (51.6) | 23 (35.9) | 57 (49.1) | ||
| Progression Disease, n (%) | 31 (44.3) | 63 (28) | 27 (42.2) | 34 (29.3) | ||
| Objective Response Rate | 20% | 20.5% | 0.923 | 21.9% | 21.6% | 0.983 |
| Disease Control Rate | 55.7% | 72% | 0.004 | 57.8% | 70.7% | 0.062 |
| Death, n (%) | 44 (55) | 122 (45.9) | 0.152 | 41 (56.2) | 70 (49.3) | 0.34 |
Abbreviations: Ate/Bev, Atezolizumab plus Bevacizumab; Len, Lenvatinib; PSM, propensity score matching.
Treatment response based on those who received image evaluation including Computer tomography or Magnetic resonance image.
Treatment related adverse events before and after PSM
Before (56.3 vs. 72%, P=0.008) and after PSM (56.2 vs. 71.1%, P=0.03), the Ate/Bev group both experienced a lower proportion of total TRAE than the Len group (Table 3). However, the occurrence rate of severer TRAE (≥ grade 3) between the two groups was similar. After PSM, the most reported TRAE in the Len group was hand-foot skin reaction, with a total of 32 patients (22.4%), followed by with fatigue with 31 patients (21.7%), and diarrhea with 20 patients (14%). In the Ate/Bev group, 56.2% of patients had incidence of total TRAE, where the incidence over 10% included 28% of patients with fatigue, 11.2% with dermatitis, and 11.2% with decreased appetite. Only 5 patients (7%) in the Ate/Bev group experienced severe TRAE needed to stop treatment.
Table 3.
Treatment related adverse events of patients receiving Ate/Bev or Len before and after PSM
| Variables | Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Ate/Bev (n=80) | Len (n=266) | Ate/Bev (n=73) | Len (n=142) | |||||
|
|
|
|
|
|||||
| Any, n (%) | Grade ≥3, n (%) | Any, n (%) | Grade ≥3, n (%) | Any, n (%) | Grade ≥3, n (%) | Any, n (%) | Grade ≥3, n (%) | |
| Total TRAE* | 45 (56.3) | 5 (6.3) | 185 (72) | 24 (9.6) | 41 (56.2) | 5 (7) | 96 (71.1) | 13 (9.1) |
| Fatigue, n (%) | 20 (25) | 1 (1.3) | 64 (25.6) | 11 (4.4) | 20 (28) | 1 (1.4) | 31 (21.7) | 5 (3.5) |
| HFSR, n (%) | 0 | 0 | 59 (23.6) | 5 (2) | 0 | 0 | 32 (22.4) | 3 (2.1) |
| Diarrhea, n (%) | 3 (3.8) | 0 | 36 (14.4) | 0 | 1 (1.4) | 0 | 20 (14) | 0 |
| Hypertension, n (%) | 1 (1.3) | 0 | 26 (10.4) | 1 (0.4) | 1 (1.4) | 0 | 9 (6.3) | 0 |
| Poor appetite, n (%) | 15 (18.8) | 0 | 26 (10.4) | 1 (0.4) | 8 (11.2) | 0 | 7 (4.9) | 0 |
| Dysphonia, n (%) | 0 | 0 | 14 (5.6) | 0 | 0 | 0 | 8 (5.6) | 0 |
| Dermatitis, n (%) | 9 (11.3) | 0 | 13 (5.2) | 0 | 8 (11.2) | 0 | 6 (4.2) | 0 |
| Proteinuria, n (%) | 1 (1.3) | 0 | 11 (4.4) | 0 | 1 (1.4) | 0 | 5 (3.5) | 0 |
| Encephalopathy, n (%) | 2 (2.5) | 0 | 6 (2.4) | 3 (1.2) | 2 (2.8) | 0 | 2 (1.4) | 2 (1.4) |
| Elevated bilirubin, n (%) | 2 (2.5) | 2 (2.5) | 5 (2) | 0 | 2 (2.8) | 2 (2.8) | 3 (2.1) | 0 |
| UGI bleeding, n (%) | 3 (3.8) | 1 (1.3) | 3 (1.2) | 2 (0.8) | 2 (2.8) | 1 (1.4) | 3 (2.1) | 2 (1.4) |
| Hepatitis, n (%) | 0 | 0 | 1 (0.4) | 1 (0.4) | 0 | 0 | 1 (0.7) | 1 (0.7) |
| Seizure, n (%) | 1 (1.3) | 1 (1.3) | 0 | 0 | 1 (1.4) | 1 (1.4) | 0 | 0 |
Abbreviations: Ate/Bev, Atezolizumab plus Bevacizumab; HFSR, hand foot skin reaction; Len, Lenvatinib; PSM, propensity score matching; TRAE, treatment related adverse event; UGI bleeding, upper gastrointestinal bleeding.
The comparison of any TRAE between two groups was 0.008 (Before PSM) and 0.03 (After PSM).
PFS and its predicting factors
Kaplan-Meier curves of PFS were 3.7 months in the Ate/Bev group and 6.8 months in the Len group, respectively (Figure 2A). Although the Len group seemed to have a better PFS, but the comparison was insignificant. After PSM, the Len group had a longer PFS than the Ate/Bev group (6 vs. 5.1 months, P=0.783), but there was no difference (Figure 2B). In Cox regression model of multivariate analyses, more microvascular invasion, higher AFP level, and fewer concurrent treatment were independent risk factors associated with PFS in the PSM cohort (Table 4). Different treatment agents using Ate/Bev or Len did not contribute to PFS, whether for univariate or multivariate analysis.
Figure 2.
Kaplan-Meier survival curves for (A) Progression-free survival (PFS) of the Ate/Bev and Len groups, (B) PFS of the Ate/Bev and Len groups after propensity score matching (PSM), (C) Overall survival (OS) of the Ate/Bev and Len groups, (D) OS of the Ate/Bev and Len groups after PSM.
Table 4.
Factors associated with Progression Free Survival in the PSM cohort
| Variable | Comparison | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| H.R. | 95% CI | p-value | H.R. | 95% CI | p-value | ||
| Age, years | Increase per year | 0.994 | 0.98-1.009 | 0.446 | |||
| Sex | Female vs. Male | 0.916 | 0.624-1.344 | 0.653 | |||
| Child-Pugh class | B vs. A | 1.495 | 0.805-2.799 | 0.203 | |||
| Etiology | Viral vs. non viral | 1.166 | 0.774-1.756 | 0.464 | |||
| BCLC stage | C vs. B | 1.701 | 0.977-2.961 | 0.061 | |||
| EHM | Yes vs. No | 0.855 | 0.611-1.196 | 0.360 | |||
| MVI | Yes vs. No | 1.623 | 1.159-2.277 | 0.005 | 1.637 | 1.168-2.294 | 0.004 |
| Tumor size, cm | >6 vs. ≤6 | 1.443 | 1.027-2.028 | 0.034 | |||
| AFP, ng/ml | >400 vs. ≤400 | 1.674 | 1.195-2.344 | 0.003 | 1.718 | 1.225-2.411 | 0.002 |
| Concurrent treatment | Yes vs. No | 0.649 | 0.461-0.914 | 0.013 | 0.6 | 0.425-0.847 | 0.004 |
| Treatment option | Ate/Bev vs. Len | 1.052 | 0.733-1.511 | 0.783 | |||
Abbreviations: AFP, alpha-fetoprotein; Ate/Bev, Atezolizumab plus Bevacizumab; BCLC stage, Barcelona Clinic Liver Cancer stage; EHM, extra-hepatic metastasis; Len, Lenvatinib; PSM, propensity score matching.
OS and its predicting factors
Comparing OS, the Ate/Bev group had a median of 10.4 months, while the Len group had 16.6 months, but there was no significant difference between the two groups (P=0.158) (Figure 2C). After PSM, the OS in the Ate/Bev group was 13.3 months, compared to 14.1 months in the Len group, still showing a statistically insignificant (P=0.945) (Figure 2D). In the multivariate analysis, after adjusting for other variables, non-viral etiology, higher AFP level, larger main tumor size, no concurrent treatment and no sequential post-treatment were associated with poor outcome in the PSM cohort (Table 5). Different treatment agents using Ate/Bev or Len did not contribute to OS, whether for univariate or multivariate analysis.
Table 5.
Factors associated with Overall Survival in the PSM cohort
| Variable | Comparison | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| H.R. | 95% CI | p-value | H.R. | 95% CI | p-value | ||
| Age, years | Increase per year | 1.005 | 0.988-1.023 | 0.537 | |||
| Sex | Female vs. Male | 0.859 | 0.561-1.314 | 0.483 | |||
| Child-Pugh class | B vs. A | 2.76 | 1.592-4.784 | <0.001 | |||
| Etiology | Viral vs. non viral | 0.58 | 0.386-0.869 | 0.008 | 0.448 | 0.295-0.681 | <0.001 |
| BCLC stage | C vs. B | 1.387 | 0.743-2.591 | 0.304 | |||
| EHM | Yes vs. No | 1.077 | 0.742-1.563 | 0.696 | |||
| MVI | Yes vs. No | 1.442 | 0.989-2.103 | 0.057 | |||
| Tumor size, cm | >6 vs. ≤6 | 1.888 | 1.277-2.792 | 0.001 | 1.809 | 1.208-2.710 | 0.004 |
| AFP, ng/ml | >400 vs. ≤400 | 1.941 | 1.335-2.823 | 0.001 | 2.063 | 1.396-3.048 | <0.001 |
| Concurrent treatment | Yes vs. No | 0.411 | 0.269-0.629 | <0.001 | 0.362 | 0.235-0.557 | <0.001 |
| Post treatment | Yes vs. No | 0.43 | 0.293-0.631 | <0.001 | 0.437 | 0.297-0.644 | <0.001 |
| Treatment option | Ate/Bev vs. Len | 0.986 | 0.666-1.461 | 0.945 | |||
Abbreviations: AFP, alpha-fetoprotein; Ate/Bev, Atezolizumab plus Bevacizumab; BCLC stage, Barcelona Clinic Liver Cancer stage; EHM, extra-hepatic metastasis; Len, Lenvatinib; PSM, propensity score matching.
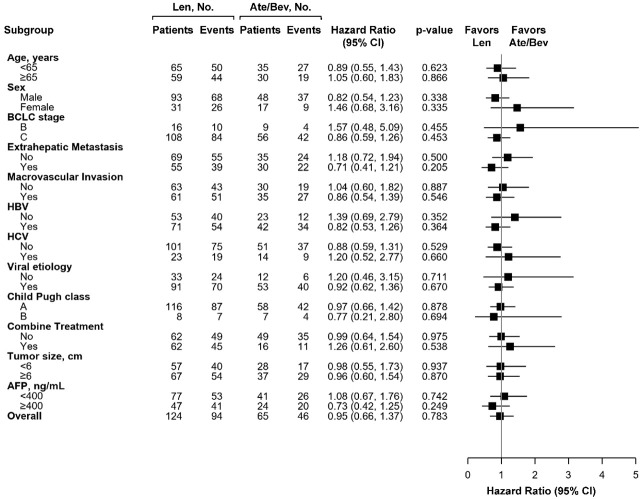
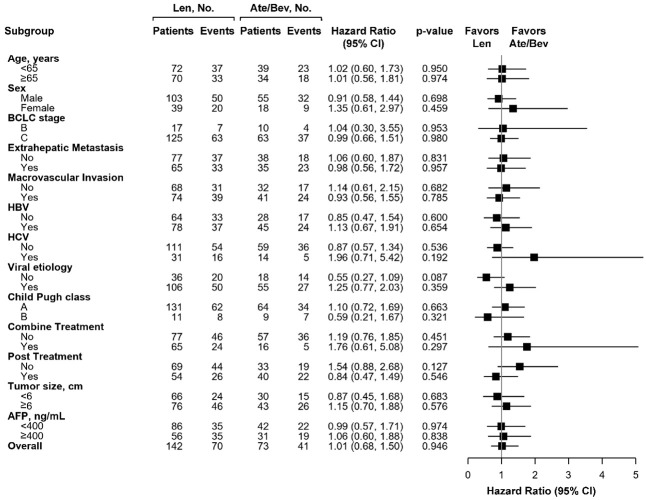
Subgroup analysis for PFS and OS after PSM
After PSM, the subgroup analysis indicated that using Ate/Bev was equal to using Len associated with PFS in all subgroups before PSM (Supplementary Figure 1) and after PSM (Figure 3). Similarly, there were still no difference in all subgroups regarding the OS in using first line Ate/Bev or Len before PSM (Supplementary Figure 2) and after PSM (Figure 4). Although non-viral patients who preferred Len over Ate/Bev tended to experience better OS (HR: 0.50, 95% CI: 0.27-0.93, P=0.028), this comparison became insignificant after PSM (HR: 0.55, 95% CI: 0.27-1.09, P=0.087).
Figure 3.
Forest plots of Progression-Free Survival in the subgroups of the Ate/Bev and Len groups after propensity score matching.
Figure 4.
Forest plots of Overall Survival in the subgroups of the Ate/Bev and Len groups after propensity score matching.
Sequential treatments following Ate/Bev or Len after PSM
After cessation of first line treatment, 40 patients (54.8%) in the Ate/Bev group and 54 (43.9%) in the Len group still afforded following therapies (Table 6). Concerning sequential systemic treatments, the Ate/Bev group had a higher proportion than the Len group (45.2 vs. 24.6%, P=0.009). A total of 30 patients (90.9%) received TKI after failure of Ate/Bev, 3 patients used sorafenib whereas 27 patients took Len-based therapies including 14 for Len, 9 for Len plus pembrolizumab, 3 for Len plus chemotherapy, and 2 for Len plus nivolumab. In the Len group, most patients decided chemotherapy or immunotherapy as the second line treatment. The most frequently used agent was chemotherapy for 13 patients, followed by Ate/Bev for 9, and nivolumab for 9.
Table 6.
Sequential treatments after failure of Ate/Bev or Len in the PSM cohort
| Variables | Ate/Bev (n=73) | Len (n=142) | P-value |
|---|---|---|---|
| Treatment Stop, n (%) | 68 (93.2) | 120 (84.5) | 0.07 |
| Post-treatment, n (%) | 40 (54.8) | 54 (43.9) | 0.14 |
| 2nd-line systemic treatments, n (%) | 33 (45.2) | 35 (24.6) | 0.009 |
| Len | 14 | 2 | |
| Len plus Pembrolizumab | 9 | 0 | |
| Len plus Nivolumab | 2 | 0 | |
| Len plus Chemotherapy | 3 | 0 | |
| Chemotherapy | 1 | 13 | |
| Ate/Bev | 0 | 9 | |
| Nivolumab plus Ipilizumab | 1 | 1 | |
| Nivolumab | 0 | 9 | |
| Sorafenib | 3 | 0 | |
| Thalidomide | 0 | 1 |
Abbreviations: Ate/Bev, Atezolizumab plus Bevacizumab; Len, Lenvatinib; PSM, propensity score matching.
Discussion
A previous study reported that, compared with sorafenib, Ate/Bev treatment provided an incremental effectiveness of 1.7 QALYs, with an additional cost of 127,607 USD. The incremental cost-effectiveness ratio was 75,192 USD per QALY, which is below the predefined willingness-to-pay threshold in Taiwan [16]. In Taiwan, the monthly cost of Ate/Bev is approximately 10,066 USD, compared to 3,452 USD for sorafenib and 1,333 USD for Len, making the high cost of Ate/Bev a limiting factor for its widespread use in clinical practice. In Taiwan, Len has been covered by insurance since January 2020, while Ate/Bev has been reimbursed since August 2023. This study examines treatment outcomes for Len and Ate/Bev in the context of these different insurance coverage periods. Compared to the Len group, patients who opted to self-pay for Ate/Bev tended to have more locally advanced tumors, larger tumor burdens, and poorer liver function at baseline, which are common factors associated with a poorer prognosis. In contrast, most patients in the Len group received the drug under National Health Insurance (NHI) reimbursement criteria and generally had better liver function and more consistent tumor patterns.
The Ate/Bev group had a comparable ORR (20% vs. 20.3%) but an inferior DCR (55.7% vs. 72%, P=0.004) compared to the Len group. After PSM, there were no statistically significant differences between the Ate/Bev and Len groups regarding ORR, DCR, and death. Additionally, the PFS of the Ate/Bev group was shorter than that of the Len group, though the difference was not statistically significant (3.7 months vs. 6.8 months, P=0.292). After PSM, the Len group still had a longer PFS than the Ate/Bev group (6 months vs. 5.1 months, P=0.783), but the difference was not significant. Regarding OS, the Ate/Bev group also had poorer survival compared to the Len group, but the difference was not significant (10.4 months vs. 16.6 months, P=0.158). After PSM, the OS of the two groups was more similar (13.3 months for the Ate/Bev group and 14.1 months for the Len group).
We found that our patients who received self-paid Ate/Bev seemed to have poorer PFS and OS compared to the clinical trials and showed a worse trend in survival compared to patients who received reimbursed Len. This may be due to these patients having more advanced tumor patterns and poorer liver function than those enrolled in clinical trials. Additionally, the observation period from the beginning of Ate/Bev treatment to the first image evaluation was significantly shorter than in the clinical trials due to the high cost of this agent. Most patients received their first image evaluation after only two to three administrations, which might lead to an underestimate of PFS. Moreover, previous studies indicated that delayed immune treatment response sometimes occurs after image pseudo-progression in HCC treatment [17,18]. Insufficient drug exposure could result in a suboptimal treatment response and worse treatment outcomes.
The present study observed that the Ate/Bev group experienced a lower proportion of TRAEs than the Len group, both before (56.3% vs. 72%, P=0.008) and after PSM (56.2% vs. 71.1%, P=0.03). However, the occurrence rate of severe TRAEs (≥ grade 3) between the two groups was similar. Following PSM, the most frequently reported TRAE in the Len group was hand-foot skin reaction, affecting a total of 32 patients (22.4%), followed by fatigue in 31 patients (21.7%), and diarrhea in 20 patients (14%). In the Ate/Bev group, 56.2% of patients experienced total TRAEs, with incidences exceeding 10% including fatigue in 28% of patients, dermatitis in 11.2%, and decreased appetite in 11.2%. Only 5 patients (7%) in the Ate/Bev group experienced severe TRAEs necessitating treatment discontinuation.
The current study also indicated that concurrent treatment was a significant contributing factor to treatment prognosis, both in univariate and multivariate analyses. In real-world practice, clinicians often combine locoregional therapies with systemic treatments to enhance treatment response [19,20]. Patients receiving concurrent treatment had superior PFS (9.3 vs. 3.4 months, P=0.012) and OS (19.2 vs. 8.9 months, P<0.001) compared to those who did not combine treatments. We observed that patients reimbursed for Len received more concurrent therapies than those self-paying for Ate/Bev (45.9% vs. 21.8%, P=0.001). In the PSM cohort, the most common concurrent treatments with Len were mono-immunotherapy with pembrolizumab or nivolumab, radiotherapy, TACE, and proton beam radiotherapy. In the Ate/Bev group, the predominant concurrent treatment was proton beam radiotherapy, followed by TACE and radiotherapy.
Len combined with pembrolizumab, a combination of TKI plus immunotherapy, had been a popular treatment option for patients with advanced HCC based on a phase Ib clinical trial [21]. Although this combination did not demonstrate superiority to Len monotherapy in phase III results [22], many patients still derived survival benefits from this combination in real-world practice. Yang et al. reported that Len plus PD-1 inhibitor treatment resulted in a longer OS of 17.8 months and a notable ORR of 19.6% and DCR of 73.5% in unresectable HCC patients [23]. The current study also observed that patients combining Len with pembrolizumab or nivolumab achieved excellent OS of 18.5 months.
In multivariate analysis, post-treatment emerged as a significant factor in reducing mortality risk for patients receiving first-line Ate/Bev or Len. Patients who underwent post-treatment experienced significantly better OS compared to those who did not (17.2 vs. 6.6 months, P<0.001). Following cessation of first-line treatment, 40 patients (54.8%) in the Ate/Bev group and 54 (43.9%) in the Len group continued to receive subsequent therapies. Regarding sequential systemic treatments, a higher proportion of patients in the Ate/Bev group received them compared to the Len group (45.2% vs. 24.6%, P=0.009). Of the patients who failed Ate/Bev, 90.9% received TKI therapy, 3 patients received sorafenib, and 27 patients received Len-based therapies, including 14 for Len alone, 9 for Len plus pembrolizumab, 3 for Len plus chemotherapy, and 2 for Len plus nivolumab. In the Len group, most patients opted for chemotherapy or immunotherapy as second-line treatment. Chemotherapy was the most frequently chosen agent for 13 patients, followed by Ate/Bev for 9, and nivolumab for 9. It appears that the primary consideration in selecting sequential treatment was to explore a different mechanism from the failed first-line agent.
Unlike previous studies, the current study found no association between the use of Ate/Bev or Len and PFS across all subgroups. Similarly, there were no differences observed in OS among all subgroups receiving first-line Ate/Bev or Len. Our study observed that non-viral patients who preferred Len over Ate/Bev tended to experience better OS (HR: 0.50, 95% CI: 0.27-0.93, P=0.028). However, this comparison became insignificant after PSM (HR: 0.55, 95% CI: 0.27-1.09, P=0.087). A larger study cohort might be necessary to elucidate this issue further in clinical practice.
The current study has several limitations. Firstly, the differential NHI-reimbursed status for Ate/Bev and Len introduces the risk of selection bias and confounding factors that could influence treatment outcomes. Although we utilized PSM to address these biases, residual confounding may still be present. Since both regimens have been reimbursed by the Taiwan NHI program since August 2023, further studies are needed to compare Ate/Bev and Len under more consistent baseline clinical characteristics. Secondly, the relatively small sample size and single-center nature of the study may restrict the generalizability of our findings to broader populations. Larger multicenter studies are necessary to validate our results and provide more robust evidence. Thirdly, the absence of long-term follow-up data may obscure the impact of treatment on survival outcomes beyond the study period. Future studies with extended follow-up durations are required to evaluate the durability of treatment responses and long-term survival benefits.
Conclusion
In summary, our study offers valuable insights into the comparative effectiveness of Health Insurance-Guided First-Line Len and self-paid Ate/Bev in patients with unresectable HCC. Despite initial differences in baseline characteristics, both treatment regimens demonstrated comparable treatment responses and survival outcomes. These findings highlight the significance of individualized treatment decisions tailored to patient-specific factors. Further research is warranted to optimize first-line treatment strategies for Len or Ate/Bev under consistent reimbursement criteria from the NHI.
Acknowledgements
The authors would like to thank Miss Nien-Tzu Hsu and the biostatistics center of Kaohsiung Chang Gung Memorial Hospital for excellent statistics works. This study was funded from Chang Gung Memorial Hospital (CMRPG8L00291) to Prof. Yuan-Hung Kuo.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Cappuyns S, Corbett V, Yarchoan M, Finn RS, Llovet JM. Critical appraisal of guideline recommendations on systemic therapies for advanced hepatocellular carcinoma: a review. JAMA Oncol. 2024;10:395–404. doi: 10.1001/jamaoncol.2023.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Ducreux M, Abou-Alfa GK, Bekaii-Saab T, Berlin J, Cervantes A, de Baere T, Eng C, Galle P, Gill S, Gruenberger T, Haustermans K, Lamarca A, Laurent-Puig P, Llovet JM, Lordick F, Macarulla T, Mukherji D, Muro K, Obermannova R, O’Connor JM, O’Reilly EM, Osterlund P, Philip P, Prager G, Ruiz-Garcia E, Sangro B, Seufferlein T, Tabernero J, Verslype C, Wasan H, Van Cutsem E. The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 24th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2022. ESMO Open. 2023;8:101567. doi: 10.1016/j.esmoop.2023.101567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 6.Kuo YH, Lu SN, Chen YY, Kee KM, Yen YH, Hung CH, Hu TH, Chen CH, Wang JH. Real-world lenvatinib versus sorafenib in patients with advanced hepatocellular carcinoma: a propensity score matching analysis. Front Oncol. 2021;11:737767. doi: 10.3389/fonc.2021.737767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsiao YW, Sou FM, Wang JH, Chen YH, Tsai MC, Hu TH, Hung CH, Chen CH, Kuo YH. Well-controlled viremia reduces the progression of hepatocellular carcinoma in chronic viral hepatitis patients treated with lenvatinib. Kaohsiung J Med Sci. 2023;39:1233–42. doi: 10.1002/kjm2.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Cheng AL, Vogel A, Tovoli F, Ueshima K, Aikata H, López CL, Pracht M, Meng Z, Daniele B, Park JW, Palmer D, Tamai T, Saito K, Dutcus CE, Lencioni R. Overall survival and objective response in advanced unresectable hepatocellular carcinoma: a subanalysis of the REFLECT study. J Hepatol. 2023;78:133–141. doi: 10.1016/j.jhep.2022.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Su CW, Teng W, Lin PT, Jeng WJ, Chen KA, Hsieh YC, Chen WT, Ho MM, Hsieh CH, Wang CT, Chai PM, Lin CC, Lin CY, Lin SM. Similar efficacy and safety between lenvatinib versus atezolizumab plus bevacizumab as the first-line treatment for unresectable hepatocellular carcinoma. Cancer Med. 2023;12:7077–7089. doi: 10.1002/cam4.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiraoka A, Kumada T, Tada T, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, Fukunishi S, Tsuji K, Ishikawa T, Tajiri K, Ochi H, Yasuda S, Toyoda H, Ogawa C, Nishimura T, Hatanaka T, Kakizaki S, Shimada N, Kawata K, Naganuma A, Kosaka H, Shibata H, Aoki T, Tanaka T, Ohama H, Nouso K, Morishita A, Tsutsui A, Nagano T, Itokawa N, Okubo T, Arai T, Imai M, Koizumi Y, Nakamura S, Joko K, Iijima H, Kaibori M, Hiasa Y, Kudo M Real-life Practice Experts for HCC (RELPEC) Study Group and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan) Does first-line treatment have prognostic impact for unresectable HCC?-Atezolizumab plus bevacizumab versus lenvatinib. Cancer Med. 2023;12:325–34. doi: 10.1002/cam4.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Yang L, Wei S, Li J, Yi P. Efficacy and safety of atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2023;149:16191–16201. doi: 10.1007/s00432-023-05342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Reimbursement Criteria of Hepatocellular Carcinoma Treatment. Ministry of Health and Welfare, ROC: The National Health Insurance Administration. May 2024 update. [Google Scholar]
- 13.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Matsui O, Kobayashi S, Sanada J, Kouda W, Ryu Y, Kozaka K, Kitao A, Nakamura K, Gabata T. Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging. 2011;36:264–272. doi: 10.1007/s00261-011-9685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Tseng CY, Tsai YW, Shiu MN. Cost-effectiveness analysis of atezolizumab plus bevacizumab versus sorafenib in first line treatment for Chinese subpopulation with unresectable hepatocellular carcinoma. Front Oncol. 2023;13:1264417. doi: 10.3389/fonc.2023.1264417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odagiri N, Tamori A, Kotani K, Motoyama H, Kawamura E, Hagihara A, Fujii H, Uchida-Kobayashi S, Enomoto M, Kawada N. A case of hepatocellular carcinoma with “pseudoprogression” followed by complete response to atezolizumab plus bevacizumab. Clin J Gastroenterol. 2023;16:392–396. doi: 10.1007/s12328-023-01761-6. [DOI] [PubMed] [Google Scholar]
- 18.Otake S, Ota Y, Aso K, Okada M, Hayashi H, Hasebe T, Nakajima S, Sawada K, Fujiya M, Okumura T. Contrast-enhanced ultrasonography features for diagnosing pseudoprogression of hepatocellular carcinoma with immunotherapy: a case report of the response after pseudoprogression. Intern Med. 2024;63:1093–1097. doi: 10.2169/internalmedicine.2349-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao C, Xiang Z, Li M, Wang H, Liu H, Yan H, Huang M. Transarterial chemoembolization combined with atezolizumab plus bevacizumab or lenvatinib for unresectable hepatocellular carcinoma: a propensity score matched study. J Hepatocell Carcinoma. 2023;10:1195–1206. doi: 10.2147/JHC.S418256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su CW, Teng W, Shen EY, Huang BS, Lin PT, Hou MM, Wu TH, Tsan DL, Hsieh CH, Wang CT, Chai PM, Lin CY, Lin SM, Lin CC. Concurrent atezolizumab plus bevacizumab and high-dose external beam radiotherapy for highly advanced hepatocellular carcinoma. Oncologist. 2024;29:e922–e931. doi: 10.1093/oncolo/oyae048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2020;38:2960–70. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llovet JM, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, Xu R, Edeline J, Ryoo BY, Ren Z, Masi G, Kwiatkowski M, Lim HY, Kim JH, Breder V, Kumada H, Cheng AL, Galle PR, Kaneko S, Wang A, Mody K, Dutcus C, Dubrovsky L, Siegel AB, Finn RS LEAP-002 Investigators. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1399–1410. doi: 10.1016/S1470-2045(23)00469-2. [DOI] [PubMed] [Google Scholar]
- 23.Yang X, Chen B, Wang Y, Wang Y, Long J, Zhang N, Xue J, Xun Z, Zhang L, Cheng J, Lei J, Sun H, Li Y, Lin J, Xie F, Wang D, Pan J, Hu K, Guan M, Huo L, Shi J, Yu L, Zhou L, Zhou J, Lu Z, Yang X, Mao Y, Sang X, Lu Y, Zhao H. Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol Int. 2023;17:709–719. doi: 10.1007/s12072-022-10480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.