Abstract
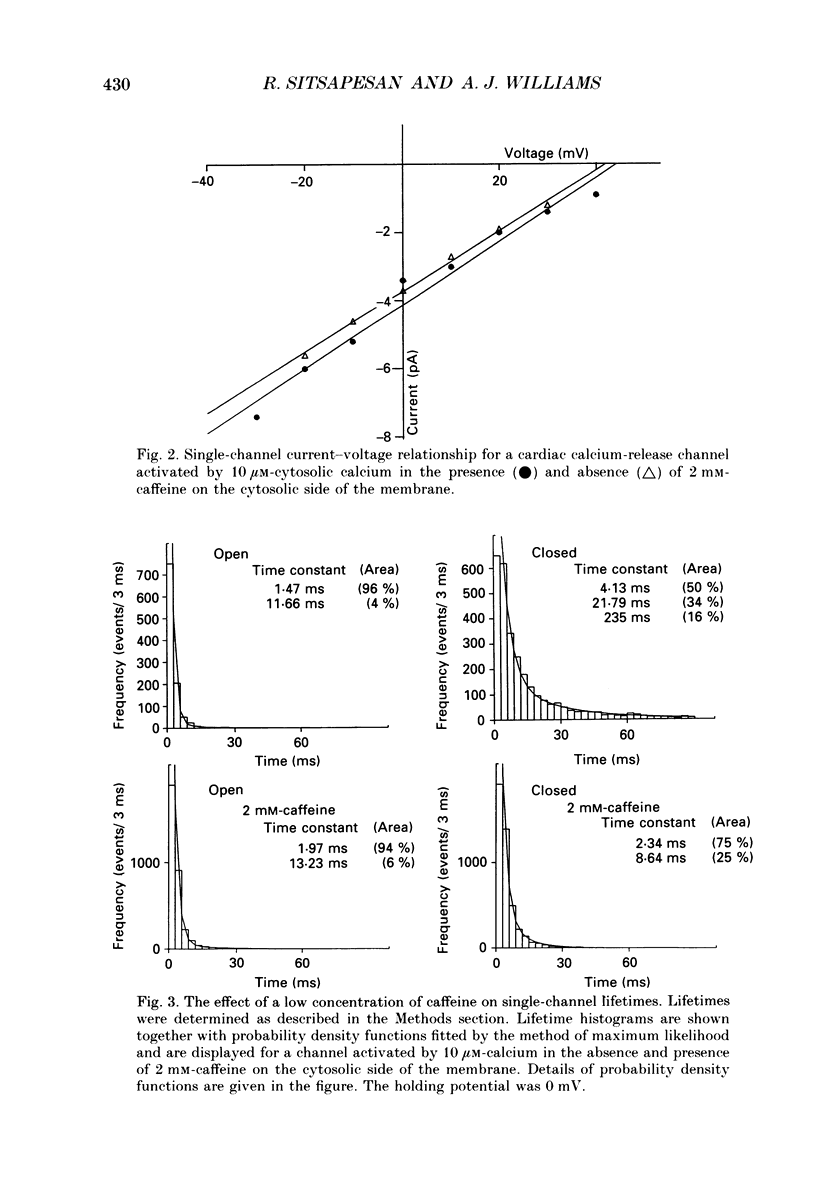
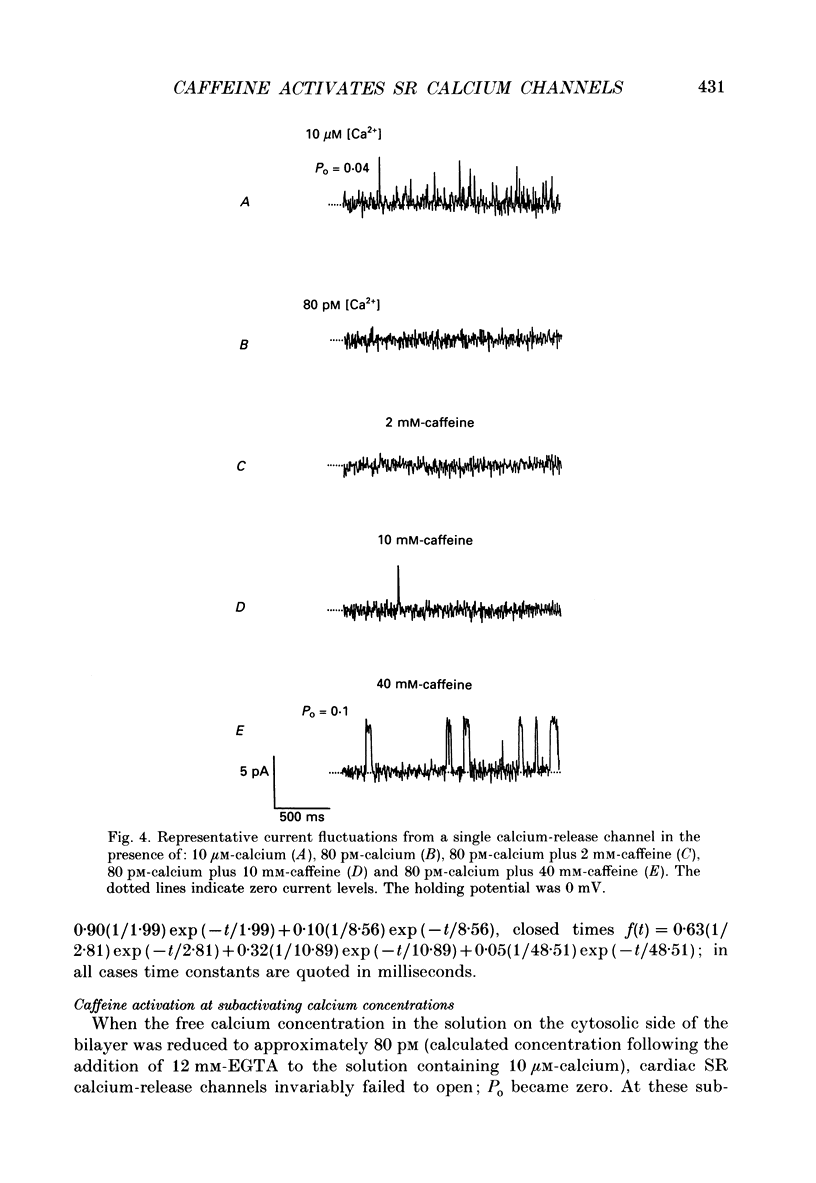
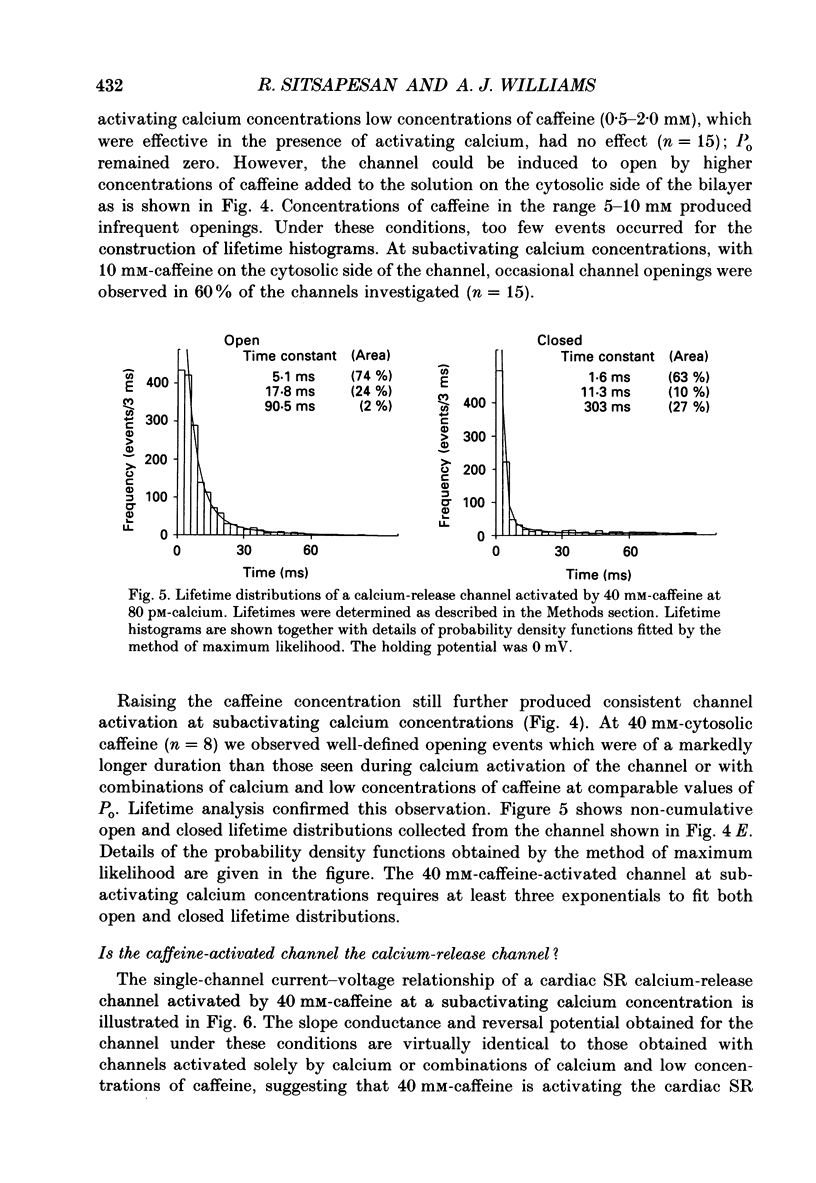
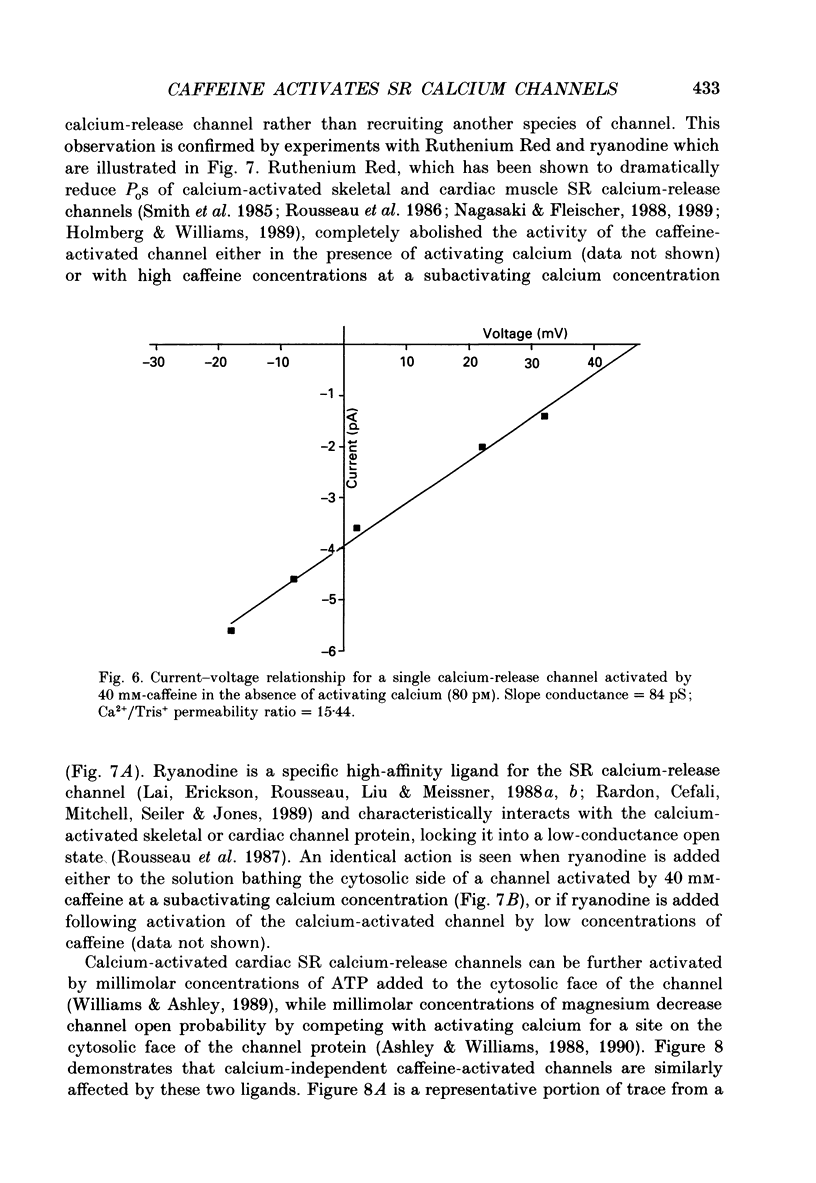
1. Calcium-release channels of sheep cardiac junctional sarcoplasmic reticulum were incorporated into planar phospholipid bilayers. Single-channel current fluctuations were recorded under voltage clamp conditions. 2. Channels incorporate into the bilayer with a fixed orientation and channel open probability is regulated by the calcium concentration at the cytosolic face of the membrane. 3. Addition of caffeine (0.5-2.0 mM) to the cytosolic side of the membrane increased the open probability of the calcium-activated calcium-release channel by increasing the frequency of opening without significant alteration to the durations of open events. This effect was observed at both 0.1 and 10 microM-activating cytosolic calcium. 4. Caffeine (0.5-2.0 mM) did not activate the channel at a subactivating cytosolic calcium concentration (80 pM). 5. At subactivating calcium concentrations, channels could be activated by higher concentrations of caffeine (greater than 5.0 mM) revealing a second, calcium-independent, mechanism for channel activation. Channel openings induced by these high concentrations of caffeine at subactivating calcium concentrations displayed different kinetics from those observed with calcium as the sole activating ligand or with combinations of calcium and low concentrations of caffeine. 6. Activation of channel opening by caffeine in the presence of calcium did not affect single-channel conductance. Channel openings produced by caffeine at subactivating cytosolic calcium concentrations had identical conductance and relative permeability to those seen on calcium activation. 7. Channels activated by caffeine at both activating and subactivating calcium concentrations were characteristically modified by ryanodine, Ruthenium Red, ATP and magnesium, implying that the same channel is involved under both conditions.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K., Lai F. A., Liu Q. Y., Rousseau E., Erickson H. P., Meissner G. Structural and functional characterization of the purified cardiac ryanodine receptor-Ca2+ release channel complex. J Biol Chem. 1989 Jan 15;264(2):1329–1335. [PubMed] [Google Scholar]
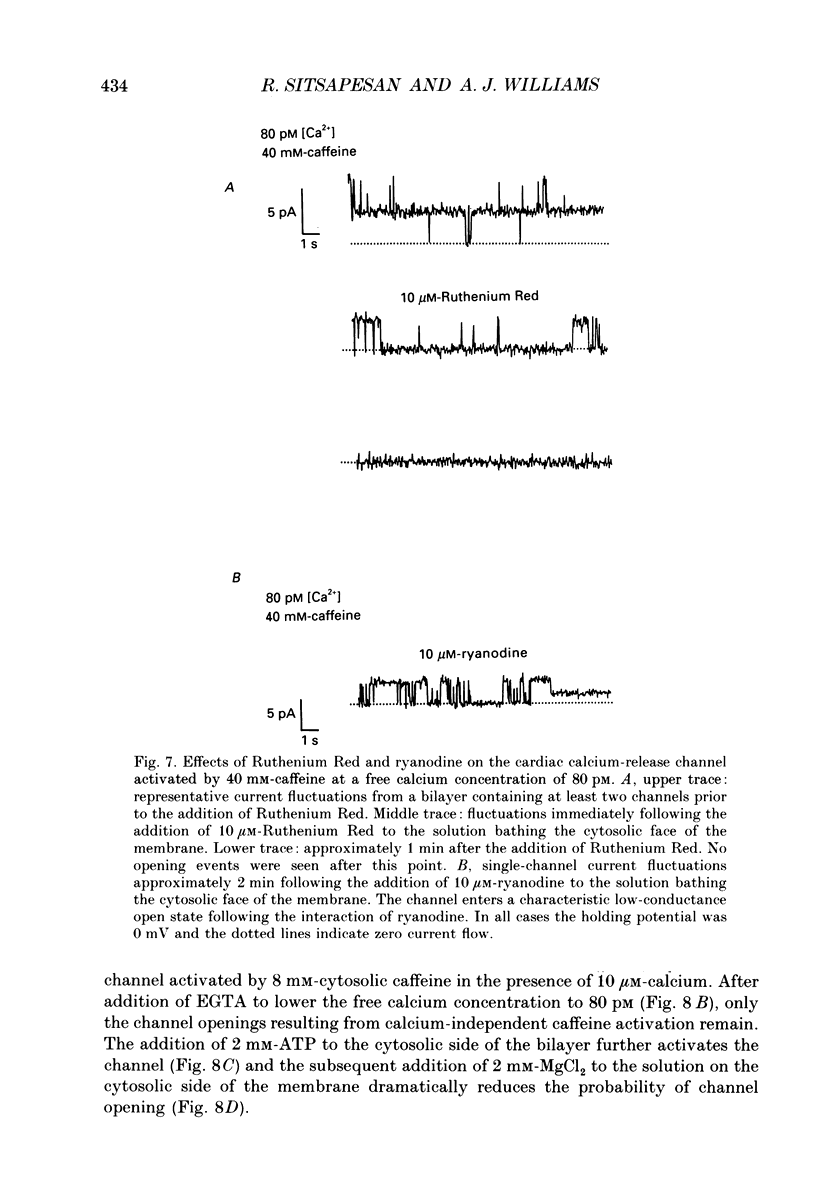
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Olson C. B., Jewell B. R., Bravený P. Influence of caffeine and other methylxanthines on mechanical properties of isolated mammalian heart muscle. Evidence for a dual mechanism of action. Circ Res. 1972 Apr;30(4):367–392. doi: 10.1161/01.res.30.4.367. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Effects of ryanodine in skinned cardiac cells. Fed Proc. 1985 Dec;44(15):2970–2976. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Activation of skinned cardiac cells. Subcellular effects of cardioactive drugs. Eur J Cardiol. 1973 Dec;1(2):143–155. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Techniques of skinned cardiac cells and of isolated cardiac fibers with disrupted sarcolemmas with reference to the effects of catecholamines and of caffeine. Recent Adv Stud Cardiac Struct Metab. 1976;9:1–94. [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg S. R., Williams A. J. Single channel recordings from human cardiac sarcoplasmic reticulum. Circ Res. 1989 Nov;65(5):1445–1449. doi: 10.1161/01.res.65.5.1445. [DOI] [PubMed] [Google Scholar]
- Imagawa T., Smith J. S., Coronado R., Campbell K. P. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem. 1987 Dec 5;262(34):16636–16643. [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Lai F. A., Anderson K., Rousseau E., Liu Q. Y., Meissner G. Evidence for a Ca2+ channel within the ryanodine receptor complex from cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun. 1988 Feb 29;151(1):441–449. doi: 10.1016/0006-291x(88)90613-4. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Meissner G., Henderson J. S. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem. 1987 Mar 5;262(7):3065–3073. [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Miller C., Rosenberg R. L. A voltage-gated cation conductance channel from fragmented sarcoplasmic reticulum. Effects of transition metal ions. Biochemistry. 1979 Apr 3;18(7):1138–1145. doi: 10.1021/bi00574a003. [DOI] [PubMed] [Google Scholar]
- Nagasaki K., Fleischer S. Modulation of the calcium release channel of sarcoplasmic reticulum by adriamycin and other drugs. Cell Calcium. 1989 Jan;10(1):63–70. doi: 10.1016/0143-4160(89)90045-6. [DOI] [PubMed] [Google Scholar]
- Nagasaki K., Fleischer S. Ryanodine sensitivity of the calcium release channel of sarcoplasmic reticulum. Cell Calcium. 1988 Feb;9(1):1–7. doi: 10.1016/0143-4160(88)90032-2. [DOI] [PubMed] [Google Scholar]
- Niedergerke R., Page S. Analysis of caffeine action in single trabeculae of the frog heart. Proc R Soc Lond B Biol Sci. 1981 Nov 13;213(1192):303–324. doi: 10.1098/rspb.1981.0068. [DOI] [PubMed] [Google Scholar]
- Näbauer M., Callewaert G., Cleemann L., Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989 May 19;244(4906):800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- Pessah I. N., Francini A. O., Scales D. J., Waterhouse A. L., Casida J. E. Calcium-ryanodine receptor complex. Solubilization and partial characterization from skeletal muscle junctional sarcoplasmic reticulum vesicles. J Biol Chem. 1986 Jul 5;261(19):8643–8648. [PubMed] [Google Scholar]
- Rardon D. P., Cefali D. C., Mitchell R. D., Seiler S. M., Jones L. R. High molecular weight proteins purified from cardiac junctional sarcoplasmic reticulum vesicles are ryanodine-sensitive calcium channels. Circ Res. 1989 Apr;64(4):779–789. doi: 10.1161/01.res.64.4.779. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Ladine J., Liu Q. Y., Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988 Nov 15;267(1):75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Henderson J. S., Meissner G. Single channel and 45Ca2+ flux measurements of the cardiac sarcoplasmic reticulum calcium channel. Biophys J. 1986 Nov;50(5):1009–1014. doi: 10.1016/S0006-3495(86)83543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature. 1985 Aug 1;316(6027):446–449. doi: 10.1038/316446a0. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+. J Gen Physiol. 1986 Nov;88(5):573–588. doi: 10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins B., Harding S. E., Kirby M. S., Poole-Wilson P. A., Williams A. J. Contamination of a cardiac sarcolemmal preparation with endothelial plasma membrane. Biochim Biophys Acta. 1986 Mar 27;856(1):137–143. doi: 10.1016/0005-2736(86)90020-9. [DOI] [PubMed] [Google Scholar]
- Tomlins B., Williams A. J., Montgomery R. A. The characterization of a monovalent cation-selective channel of mammalian cardiac muscle sarcoplasmic reticulum. J Membr Biol. 1984;80(2):191–199. doi: 10.1007/BF01868775. [DOI] [PubMed] [Google Scholar]
- Williams A. J., Ashley R. H. Reconstitution of cardiac sarcoplasmic reticulum calcium channels. Ann N Y Acad Sci. 1989;560:163–173. doi: 10.1111/j.1749-6632.1989.tb24093.x. [DOI] [PubMed] [Google Scholar]


