Abstract
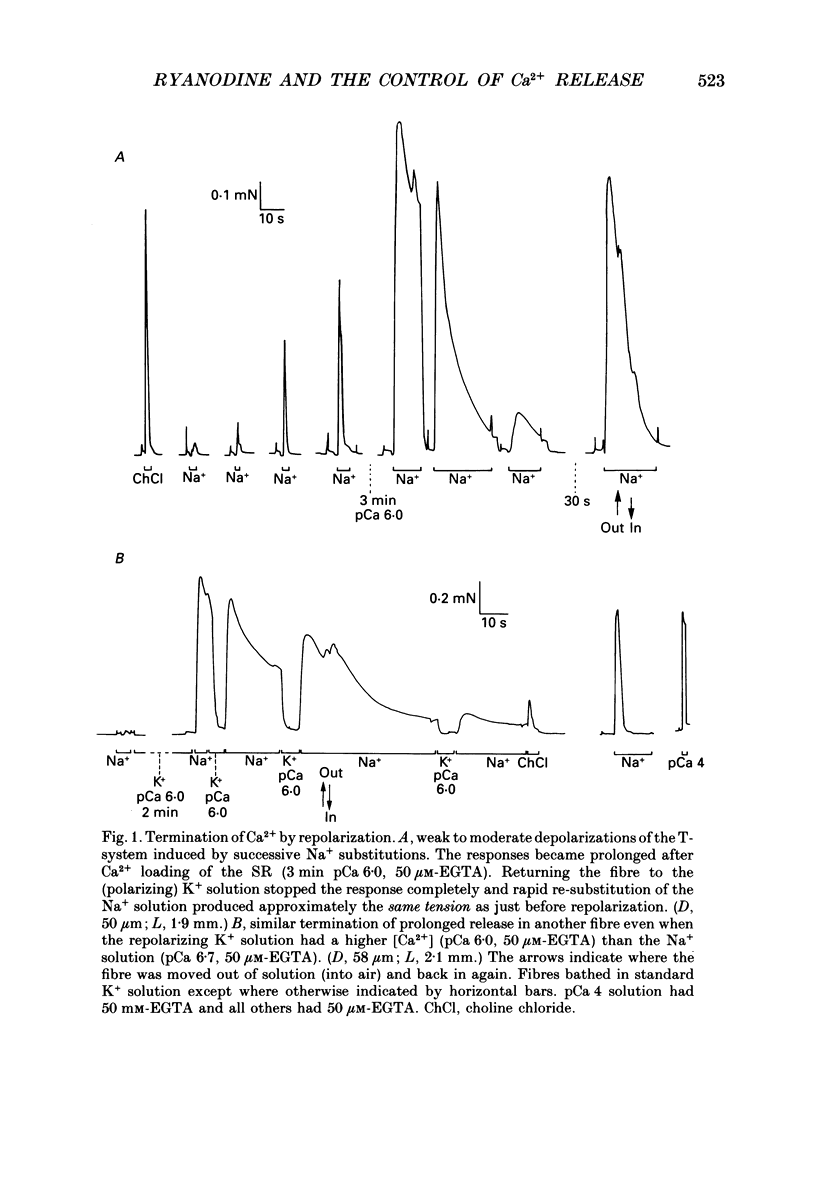
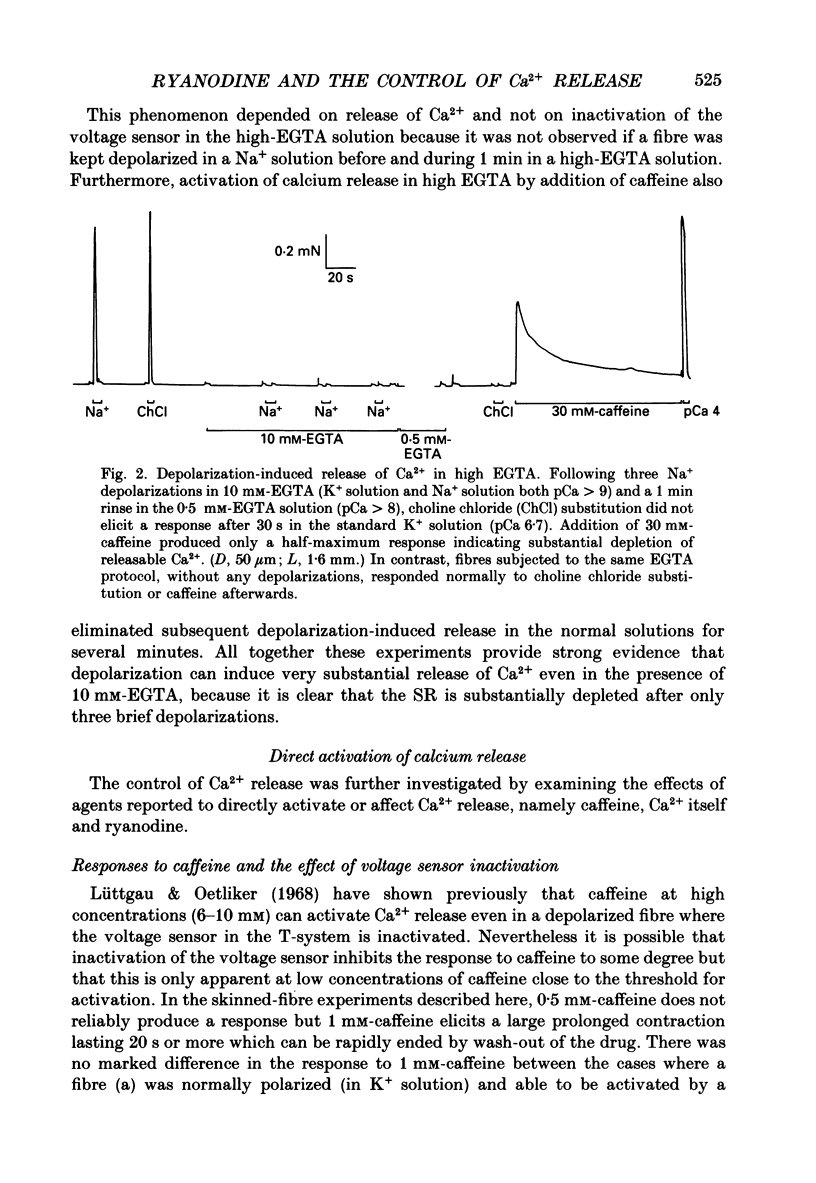
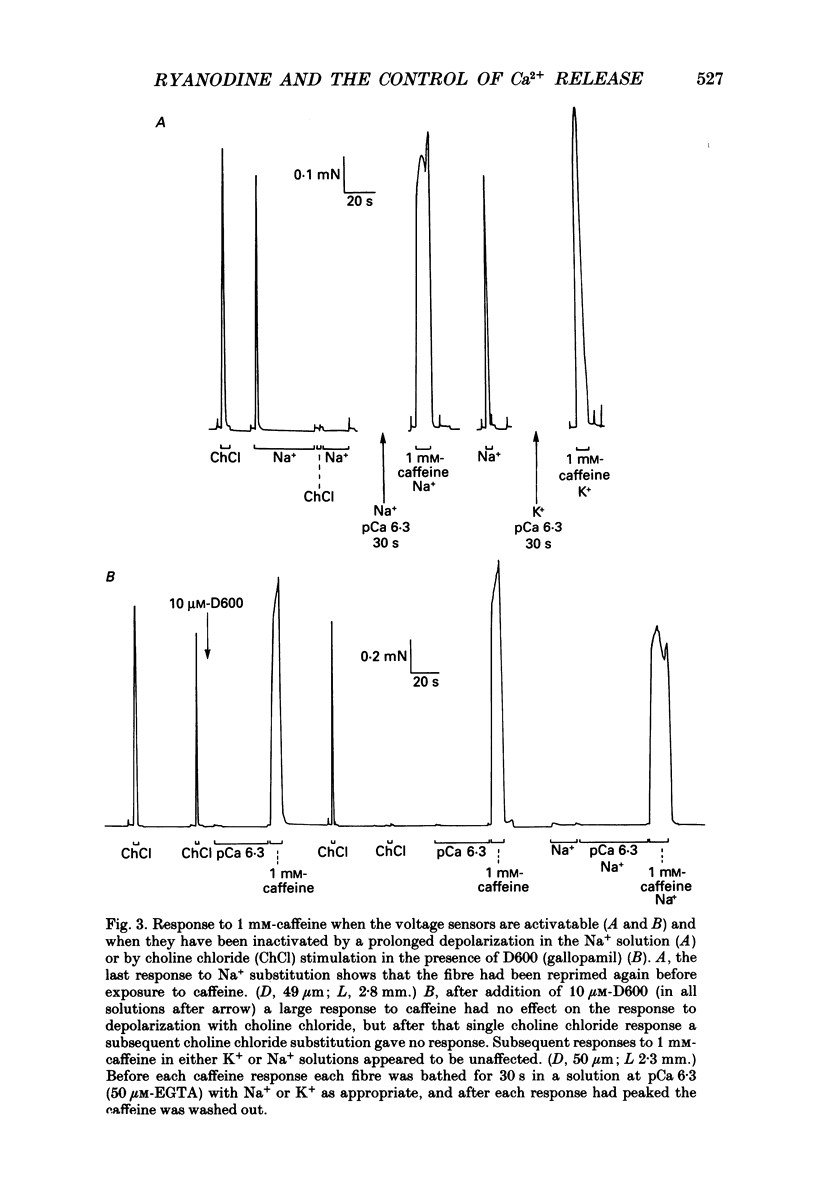
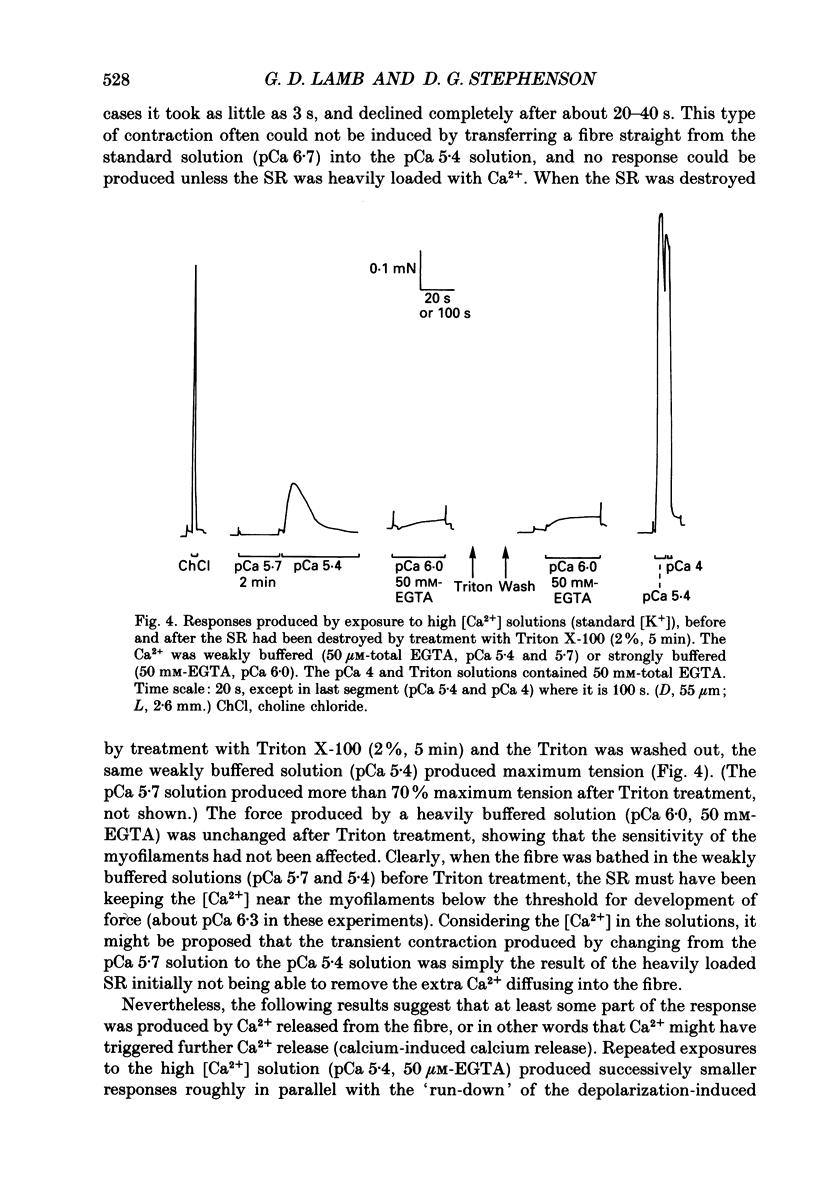
1. Skinned muscle fibres from the toad were used to investigate the roles of T-system membrane potential and Ca2+ in controlling the calcium release channels of the sarcoplasmic reticulum (SR). 2. Replacement of K+ in the bathing solution with Na+ produced a large contraction which could last for 30 s or more under certain circumstances. This prolonged contraction could be quickly and completely terminated by repolarizing the fibre in the K+ solution and then immediately re-initiated by returning to the Na+ solution. These data indicate that the membrane potential tightly controlled the substantial and prolonged release of calcium. 3. T-system depolarization in the presence of 10 mM-free EGTA (pCa greater than 9) markedly depleted the SR of Ca2+. This implies that depolarization of the T-system can still trigger substantial release of Ca2+ from the SR even when the myoplasmic [Ca2+] is very low and very heavily buffered by EGTA. 4. When the SR was heavily loaded with Ca2+, substitution of a weakly buffered high [Ca2+] solution (pCa 5.4, 50 microM-EGTA) could produce a small to moderate, transient contraction taking between 3 and 12 s to reach a peak and lasting 30 s or more. 5. This contraction may be produced at least partly by 'calcium-induced calcium release' as ruthenium red (2 microM) completely blocked the responses. Moreover, repeated substitutions produced successively smaller responses in parallel with the 'run-down' of the depolarization-induced contractions. 6. Depolarization could always produce an additional large and fast response at any stage during a 'Ca2(+)-induced' response. 7. In the presence of 25 microM-ryanodine, the rapid contraction produced by T-system depolarization was prolonged and could not be stopped by repolarization. During and after this contraction no depolarizing stimulus could induce a further contraction, even though in some fibres addition of 30 mM-caffeine produced a maximum response which indicated that there was still a substantial amount of calcium in the SR. 8. At pCa 6.4, 25 microM-ryanodine could itself induce a substantial slow contracture in a normally polarized fibre within 30-60 s, after which little or no response could be induced by T-system depolarization. At higher concentrations (25 microM) ryanodine produced a near-maximum contraction in only a few seconds.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






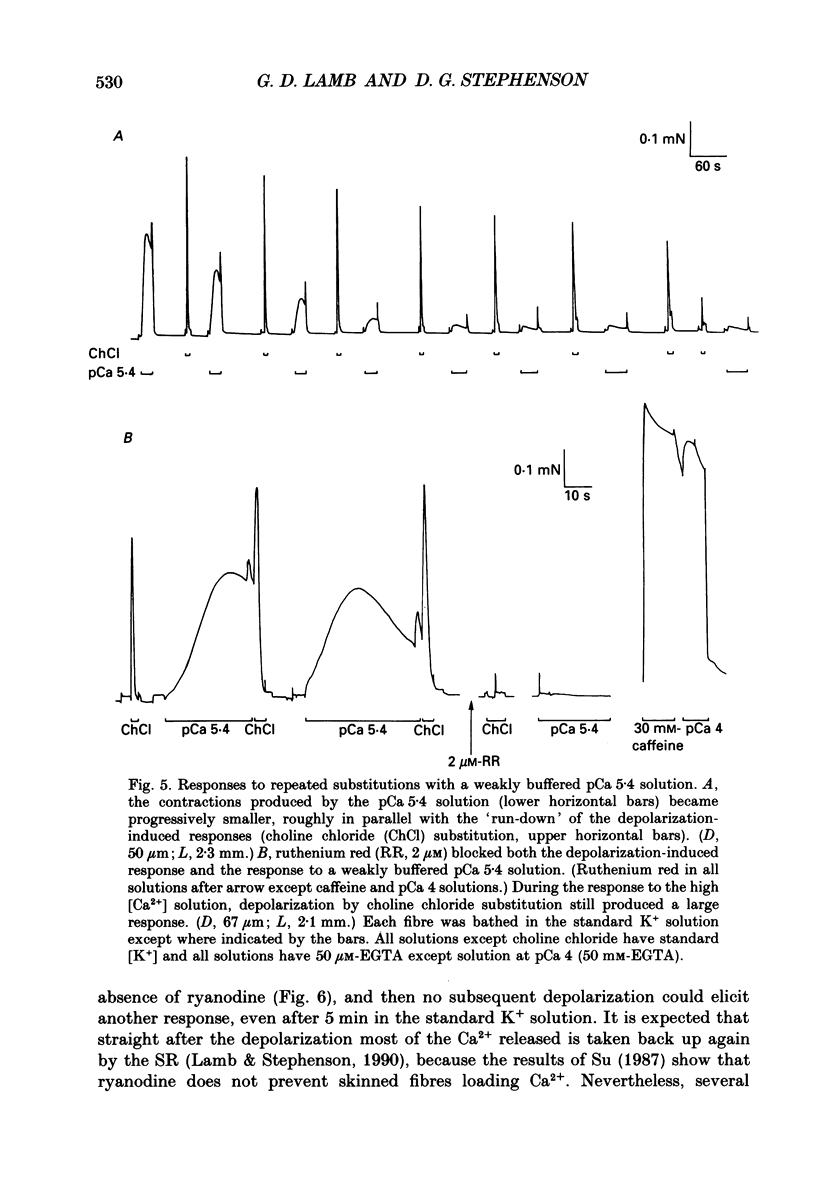



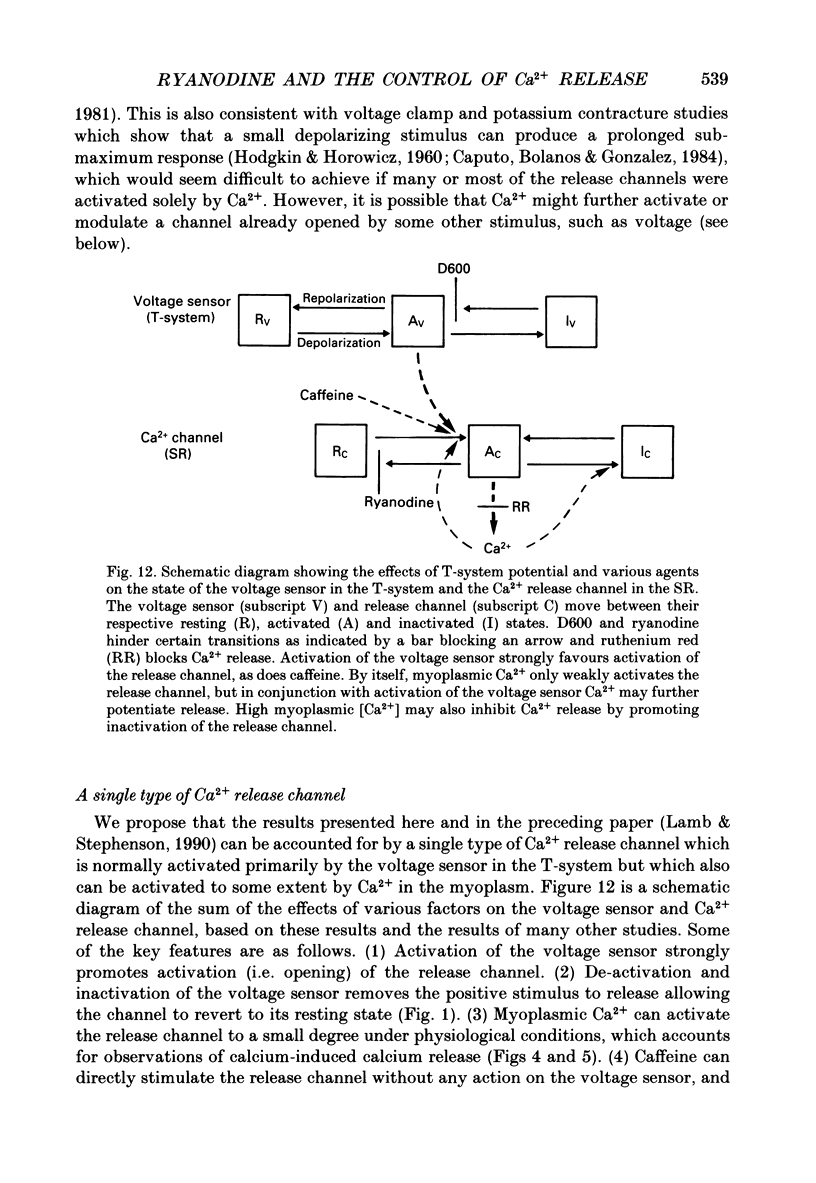



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
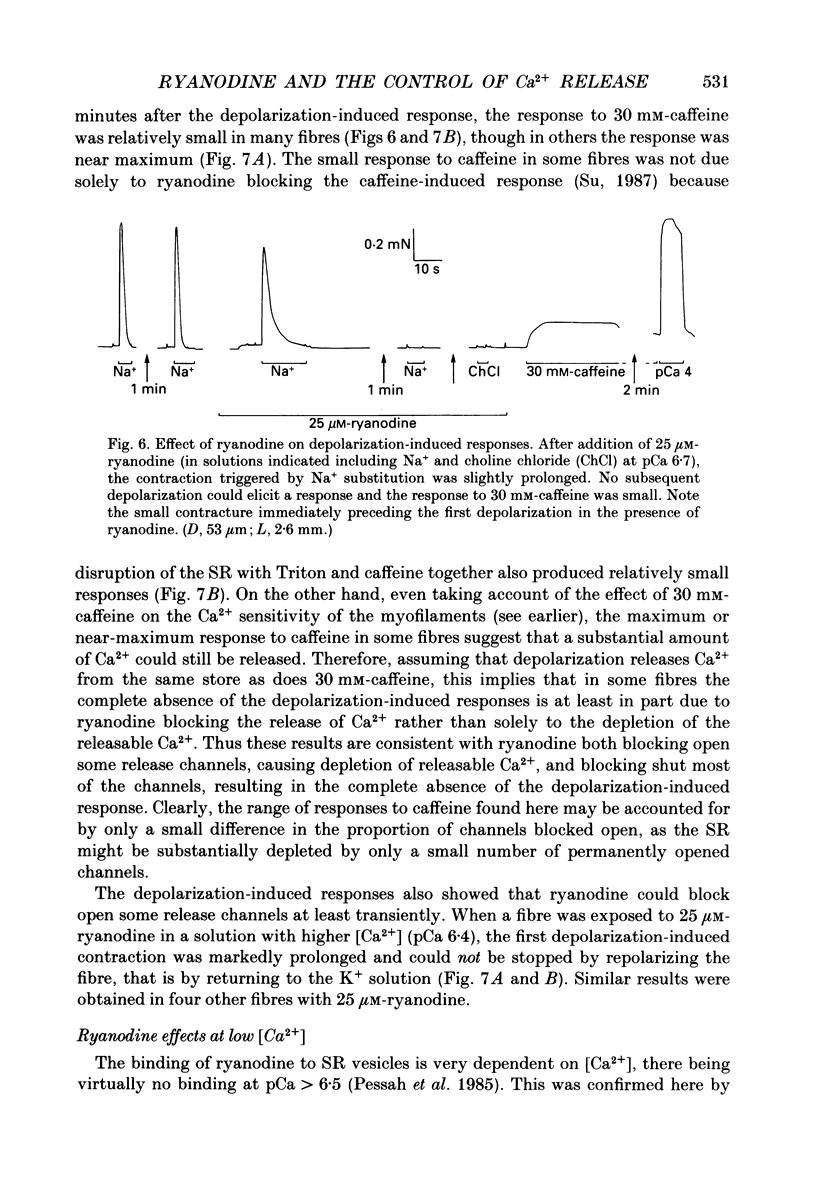
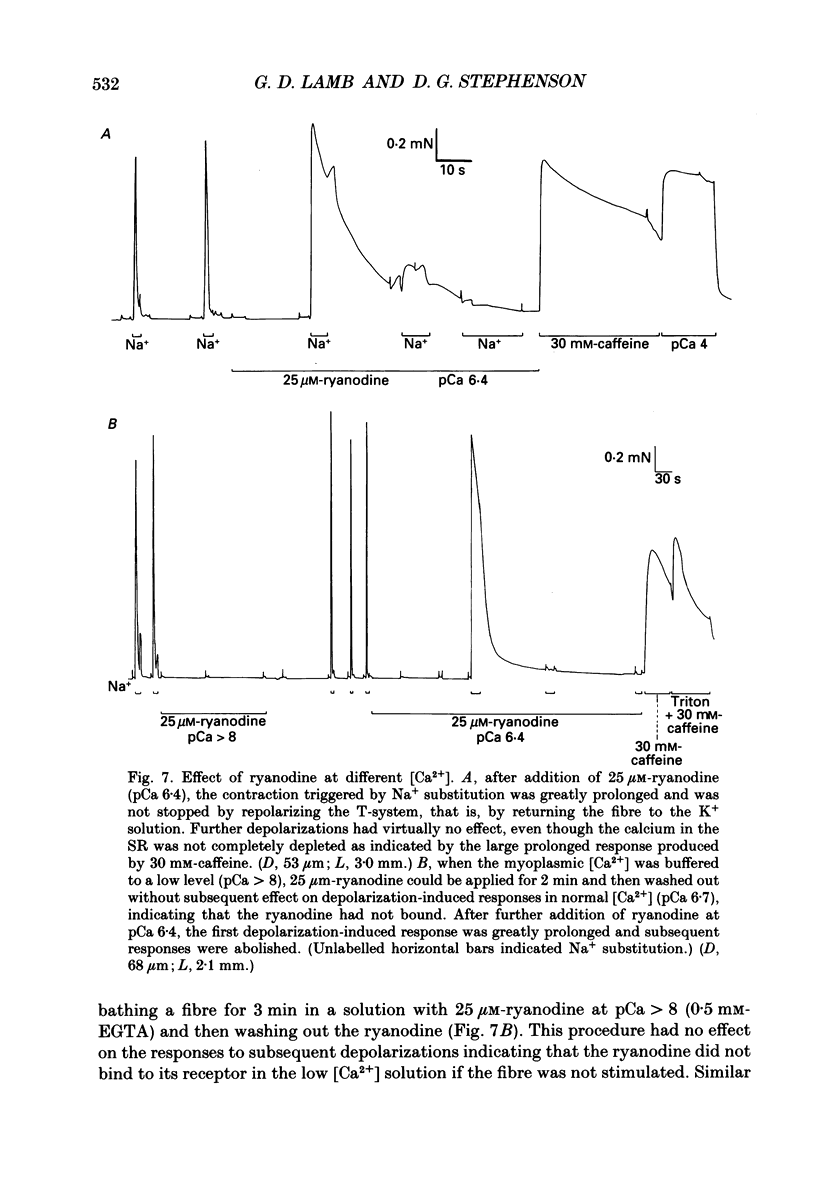
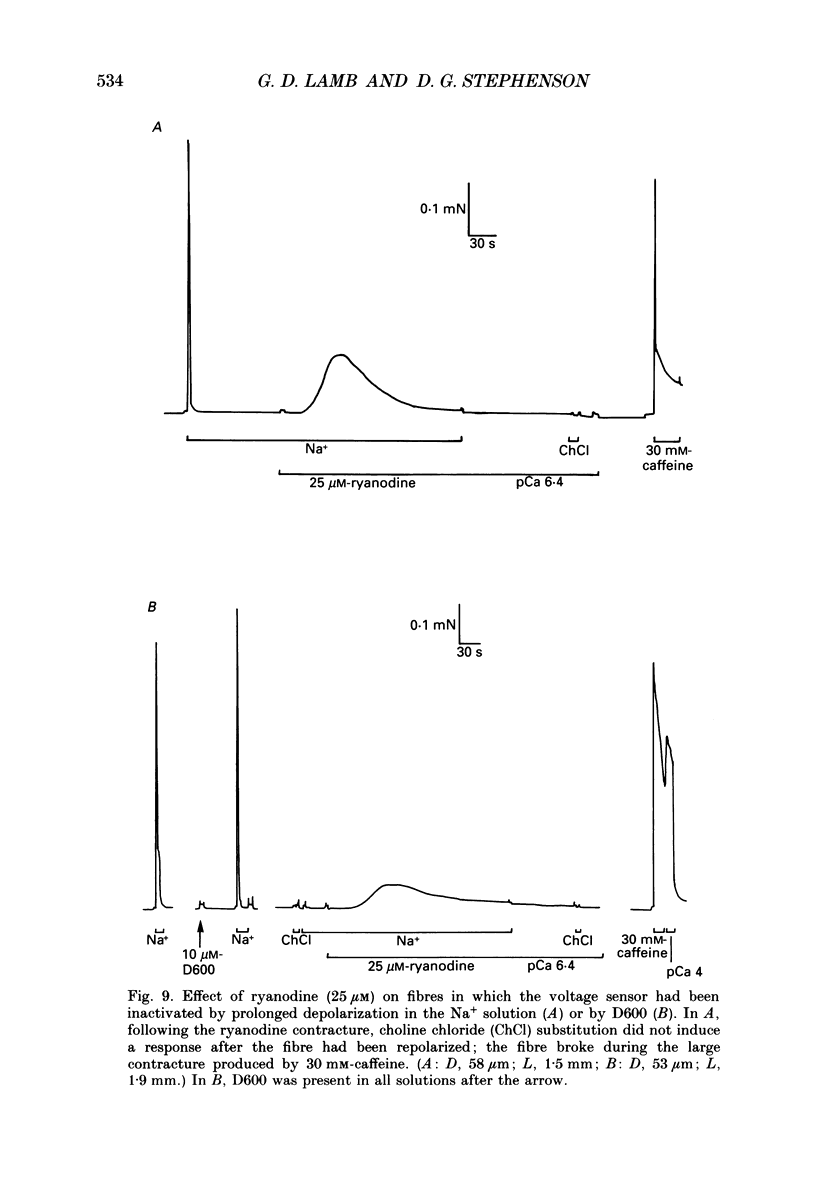
- Caputo C., Bolaños P., Gonzalez G. F. Effect of membrane polarization on contractile threshold and time course of prolonged contractile responses in skeletal muscle fibers. J Gen Physiol. 1984 Dec;84(6):927–943. doi: 10.1085/jgp.84.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
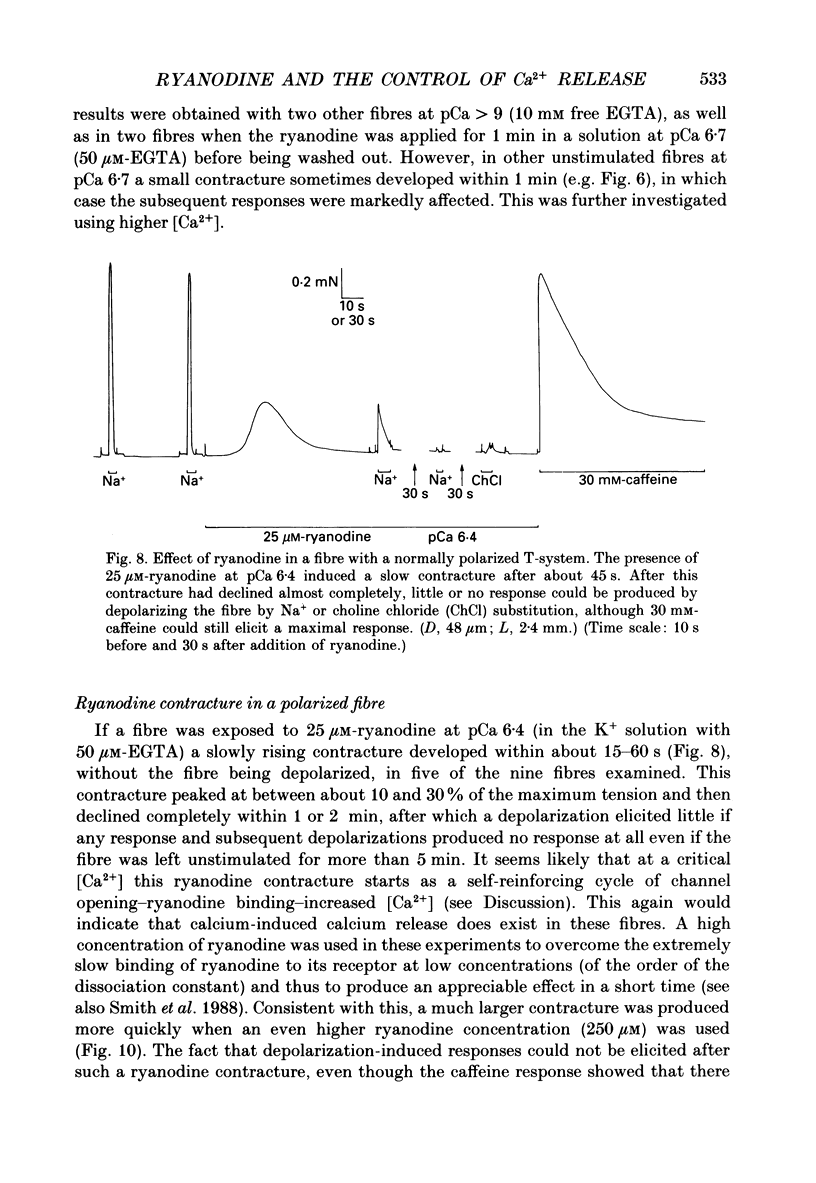
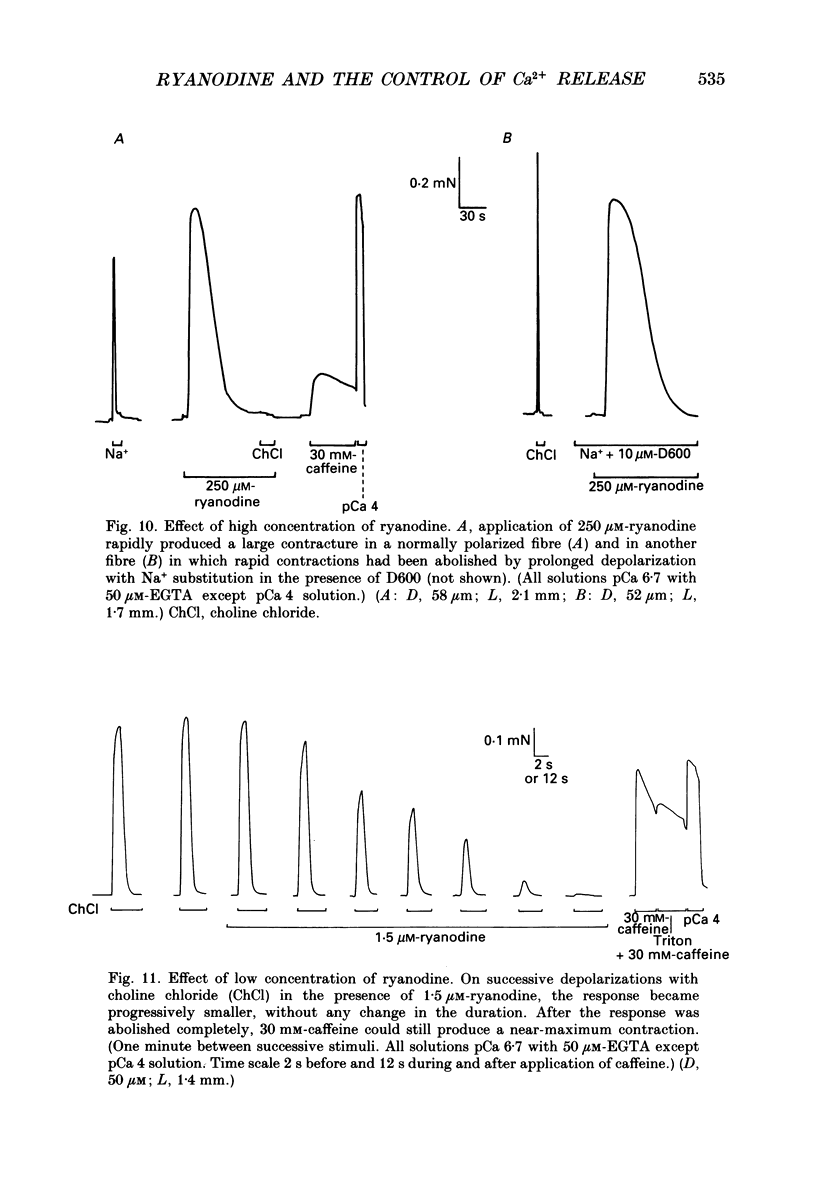
- Caputo C., Fernandez de Bolaños P. Membrane potential, contractile activation and relaxation rates in voltage clamped short muscle fibres of the frog. J Physiol. 1979 Apr;289:175–189. doi: 10.1113/jphysiol.1979.sp012731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. Effects of glycerol treatment and maintained depolarization on charge movement in skeletal muscle. J Physiol. 1976 Jan;254(2):285–316. doi: 10.1113/jphysiol.1976.sp011233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., McCarthy R. T., Milton R. L. Paralysis of frog skeletal muscle fibres by the calcium antagonist D-600. J Physiol. 1983 Aug;341:495–505. doi: 10.1113/jphysiol.1983.sp014819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M. D., Best P. M. Block of contracture in skinned frog skeletal muscle fibers by calcium antagonists. J Gen Physiol. 1989 Mar;93(3):429–449. doi: 10.1085/jgp.93.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink R. H., Stephenson D. G., Williams D. A. Potassium and ionic strength effects on the isometric force of skinned twitch muscle fibres of the rat and toad. J Physiol. 1986 Jan;370:317–337. doi: 10.1113/jphysiol.1986.sp015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G. B. The effects of reducing the extracellular calcium concentration on the twitch in isolated frog's skeletal muscle fibres. Jpn J Physiol. 1982;32(4):589–608. doi: 10.2170/jjphysiol.32.589. [DOI] [PubMed] [Google Scholar]
- Fryer M. W., Lamb G. D., Neering I. R. The action of ryanodine on rat fast and slow intact skeletal muscles. J Physiol. 1989 Jul;414:399–413. doi: 10.1113/jphysiol.1989.sp017695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa T., Smith J. S., Coronado R., Campbell K. P. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem. 1987 Dec 5;262(34):16636–16643. [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Calcium release in skinned muscle fibres of the toad by transverse tubule depolarization or by direct stimulation. J Physiol. 1990 Apr;423:495–517. doi: 10.1113/jphysiol.1990.sp018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Schlatterer R. G., Nicar M., Campbell K. P., Sutko J. L. The effects of ryanodine on passive calcium fluxes across sarcoplasmic reticulum membranes. J Biol Chem. 1987 Feb 25;262(6):2711–2718. [PubMed] [Google Scholar]
- Lea T. J., Ashley C. C. Ca-induced Ca release from the sarcoplasmic reticulum of isolated myofibrillar bundles of barnacle muscle fibres. Pflugers Arch. 1989 Feb;413(4):401–406. doi: 10.1007/BF00584490. [DOI] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew S. G., Wolleben C., Siegl P., Inui M., Fleischer S. Positive cooperativity of ryanodine binding to the calcium release channel of sarcoplasmic reticulum from heart and skeletal muscle. Biochemistry. 1989 Feb 21;28(4):1686–1691. doi: 10.1021/bi00430a039. [DOI] [PubMed] [Google Scholar]
- Meissner G., Darling E., Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986 Jan 14;25(1):236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Mészáros L. G., Ikemoto N. Ruthenium red and caffeine affect the Ca2+-ATPase of the sarcoplasmic reticulum. Biochem Biophys Res Commun. 1985 Mar 29;127(3):836–842. doi: 10.1016/s0006-291x(85)80019-x. [DOI] [PubMed] [Google Scholar]
- Ohta T., Endo M., Nakano T., Morohoshi Y., Wanikawa K., Ohga A. Ca-induced Ca release in malignant hyperthermia-susceptible pig skeletal muscle. Am J Physiol. 1989 Feb;256(2 Pt 1):C358–C367. doi: 10.1152/ajpcell.1989.256.2.C358. [DOI] [PubMed] [Google Scholar]
- Pessah I. N., Waterhouse A. L., Casida J. E. The calcium-ryanodine receptor complex of skeletal and cardiac muscle. Biochem Biophys Res Commun. 1985 Apr 16;128(1):449–456. doi: 10.1016/0006-291x(85)91699-7. [DOI] [PubMed] [Google Scholar]
- Siebler M., Schmidt H. D600 prolongs inactivation of the contractile system in frog twitch fibres. Pflugers Arch. 1987 Sep;410(1-2):75–82. doi: 10.1007/BF00581899. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+. J Gen Physiol. 1986 Nov;88(5):573–588. doi: 10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson E. W. Activation of fast skeletal muscle: contributions of studies on skinned fibers. Am J Physiol. 1981 Jan;240(1):C1–19. doi: 10.1152/ajpcell.1981.240.1.C1. [DOI] [PubMed] [Google Scholar]
- Stephenson E. W. Excitation of skinned muscle fibers by imposed ion gradients. I. Stimulation of 45Ca efflux at constant [K][Cl] product. J Gen Physiol. 1985 Dec;86(6):813–832. doi: 10.1085/jgp.86.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J. Y. Effects of ryanodine on skinned skeletal muscle fibers of the rabbit. Pflugers Arch. 1987 Nov;410(4-5):510–516. doi: 10.1007/BF00586534. [DOI] [PubMed] [Google Scholar]
- Vergara J., Tsien R. Y., Delay M. Inositol 1,4,5-trisphosphate: a possible chemical link in excitation-contraction coupling in muscle. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6352–6356. doi: 10.1073/pnas.82.18.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]



