Abstract
Objective: To explore the characteristics of the ploidy status of Small Cell Lung Cancer (SCLC) cells and assesses its efficacy in cytopathological diagnosis of SCLC. Methods: A retrospective analysis was performed on patients who agreed to DNA image cytometry (DNA ICM) and serum tumor biomarker testing. Samples for ploidy assessment, all from bronchial procedures, were analyzed using DNA ICM. Data on demographic features, pathological characteristics, staging results, biomarkers such as neuron specific enolase (NSE) and Pro-gastrin-releasing peptide (ProGRP) data, and ploidy status across different groups were collected. Corresponding statistical analyses were conducted to examine differences in aneuploid status among the groups. Receiver operating characteristic (ROC) curve analysis was used to evaluate the diagnostic efficacy of DNA ICM and tumor markers for diagnosing SCLC. Results: A total of 74 patients with SCLC, 108 patients with NSCLC, and 74 patients with benign lesions were included. The age range of all patients was 26 to 85 years, with 69% being male and 31% female. In terms of ploidy status, single aneuploid cell peaks and double aneuploid peaks were observed in both SCLC and NSCLC groups. The proportions of two types of aneuploid cell peaks differed significantly between the two groups (P=0.008). The aneuploid cell ratio showed a high accuracy for small-cell lung cancer, with a sensitivity of 91.2% and a specificity of 88.1%. There was no statistical difference in ploidy status between limited and extensive stages of SCLC. As for biomarkers, the AUC (area under the curve) values were 0.921 for NSE and 0.928 for ProGRP, with a significant difference in NSE between two stages of SCLC (P=0.015), while no significant difference was observed in ProGRP. Conclusion: Aneuploid cell peaks in SCLC patients are mostly distributed in the near triploid range, possibly serving as a specific cytological marker for SCLC. DNA ICM is a highly sensitive tool that may be an adjunct for SCLC diagnosis.
Keywords: Small cell lung cancer, DNA image cytometry, aneuploid peak, tumor biomarker
Introduction
Lung cancer continued to have the highest morbidity and mortality rates among malignant tumors worldwide in 2022, according to global cancer statistics [1]. Most cases were histologically confirmed to be non-small cell lung cancer (NSCLC), while small cell lung cancer (SCLC) accounted for approximately 15% of all lung cancer cases [2]. However, SCLC is characterized by its high aggressiveness, rapid growth, and tendency to metastasize early. Most patients develop therapeutic resistance rapidly, resulting in a five-year survival rate of merely 5-6% [3]. The 2-year relative survival rate for extensive-stage patients is approximately 7% to 8%, with a median survival time of 7 months [3]. As a result, early identification and accurate staging are critical for improving SCLC prognosis.
Currently, chest CT, sputum cytology, serum tumor marker detection, and liquid biopsy are commonly used in clinical practice to screen for lung cancer. Low-dose spiral CT (LDCT) has been found to improve the early identification of lung cancer and dramatically lower mortality [4]. However, as detection rates improved, the false-positive rate associated with LDCT in lung cancer screening has also increased [5]. A study conducted in China indicated that the false-positive rate may reach 97.9% in individuals with lung nodules [6]. While serum tumor indicators offer a noninvasive and convenient method, they often lack the necessary sensitivity and specificity. Although histology results are considered as a gold standard, they can be influenced by many factors, including tumor size, restricted tissue sampling, and interobserver variability [7,8].
Cytology is a key step in screening for lung cancer. Nonetheless, its accuracy is influenced by various factors, such as the expertise of the diagnostician, the locations of the sample, and inadequate sampling, which can pose a risk of false negatives [9]. With advances in cytopathology, bronchoscopy combined with liquid-based cytology has greatly improved the sensitivity and specificity of lung cancer diagnosis compared to conventional cytology [10]. Moreover, the development of DNA quantitative analysis technology, characterized by its objectivity, convenience, and capacity to detect cancer cells before cell morphologic changes, has introduced a new dimension to cytopathology. Although its current use in lung cancer is limited, the diagnostic criteria have yet to be fully established, and the results of some studies vary widely [11-14].
DNA aneuploidy is a common characteristic of malignant tumors and is associated with tumor initiation, progression, recurrence, and therapeutic resistance in cancer. It results from genetic instability and uncontrolled proliferation of tumor cells [15]. The detection and analysis of DNA aneuploidy can be performed by flow cytometry (FCM) and imaging cytometry (ICM). DNA-FCM is a cellular analysis approach that utilizes hydrodynamic principles to achieve high-speed and high-throughput analysis. DNA-FCM is suitable for the rapid analysis of large numbers of living cells or fresh tissue samples. This technique is especially valuable in the evaluation of hematologic and lymphoid malignancies. DNA-ICM utilizes microscopic image analysis to scan cell nuclei and measure their Integrated Optical Density (IOD), thus indirectly quantifying the relative DNA content. The DNA index (DI) for each cell is calculated by comparing its DNA content with that of a standard diploid cell. A DI value above 1.0 typically indicates DNA is hyperdiploid, whereas less than 1.0 is hypodiploid. Aneuploidy, defined as the gain or loss of chromatid or chromosome regions, is a hallmark of malignant tumors [16]. According to the European Society for Analytical Cellular Pathology (ESACP) [17], DNA aneuploidy includes DNA stemline aneuploidy and rare events. The former refers to a deviation of more than 10% of the tumor cell population from the diploid (2c ± 0.2c) or tetraploid (4c ± 0.4c) regions, while the latter indicates the presence of nuclei with DNA content ≥ 5c, referred to as 5c exceeding events (5cEE) or 5c exceeding rate (5cER). Several studies have shown that the DNA content distributions of some solid malignancies, including lung cancer, tend to be in the near-triploid range [18-20]. Combined with cellular morphology, DNA-ICM provides useful information for tumor diagnosis, grading, prognostic assessment, and efficacy monitoring [21]. ICM is used in clinical cytopathologic screening for cervical and oral cancers due to its objectivity and ability to detect cancer cells before morphologic changes become apparent. Thus, the ICM technique was chosen to examine ploidy status in this study.
As an important cytopathologic detection method, DNA ICM has been widely applied in research on various cancers, including cervical, esophageal, and oral mucosal cancers [14,22,23]. However, its application and in-depth study in lung tumors, particularly in SCLC, remain limited. Genomic research on SCLC, especially concerning DNA ICM, has received little attention to date.
Given the predictive potential and clinical value of aneuploidy in other types of cancers, further exploration of DNA aneuploidy detection using DNA image cytometry for SCLC is necessary. Therefore, our study collected DNA ploidy data from 74 patients with SCLC and retrospectively analyzed their clinical characteristics, tumor marker levels, and DNA ploidy. We compared these patients with those who suffered from NSCLC and benign lung diseases to define the characteristics of the distribution of ploidy peaks in SCLC. Furthermore, we explored a cutoff value of DNA ICM for diagnosing SCLC and compared the different values between DNA ICM and tumor markers for different stages of SCLC.
Materials and methods
Patients and methods
The research was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (Approval No. 2024-069-01). Patients with lung cancer who underwent tracheoscopy and DNA ICM examination at the First Affiliated Hospital of Chongqing Medical University were retrospectively enrolled from July 2022 to July 2023. The inclusion criteria were as follows: (1) patients with primary lung cancer were diagnosed by histopathology or cytology combined with immunohistochemistry; (2) all samples were collected through bronchoscopy procedures, including bronchoalveolar lavage fluid (BALF), bronchial brushing samples, endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) and fine-needle aspiration samples; (3) all patients were newly diagnosed and had not received prior treatments such as chemotherapy or radiotherapy; (4) all patients underwent tumor marker testing, including ProGRP and NSE. Exclusion criteria: (1) patients with primary malignancies from other systems; (2) insufficient cells for DNA-ICM. Finally, the study enrolled 74 patients with SCLC patients, 108 patients with NSCLC, and 74 patients with benign lung diseases. The benign pulmonary diseases included 34 cases of lung infection, 11 cases of interstitial lung disease, 16 cases of tuberculosis, 5 cases of COPD, 3 cases of bronchial asthma, and 5 cases of other benign diseases. Lung cancer staging was performed according to the UICC/AJCC 8th edition TNM staging system, in which small-cell lung cancer was staged in conjunction with the Veterans Association of the United States of America (VALG).
Sample acquisition and processing
All samples were acquired through bronchoscopy procedures conducted by qualified bronchoscopists at the First Affiliated Hospital of Chongqing Medical University. Prior to the procedure, the experts preliminarily localized the lesion site according to CT scan result. Bronchoscopes or EBUS were used according to an established process with the use of cone beam computed tomography (CBCT) or virtual navigation planners if necessary. Upon reaching the target bronchial segment or suspicious malignant area, techniques such as brush cytology, bronchoalveolar lavage, fine-needle aspiration, or EBUS-TBNA were used for sample collection. Samples were immediately transferred to bottles containing a cell preservation solution and then sent to the cytopathology department. The technician added mucus treatment solutions into these bottles and shook them on a vortex mixer for 10-15 minutes. The resulting mixture was collected into 50-mL centrifuge tubes and centrifuged at 2000 rpm for 5 min. The supernatant was discarded, and the cell precipitate was added to 1 mL tris buffer (pH=7.4). Then, the cell suspensions were mixed by shaking on the vortex mixer. Half of the cell suspension was prepared for liquid-based cytology with H&E staining for cytological diagnosis; the other half was stained with Feulgen stain for DNA ICM, whose slides were automatically scanned by the MotiCyte-auto system (Motic, Xiamen, China). The cell preservation solution and liquid-based staining machine were from Guangzhou Anbiping Medical Corporation.
Automated DNA-ICM
All Feulgen-stained slides were scanned and processed using a fully automated DNA image cytology analysis system, which consists of a computer and an automated digital microscope that automatically scans each cell nucleus on the slides and measures parameters like the IOD value and nuclear area, thereby calculating a relative DNA content of the nuclei. The number of cells on the slides used for scanning should be not less than 2000, and at least 500 epithelial cells had to be present. Ploidy analysis of cellular DNA was performed according to the software integrated into the ICM system. The system automatically generated various ploidy parameters for each slide, including the maximum DNA Index (DI) value, the number of aneuploid cells, and the 5cER. Additionally, DI values for each cell nucleus were provided, and DNA histograms were created. The median DI was determined by calculating the median of the top 20 highest DI values within each slide. The system also supplied data regarding the number of cells in the histogram, the number of cells in the peaks, and the peak DI value.
Moreover, the analysis and interpretation of DNA ploidy status and histograms followed the consensus of ESACP. Specifically, DNA stemlines in histograms were automatically identified as peaks. DNA stemline aneuploidy is exhibited when the mean nuclear DNA content of a stemline deviates by more than ±10% from the diploid (2c) or tetraploid (4c) values. Accordingly, the ploidy status was categorized as follows: (i) Diploid: The main peak is located in the 2C region (DI=0.9-1.1). (ii) Tetraploid: The main peak is located in the 4C region (DI=1.80-2.20). (iii) Aneuploid: The main peak is not located in the 2C or 4C region, with a DI between 1.1-1.8 or DI > 2.2.
Serum tumor markers test
The tumor markers detected included ProGRP and NSE. A morning fasting venous blood sample of 3 mL was collected from patients, and serum was obtained by centrifugation at 3000 rpm. Serum tumor markers were detected using an electrogenerated chemiluminescence machine (Cobas e 602). The reference ranges of serum tumor markers were 25.3-77.8 pg/mL for ProGRP and 0-16.3 ng/mL for NSE.
Statistical analysis
All analyses were performed using SPSS 26.0 software. The Mann-Whitney U test was used to analyze the difference in ploidy-related parameters between different groups. Pearson’s chi-square test was used to compare the differences in the distribution of ploidy peaks between groups. All statistical tests were two-sided tests, and P < 0.05 was considered significant. The optimal cutoff values for continuous variables were calculated using the Youden index method. Stacked bar graphs and ROC curves of the ratio of peak number composition of aneuploid cells were plotted using GraphPad Prism 8.0, and the DeLong method was used to compare the statistical differences between the ROC curves.
Results
Clinicopathologic features
We enrolled a total of 256 patients with lung diseases, detailed in in Table 1. Among the 74 patients with benign lung diseases, the median age was 57.5 years, ranging from 26 to 84 years, with 48 (64.9%) males and 26 (35.1%) females, and the majority of 34 (45.9%) patients had lung infections, as mentioned previously. Among the 74 patients with SCLC and 108 patients with NSCLC, the median age of both groups was 66 years, of which 43 (58.1%) patients in the SCLC group were ≥ 65 years old. Most SCLC cases were male (83.8%), and most of them (87.9%) were in the intermediate-advanced stage at the time of initial diagnosis.
Table 1.
Clinical characteristics of lung cancer patients at baseline
| Characteristic | NSCLC | SCLC | Benign |
|---|---|---|---|
| (n=108) | (n=74) | (n=74) | |
| Age | |||
| Median (range) | 66 (37-85) | 66 (40-84) | 57.5 (26-84) |
| < 65, n (%) | 49 (45.4) | 31 (41.9) | 49 (66.2) |
| ≥ 65, n (%) | 59 (54.6) | 43 (58.1) | 25 (33.8) |
| Gender, n (%) | |||
| Male | 68 (63.0) | 62 (83.8) | 48 (64.9) |
| Female | 40 (37.0) | 12 (16.2) | 26 (35.1) |
| Pathology type, n (%) | |||
| Adenocarcinoma | 63 (58.3) | - | - |
| Squamous | 37 (34.3) | - | - |
| NSCLC | 8 (7.4) | - | - |
| SCLC | - | 74 | - |
| Stage, n (%) | |||
| I | 7 (6.5) | 1 (1.4) | - |
| II | 5 (4.6) | 3 (4.1) | - |
| III | 26 (24.1) | 9 (12.2) | - |
| IV | 51 (47.2) | 56 (75.7) | - |
| Unknown | 19 (17.6) | 5 (6.8) | - |
59 (54.6%) patients in the NSCLC group were ≥ 65 years of age, 63% of patients were male, and 71.3% of patients were in stage III and IV at the initial diagnosis. The pathology results showed 63 adenocarcinomas, 37 squamous carcinomas, 1 adenosquamous carcinoma, and 8 non-small cell lung cancers-not otherwise specified (NSCLC-NOS).
Cellular morphology and ploidy status of SCLC cells
SCLC cells usually present with basophilic, relatively large nuclei, which tend to exhibit a high nuclear/cytoplasmic ratio due to scant cytoplasm (Figure 1A). The nuclei of these cells are sorted according to their DI values from smallest to largest. In general, SCLC cells follow the principle of a positive correlation between nuclear size and DI value, meaning that a larger nucleus corresponds to a higher DI value. Moreover, the size of the DI value is related to the area of the nucleus as well as the staining depth of the nucleus (Figure 1). Additionally, we analyzed the DNA ploidy status of each patient. Table 2 presents the comparison of ploidy status between SCLC and NSCLC groups. There was no significant difference in the maximum DI values (referred to as ‘max DI’) or in the median DI of the top 20 cells exceeding 5c (referred to as ‘median DI’) between the two groups. Nevertheless, significant differences were observed in the percentage of cells with DI greater than 5c (referred to as ‘5cER’) and in the distribution of aneuploid peaks between the two groups.
Figure 1.
Morphologic features and DNA ploidy results by DNA ICM analysis in liquid-based cytology specimens from two patients with SCLC. The nuclei exhibited a round or ovoid shape, the nucleoli were finely structured, the nucleoplasm ratio was severely dysregulated, and nuclear mosaicism was less prominent in some tumor cells. The phenomenon of clustered distribution of cancer cells in small clusters was seen. A. Liquid-based cytology pictures of small-cell carcinoma cells (×400). Scale bars are 50 µm. B. Immunohistochemical staining of the cellular wax block with synaptophysin antibody (+, brown) (×400 magnification). Scale bars are 50 µm. C. DNA ploidy results from patient samples using ICM analysis, with carcinoma cells arranged in descending order of DI values.
Table 2.
Comparison of DNA ploidy parameters between SCLC and NSCLC groups
| SCLC | NSCLC | U/χ2 | P | |
|---|---|---|---|---|
| Max DI, M (P25, P75) | 5.26 (3.83, 6.86) | 5.92 (4.17, 7.39) | 2507.00 | 0.122 |
| Median DI, M (P25, P75) | 3.38 (2.43, 3.98) | 3.62 (2.74, 4.18) | 2722.00 | 0.444 |
| 5cER, M (P25, P75) | 0.1217 (0.0254, 0.2093) | 0.1506 (0.0642, 0.3343) | 2392.00 | 0.05* |
| Distribution of aneuploid peaks, DI value (%) | - | - | 11.64 | 0.008* |
| No aneuploid peak | (6%) | (24.4%) | - | |
| Single aneuploid peak | 1.2-1.8 (47.7%) | 1.2-1.8 (32.9%) | ||
| Double aneuploid peak | 2.5-3.5 (40.3%) | 2.5-4.2 (31.9%) | ||
| Multiple aneuploid peak | 2.5-3.2 (6%) | 3.0-5.0 (10.8%) | ||
Notes: Max DI represents for the maximum DI; median DI, namely, the median DI of the first 20 cells greater than 5C; 5cER: 5c exceeding rate. We used 5cER to represent the percentage of cells with DI greater than 5c in this study. The DI value of a single aneuploid peak refers to the DI value of the first aneuploid peak, the DI value of double aneuploid peaks refers to the DI vale of the second one, and the DI value of multiple aneuploid peaks refers to the DI value of the third one.
P < 0.05.
Analysis of aneuploid peak in the SCLC group
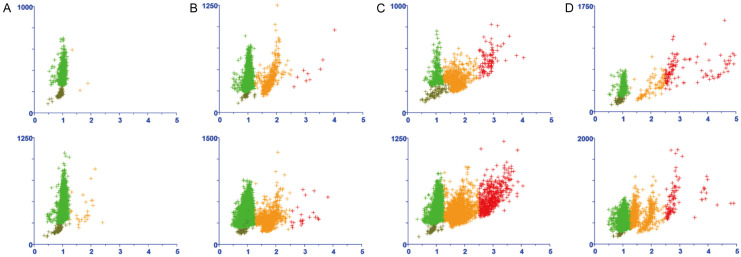
Figure 2 shows several representative cases of different types of aneuploid peaks. In small cell lung cancer, aneuploid cell peaks are categorized as: (1) single aneuploid peaks, which are mostly observed between 2C and 4C (i.e., DI value between 1.2 and 1.8); (2) double aneuploid peaks, with the first peak appearing between 2C and 4C (i.e., DI value between 1.2 and 1.8), and the second peak often having a DI value twice that of the first; (3) multiple aneuploid peaks, with varying heights in histograms like skyscrapers, indicating extreme chromosomal instability in tumor cells. The analysis of aneuploid peaks in the SCLC group, as shown in Table 2 and Figure 3, revealed that among 74 patients, single aneuploid peaks were predominant (47.7%), followed by double aneuploid peaks (40.3%). Multiple aneuploid peaks (denoting the presence of three or more aneuploid peaks) were rare (6%). It is worth noting that a majority of the cases (94%) each exhibited a peak with DI values between 1.2 and 1.8. As for the second peak in the double aneuploid peak cases and the third peak in the multiple aneuploid peak cases, the range of these peaks’ DI values in NSCLC varied more extensively than that in SCLC (Table 2).
Figure 2.
Different types of aneuploid peaks in representative cases as analyzed by DNA ICM. A. Absence of abnormal aneuploid peaks: the pattern is commonly observed in specimens from benign diseases or those with a very low percentage of tumor cells; B. Single aneuploid peak (represented by the cluster of orange points): this pattern was observed in two representative cases of SCLC; C. Double aneuploid peak (the two clusters of orange and red points): this pattern was observed in two representative cases of SCLC; D. Multiple aneuploid peak (several clusters of orange and red points), which were observed in two representative cases of SCLC.
Figure 3.

Proportions of aneuploid peak counts in patient samples with NSCLC, SCLC, and benign lesions.
Application of DNA ICM to the screening of small cell lung cancer
The area under the curves (AUCs) for 5cER, the number of cells > 5c, median DI, and max DI in the SCLC group were 0.966, 0.962, 0.862, and 0.838, respectively (Table 3). Furthermore, we applied the Youden Index to confirm the optimal threshold for each parameter in SCLC. The thresholds lay on the largest Youden index point of each ROC curve, which were 0.0142 for 5cER, 5.5 for the number of cells > 5c, 2.557 for median DI, and 2.6395 for max DI (Figure 4; Table 3). Specifically, 0.0142 for 5cER and 5.5 for the number of cells > 5c were the appropriate cutoff points for SCLC.
Table 3.
Cutoff points of ploidy and tumor markers for diagnosing SCLC
| AUC | 95% CI | Sensitivity | Specificity | Cutoff value | |
|---|---|---|---|---|---|
| ProGRP | 0.928 | 0.877-0.978 | 0.824 | 1 | 151.05 |
| NSE | 0.921 | 0.884-0.975 | 0.853 | 0.898 | 15.8 |
| Max DI | 0.838 | 0.769-0.907 | 0.956 | 0.593 | 2.6395 |
| Median DI | 0.862 | 0.795-0.930 | 0.75 | 0.949 | 2.557 |
| Number of cells with DI greater than 5c | 0.962 | 0.933-0.991 | 0.956 | 0.831 | 5.5 |
| 5cER | 0.966 | 0.933-0.991 | 0.912 | 0.881 | 0.0142 |
Notes: Max DI represents the maximum DI. Median DI is the median DI of the first 20 cells greater than 5C. 5cER: 5c exceeding rate. We used 5cER to represent the percentage of cells with DI greater than 5c in this study.
Figure 4.

ROC curve of ploidy values and tumor markers for diagnosing SCLC. 5cER: 5c exceeding rate. We used 5cER to represent the percentage of cells with DI greater than 5c in this study. The number of cells > 5c means the number of cells with DI greater than 5c. Median DI is the median DI of the first 20 cells greater than 5C. Max DI represents for the maximum DI.
Furthermore, we also evaluated the efficacy of serum tumor markers specific to SCLC, as depicted in Figure 4 and Table 3. NSE and ProGRP demonstrated high accuracy with AUCs of 0.921 and 0.928, respectively. At the defined cutoff values, their sensitivities were 0.853 and 0.824, and the specificities were 0.898 and 1, respectively. We then compared the statistical differences in the AUC of each parameter by DeLong’s method. We found that the AUCs of several ploidy metrics and tumor markers mentioned above were statistically different, as shown in Table 4.
Table 4.
Comparison of AUC of ploidy parameters and tumor markers in SCLC
| Statistic | P value | Difference of value of AUC | |
|---|---|---|---|
| ①-② | -0.692 | 0.489 | -0.024 |
| ①-⑤ | -2.145 | 0.032* | -0.091 |
| ①-⑥ | -2.088 | 0.037* | -0.089 |
| ①-④ | -3.552 | < 0.001* | -0.127 |
| ①-③ | -4.067 | < 0.001* | -0.124 |
| ②-⑤ | -1.606 | 0.108 | -0.067 |
| ②-⑥ | -1.45 | 0.147 | -0.065 |
| ②-④ | -3.041 | 0.002* | -0.103 |
| ②-③ | -3.256 | 0.001* | -0.100 |
| ⑤-⑥ | 0.05 | 0.96 | 0.002 |
| ⑤-④ | -1.419 | 0.156 | -0.036 |
| ⑤-③ | -1.164 | 0.244 | -0.032 |
| ⑥-④ | -1.254 | 0.21 | -0.038 |
| ⑥-③ | -1.117 | 0.264 | -.034 |
| ④-③ | 0.220 | 0.826 | 0.004 |
① The maximum DI; ② The median DI of the first 20 cells greater than 5C; ③ The number of cells with DI greater than 5c; ④ The percentage of cells with DI greater than 5c; ⑤ NSE; ⑥ ProGRP;
De Long test, P < 0.05.
DNA ICM and the stages of SCLC
Among the 74 patients with SCLC, 9 patients were in the limited stage, and 65 patients were in the extensive stage. As Table 5 and Figure 5 show, the results of Mann-Whitney U test indicated no significant difference in each ploidy parameter between limited and extensive stages of small cell lung cancer (P > 0.05). In addition, we analyzed and compared the differences of tumor markers between the stages of small cell lung cancer. The results showed that ProGRP had no significant difference between the limited and extensive stage of SCLC (P=0.087), while NSE had a significant difference between the two stages (P=0.015).
Table 5.
Comparison of ploidy values and tumor markers for SCLC staging
| Stage M (P25, P75) | U | P | ||
|---|---|---|---|---|
|
| ||||
| Limited stage | Extensive stage | |||
| Max DI | 3.98 (3.81, 7.32) | 5.35 (3.88, 6.98) | 201 | 0.42 |
| Median DI | 3.12 (2.35, 3.55) | 3.48 (2.52, 4.13) | 175 | 0.196 |
| Number of cells with DI greater than 5c | 63 (7, 97) | 64 (15, 236) | 195.5 | 0.404 |
| 5cER | 0.0516 (0.0371, 0.2374) | 0.0927 (0.2226, 0.2359) | 233 | 0.837 |
| ProGRP | 398.2 (62.8, 521.6) | 1083 (310.35, 4774) | 189 | 0.087 |
| NSE | 15.9 (11.9, 21.3) | 57 (27.7, 114.05) | 122 | 0.015* |
P < 0.05.
Figure 5.
Difference in ploidy parameters and tumor markers between limited stage (LS) and extensive stage (ES) of SCLC. A. No statistical difference in the maximum DI value between LS and ES of SCLC; B. No statistical difference in the percentage of cells with DI greater than 5c between LS and ES of SCLC; C. A statistical difference in NSE between LS and ES of SCLC; D. No statistical difference in the median DI of the first 20 cells greater than 5C between LS and ES of SCLC; E. No statistical difference in the number of aneuploid cell peaks between LS and ES of SCLC; F. No statistical difference in ProGRP between LS and ES of SCLC. Error bars depict standard error of the mean (SEM). *, P < 0.05; NS, not significant.
Discussion
DNA aneuploidy serves as a marker of malignant cells [24]. Through special staining of DNA nuclei, digital scanning, and optical measurement of IOD, a computerized DNA quantitative analysis system can provide information about the content and changes in ploidy status of nuclei and give objective values. Thus, it can assist pathologists in identifying benign and malignant cells and offer an objective and scientific basis for diagnosis. In our study, DNA ICM technology was applied to evaluate the ploidy status of SCLC cells and the application of DNA ploidy analysis for cytopathologic diagnosis of SCLC.
The efficacy and threshold of DNA ICM for lung cancer detection remain controversial. Several studies have investigated the application of ICM for lung cancer detection, with reported thresholds for aneuploid cell counts ranging from 3-4. Sensitivity across these studies varied from 62.3%-82.8, while specificity ranged from 87.5%-100% [11,12,25-27]. These findings suggest a trend where the specificity of ICM tends to be higher than its sensitivity. However, the current study showed that the sensitivity was 95.6% and the specificity was 59.3% at a threshold of 2.64 for the maximum value of DI. The sensitivity was 95.6%, and the specificity was 83.1% at an aneuploid cell number of 5.5. These results indicate a markedly different trend compared to earlier studies in which specificity generally exceeded sensitivity. This may be explained by the current study also using tumor marker testing as an inclusion criterion during the process of data collection, which led to selection bias and, consequently, a high rate of true positives. Another cause for this discrepancy may have been the varying rates of positive diagnosis for different specimen types. Apart from that, primary malignant tumors at different sites have various thresholds for DNA ICM [25]. A multicenter large-sample esophageal cancer screening study [13] revealed that a DNA content of 5.8c and an aneuploid cell number of 3 were the optimal cut-off values for screening esophageal cancer. A prospective diagnostic experiment by Li [28] et al. indicated that the diagnostic criteria for the aneuploid using DNA ICM as a noninvasive detection of oral precancerous lesions and cancer in the Chinese population were DI ≥ 2.3 and DI ≥ 3.5, respectively. Meng [11] et al. reported that the optimal aneuploidy threshold for DNA quantitative image cytometry in the diagnosis of malignant pleural effusion was 4 cells with DI values exceeding 2.5c. Therefore, the results of these studies varied widely, which may be influenced by several factors such as different sample sizes, heterogeneity of the enrolled subjects, differences in study design, and tumor origin [29]. This finding suggests that the combination of multiparametric diagnosis by DNA ICM could be considered in some specific tumors like SCLC, despite the fact that DNA ploidy analysis is often used as a positive criterion (at least 3 aneuploid cells with DI > 2.5) in the screening of most tumors. Further studies are needed to prove whether the combination of several parameters of ploidy analysis is helpful for diagnosing SCLC.
Aneuploidy peaks, a typical feature of genetic instability, are often closely associated with the initiation and progression of malignant tumors. A meta-analysis integrating 35 relevant studies pointed out that the incidence of aneuploidy in NSCLC was 65% (95% CI: 64%-67%) [30]. Given this significant association, we examined the distribution of DNA aneuploidy peaks in SCLC (shown in Figures 2, 3). Even though the maximum and median DI values of 74 SCLC patients were not statistically different from those of the NSCLC patients, these two values of SCLC were lower than those of NSCLC in general. Our study also found that the 5cER, the number of cells > 5c and the proportion of aneuploid peaks differed between the two groups, which led to the conclusion that these three values were higher in the SCLC group. Surprisingly, our findings in the present study were similar to the results reported by Hu et al. [26]. Specifically, while NSCLC exhibited a higher level of DI value compared to SCLC, this conclusion seems to conflict with the observation that both the counts and proportions of aneuploid cells were higher in SCLC than in NSCLC. However, these findings are consistent with the morphologic features and aggressive biological behavior of SCLC. In other words, the smaller nuclear size of SCLC cells resulted in a lower DI value, and its more aggressive behavior led to a larger number of aneuploid cells.
Small cell lung cancer has been shown to have a rapid doubling time, with an average doubling time of 2-3 months. This rapid cell proliferation is usually associated with cell cycle acceleration, and the DNA index is one crucial metric for assessing DNA content. In rapidly proliferating tumors, more cells may be in the DNA synthesis phase (S phase) and have abnormal mitosis due to the accelerated cell cycle [15]. Consequently, this can result in the presence of aneuploid cells, which possess DNA content of more than normal diploid (2N) cells, approaching or exceeding triploid (3N) levels. As observed in this study, 94% cases with SCLC showed peaks of DI value between 1.2-1.8, suggesting that SCLC belongs to the group of tumor types with a considerable number of near triploid cancer samples. Further observation found that there were only 40.3% of cases with SCLC that showed another replication peak of the 1.2-1.8 peak. The lower incidence of double aneuploid cell peaks in some samples may be due to the relatively low total number of tumor cells, which are insufficient to produce a significant double peak phenomenon. For samples with sufficient cell numbers, the mechanism behind the failure to observe a second replication peak may require in-depth discussion with the help of genomic results. The small number of cases with no aneuploid cell peak may be related to a limited number of cells collected by bronchoscopy or restriction of the sampling site. For small cell lung cancer with multiple aneuploid cell peaks, although this phenomenon is observed in only a small proportion of cases (6%), it remains unclear whether further research into this subset is warranted. Additional case data are needed to reveal further biological characteristics of small-cell carcinoma in combination with genomics.
In summary, this is a small sample, single-center retrospective study that considers and compares multimodal data, and the conclusions need to be verified in multicenter and prospective studies. Given that many studies [31-33] point out the importance of aneuploidy in the prognostic assessment of solid tumors, this controversy is particularly important in the overall context of precision medicine. Despite some studies indicating that aneuploidy is not an independent prognostic factor by multivariate analysis [34,35], the broader implications of aneuploidy remain critical for understanding tumor biology and guiding therapeutic strategies. In the future, we plan to construct a diagnostic predictive model using machine learning methods, with external validation. We will also conduct a longitudinal study to evaluate the efficacy of DNA-ICM for monitoring the progression and prognosis of lung cancer. The elevated DI values observed in the nuclei of SCLC cells reflect the genomic instability and aneuploidy characteristic of this tumor type. The genomic disorganization of SCLC is reflected by elevated DI values, which serve as a quantitative measure of the degree of nuclear abnormality and, in turn, may reflect highly aggressive behavior and poor prognosis in SCLC. Therefore, it is worthwhile to continue promoting this technology in primary hospitals, and possibly combine it with deep learning to improve the accuracy and reliability of diagnosis.
Acknowledgements
The authors would like to express gratitude to the staff in the Cytopathology Laboratory at the First Affiliated Hospital of Chongqing Medical University for their technical assistance with laboratory work and sample preparation. This work was supported by the Graduate Tutor Team Construction Project of the First Affiliated Hospital of Chongqing Medical University (Project Number: CYYY-DSTDXM-202408) and In Vitro Diagnostics Project of Chongqing Medical University International Institute.
Disclosure of conflict of interest
None.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 2.Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7:3. doi: 10.1038/s41572-020-00235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, Tang J, Sun T, Zheng X, Li J, Sun H, Zhou X, Zhou C, Zhang H, Cheng Z, Ma H, Sun H. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep. 2017;7:1339. doi: 10.1038/s41598-017-01571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oudkerk M, Liu S, Heuvelmans MA, Walter JE, Field JK. Lung cancer LDCT screening and mortality reduction - evidence, pitfalls and future perspectives. Nat Rev Clin Oncol. 2021;18:135–151. doi: 10.1038/s41571-020-00432-6. [DOI] [PubMed] [Google Scholar]
- 5.Sands J, Tammemägi MC, Couraud S, Baldwin DR, Borondy-Kitts A, Yankelevitz D, Lewis J, Grannis F, Kauczor HU, Von Stackelberg O, Sequist L, Pastorino U, McKee B. Lung screening benefits and challenges: a review of the data and outline for implementation. J Thorac Oncol. 2021;16:37–53. doi: 10.1016/j.jtho.2020.10.127. [DOI] [PubMed] [Google Scholar]
- 6.Ji G, Bao T, Li Z, Tang H, Liu D, Yang P, Li W, Huang Y. Current lung cancer screening guidelines may miss high-risk population: a real-world study. BMC Cancer. 2021;21:50. doi: 10.1186/s12885-020-07750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lami K, Bychkov A, Matsumoto K, Attanoos R, Berezowska S, Brcic L, Cavazza A, English JC, Fabro AT, Ishida K, Kashima Y, Larsen BT, Marchevsky AM, Miyazaki T, Morimoto S, Roden AC, Schneider F, Soshi M, Smith ML, Tabata K, Takano AM, Tanaka K, Tanaka T, Tsuchiya T, Nagayasu T, Fukuoka J. Overcoming the interobserver variability in lung adenocarcinoma subtyping: a clustering approach to establish a ground truth for downstream applications. Arch Pathol Lab Med. 2023;147:885–895. doi: 10.5858/arpa.2022-0051-OA. [DOI] [PubMed] [Google Scholar]
- 8.Poller DN, Bongiovanni M, Cochand-Priollet B, Johnson SJ, Perez-Machado M. A human factor event-based learning assessment tool for assessment of errors and diagnostic accuracy in histopathology and cytopathology. J Clin Pathol. 2020;73:681–685. doi: 10.1136/jclinpath-2020-206538. [DOI] [PubMed] [Google Scholar]
- 9.Ren WH, Zou SM, Zhang YM, Zhang L, Zhao LL, Lu N, Cao J. The role of cytology in endobronchial ultrasound-guided transbronchial needle aspiration: a study of 813 cases focusing on diagnostic yield, an analysis of misdiagnosed cases and diagnostic accordance rate of cytological subtyping. Diagn Cytopathol. 2021;49:119–126. doi: 10.1002/dc.24608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han S, Yang W, Li H. A study of the application of fiberoptic bronchoscopy combined with liquid‑based cytology test in the early diagnosis of lung cancer. Oncol Lett. 2018;16:5807–5812. doi: 10.3892/ol.2018.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng Z, Shi J, Zhu C, Gu J, Zhou C. Automated quantification of DNA aneuploidy by image cytometry as an adjunct for the cytologic diagnosis of malignant effusion. Anal Cell Pathol (Amst) 2013;36:107–115. doi: 10.3233/ACP-130085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo C, Zhang Q, Hu Y, Huang J, Wang S, Wang G. DNA image cytometry of bronchial washing as a diagnostic adjunct to radial endobronchial ultrasound-guided sampling of peripheral lung lesions: a single center prospective study. Clin Respir J. 2024;18:e13703. doi: 10.1111/crj.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M, Hao C, Ma Q, Song G, Ma S, Zhao D, Zhao L, Li X, Wei W. DNA image cytometry test for primary screening of esophageal cancer: a population-based multi-center study in high-risk areas in China. Chin J Cancer Res. 2016;28:404–412. doi: 10.21147/j.issn.1000-9604.2016.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Böcking A, Friedrich D, Schramm M, Palcic B, Erbeznik G. DNA karyometry for automated detection of cancer cells. Cancers (Basel) 2022;14:4210. doi: 10.3390/cancers14174210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garribba L, Santaguida S. The dynamic instability of the aneuploid genome. Front Cell Dev Biol. 2022;10:838928. doi: 10.3389/fcell.2022.838928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasudevan A, Schukken KM, Sausville EL, Girish V, Adebambo OA, Sheltzer JM. Aneuploidy as a promoter and suppressor of malignant growth. Nat Rev Cancer. 2021;21:89–103. doi: 10.1038/s41568-020-00321-1. [DOI] [PubMed] [Google Scholar]
- 17.Haroske G, Baak JP, Danielsen H, Giroud F, Gschwendtner A, Oberholzer M, Reith A, Spieler P, Böcking A. Fourth updated ESACP consensus report on diagnostic DNA image cytometry. Anal Cell Pathol. 2001;23:89–95. doi: 10.1155/2001/657642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vainshelbaum NM, Zayakin P, Kleina R, Giuliani A, Erenpreisa J. Meta-analysis of cancer triploidy: rearrangements of genome complements in male human tumors are characterized by XXY karyotypes. Genes (Basel) 2019;10:613. doi: 10.3390/genes10080613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulze S, Petersen I. Gender and ploidy in cancer survival. Cell Oncol (Dordr) 2011;34:199–208. doi: 10.1007/s13402-011-0013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotb WF, Petersen I. Morphology, DNA ploidy and HPV in lung cancer and head and neck cancer. Pathol Res Pract. 2012;208:1–8. doi: 10.1016/j.prp.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Danielsen HE, Hveem TS, Domingo E, Pradhan M, Kleppe A, Syvertsen RA, Kostolomov I, Nesheim JA, Askautrud HA, Nesbakken A, Lothe RA, Svindland A, Shepherd N, Novelli M, Johnstone E, Tomlinson I, Kerr R, Kerr DJ. Prognostic markers for colorectal cancer: estimating ploidy and stroma. Ann Oncol. 2018;29:616–623. doi: 10.1093/annonc/mdx794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Li Y, Fu Y, Liu T, Liu X, Zhang X, Fu J, Guan X, Chen T, Chen X, Sun Z. Quantitative prediction of oral cancer risk in patients with oral leukoplakia. Oncotarget. 2017;8:46057–46064. doi: 10.18632/oncotarget.17550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cang W, Li Q, Gu L, Hong Z, Hu Y, Di W, Qiu L. Clinical evaluation of DNA ploidy for the triage of HPV-positive Chinese women during cervical cancer screening. Cancer Prev Res (Phila) 2021;14:355–362. doi: 10.1158/1940-6207.CAPR-20-0229. [DOI] [PubMed] [Google Scholar]
- 24.Danielsen HE, Pradhan M, Novelli M. Revisiting tumour aneuploidy - the place of ploidy assessment in the molecular era. Nat Rev Clin Oncol. 2016;13:291–304. doi: 10.1038/nrclinonc.2015.208. [DOI] [PubMed] [Google Scholar]
- 25.Shi A, Min W, Xiang L, Xu W, Jiang T. Value of automatic DNA image cytometry for diagnosing lung cancer. Oncol Lett. 2018;16:915–923. doi: 10.3892/ol.2018.8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y, Yu Q, Guo C, Wang G. DNA image cytometric analysis of bronchial washings as an adjunct for the detection of lung cancer in a clinical setting. Cancer Med. 2022;11:1860–1868. doi: 10.1002/cam4.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo C, Hu Y, Yu Q, Zhang Q, Wang G. DNA aneuploidy combined with radial EBUS in the diagnosis of peripheral lung lesions. Diagn Cytopathol. 2022;50:565–571. doi: 10.1002/dc.25039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Wu L, Deng Y, Shen X, Liu W, Shi L. DNA aneuploidy with image cytometry for detecting dysplasia and carcinoma in oral potentially malignant disorders: a prospective diagnostic study. Cancer Med. 2020;9:6411–6420. doi: 10.1002/cam4.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Datta M, Laronde D, Palcic B, Guillaud M. The role of DNA image cytometry in screening oral potentially malignant lesions using brushings: a systematic review. Oral Oncol. 2019;96:51–59. doi: 10.1016/j.oraloncology.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Choma D, Daurès JP, Quantin X, Pujol JL. Aneuploidy and prognosis of non-small-cell lung cancer: a meta-analysis of published data. Br J Cancer. 2001;85:14–22. doi: 10.1054/bjoc.2001.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Zhu R, Fan L, Ge S, Wei W, Li X, Da L, Jia Z, Zhao Z, Ning J, Da J, Peng W, Gu K, Sun G. Prognostic value of DNA aneuploidy in gastric cancer: a meta-analysis of 3449 cases. BMC Cancer. 2019;19:650. doi: 10.1186/s12885-019-5869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alaizari NA, Sperandio M, Odell EW, Peruzzo D, Al-Maweri SA. Meta-analysis of the predictive value of DNA aneuploidy in malignant transformation of oral potentially malignant disorders. J Oral Pathol Med. 2018;47:97–103. doi: 10.1111/jop.12603. [DOI] [PubMed] [Google Scholar]
- 33.Spurr LF, Weichselbaum RR, Pitroda SP. Tumor aneuploidy predicts survival following immunotherapy across multiple cancers. Nat Genet. 2022;54:1782–1785. doi: 10.1038/s41588-022-01235-4. [DOI] [PubMed] [Google Scholar]
- 34.Tang R, Ho YS, Chen HH, See LC, Wang JY. Different prognostic effect of postoperative chemoradiation therapy on diploid and nondiploid high-risk rectal cancers. Dis Colon Rectum. 1998;41:1494–1499. doi: 10.1007/BF02237295. [DOI] [PubMed] [Google Scholar]
- 35.Chen HS, Sheen-Chen SM, Lu CC. DNA index and S-phase fraction in curative resection of colorectal adenocarcinoma: analysis of prognosis and current trends. World J Surg. 2002;26:626–630. doi: 10.1007/s00268-001-0280-4. [DOI] [PubMed] [Google Scholar]