Abstract
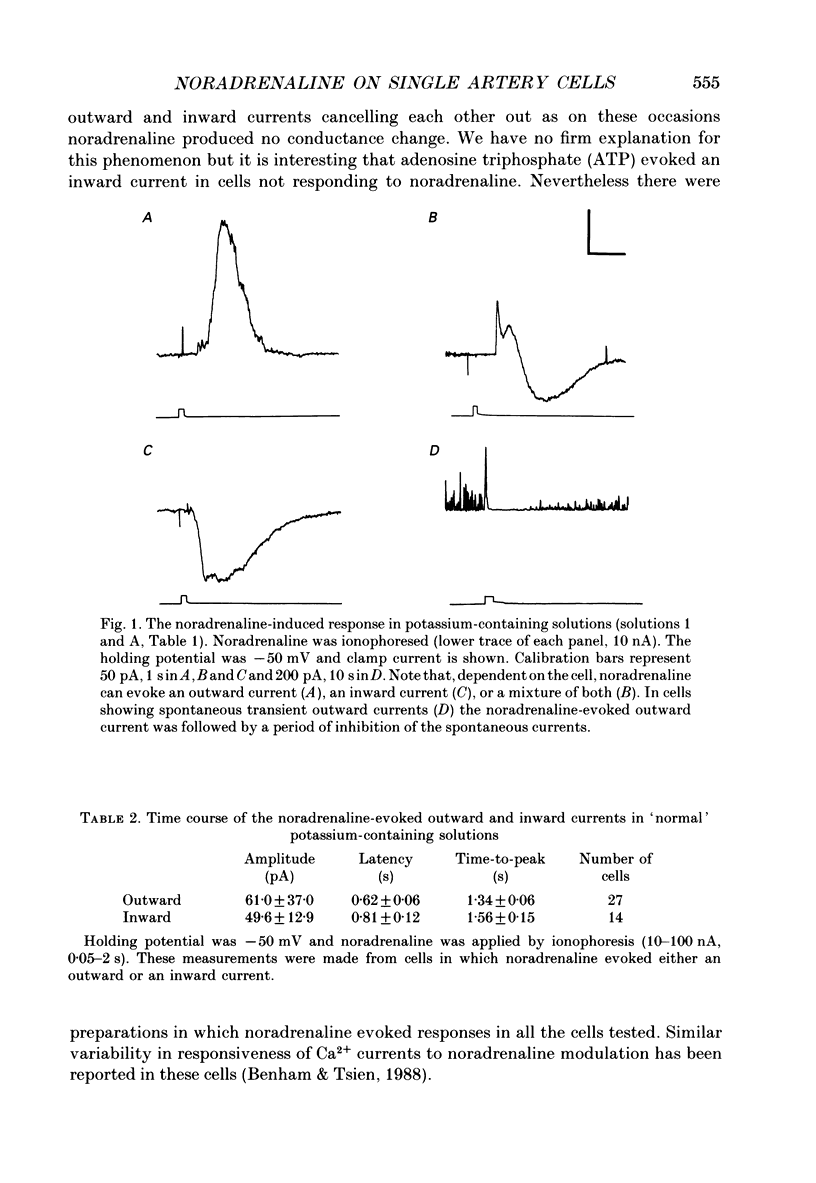
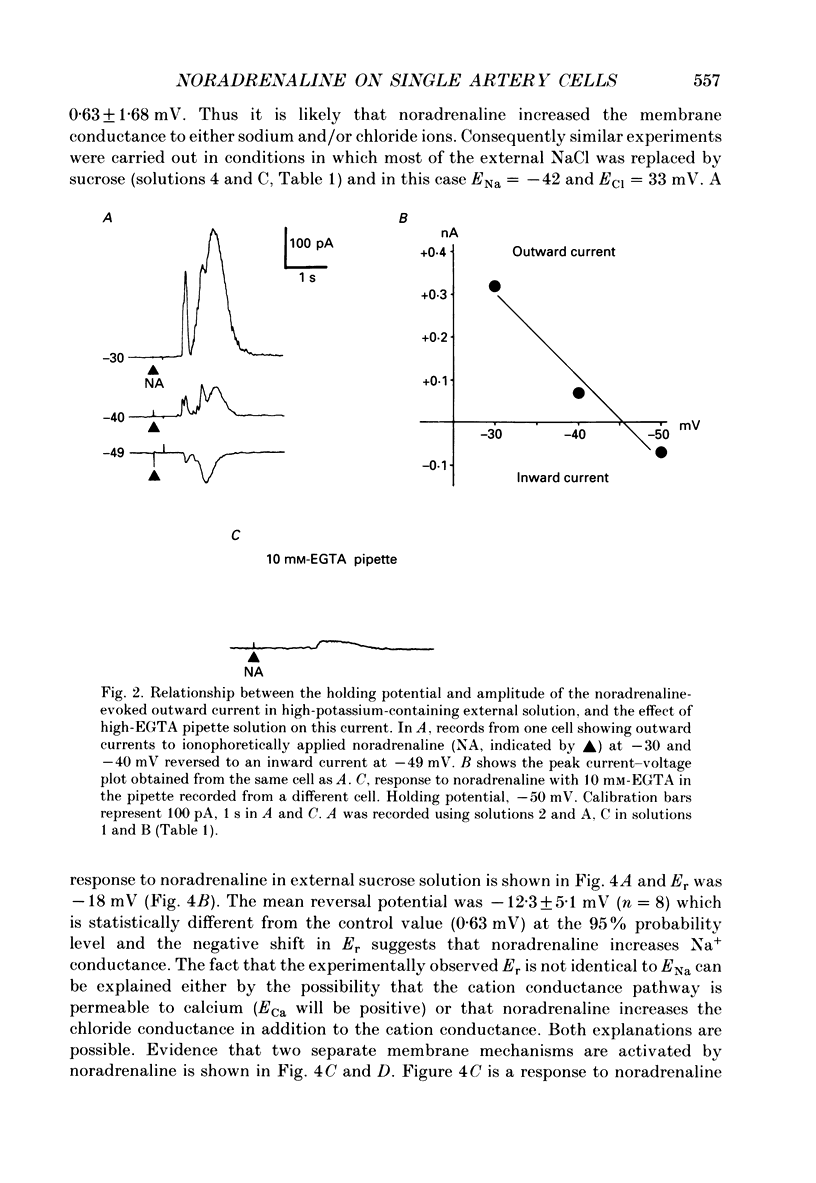
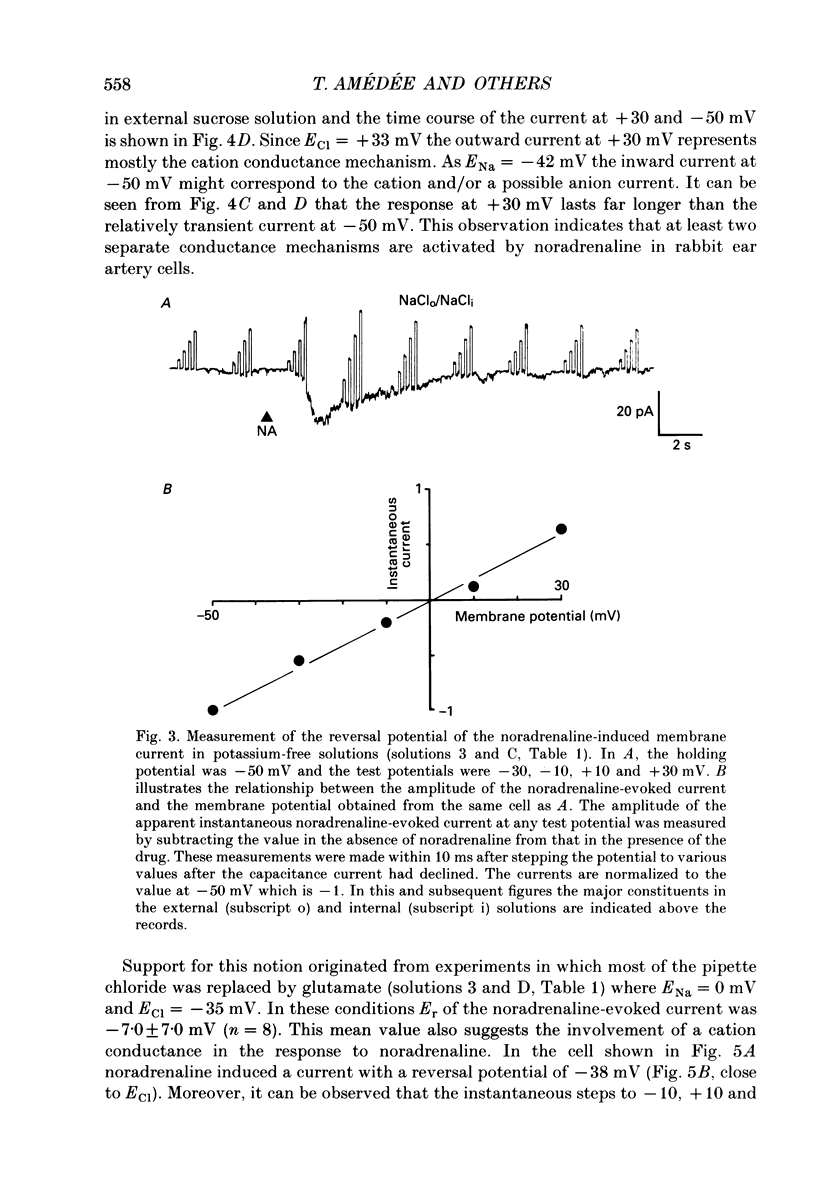
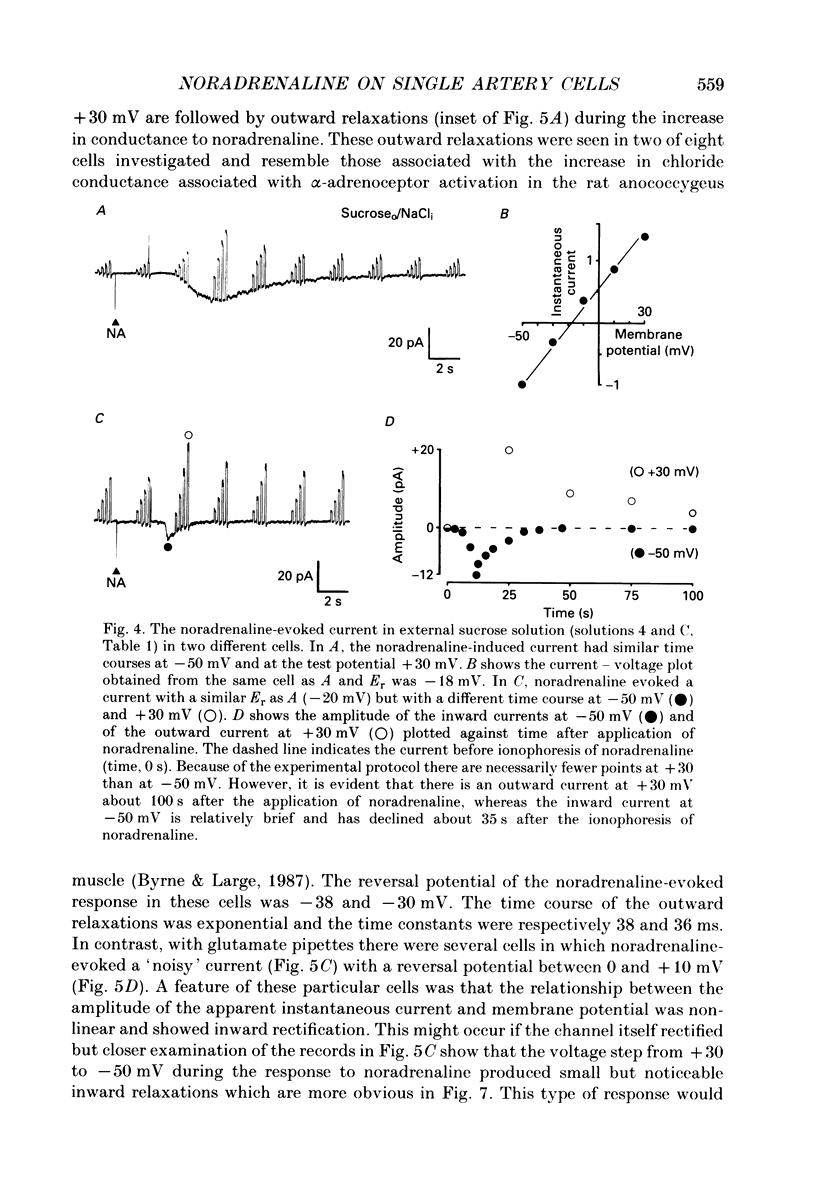
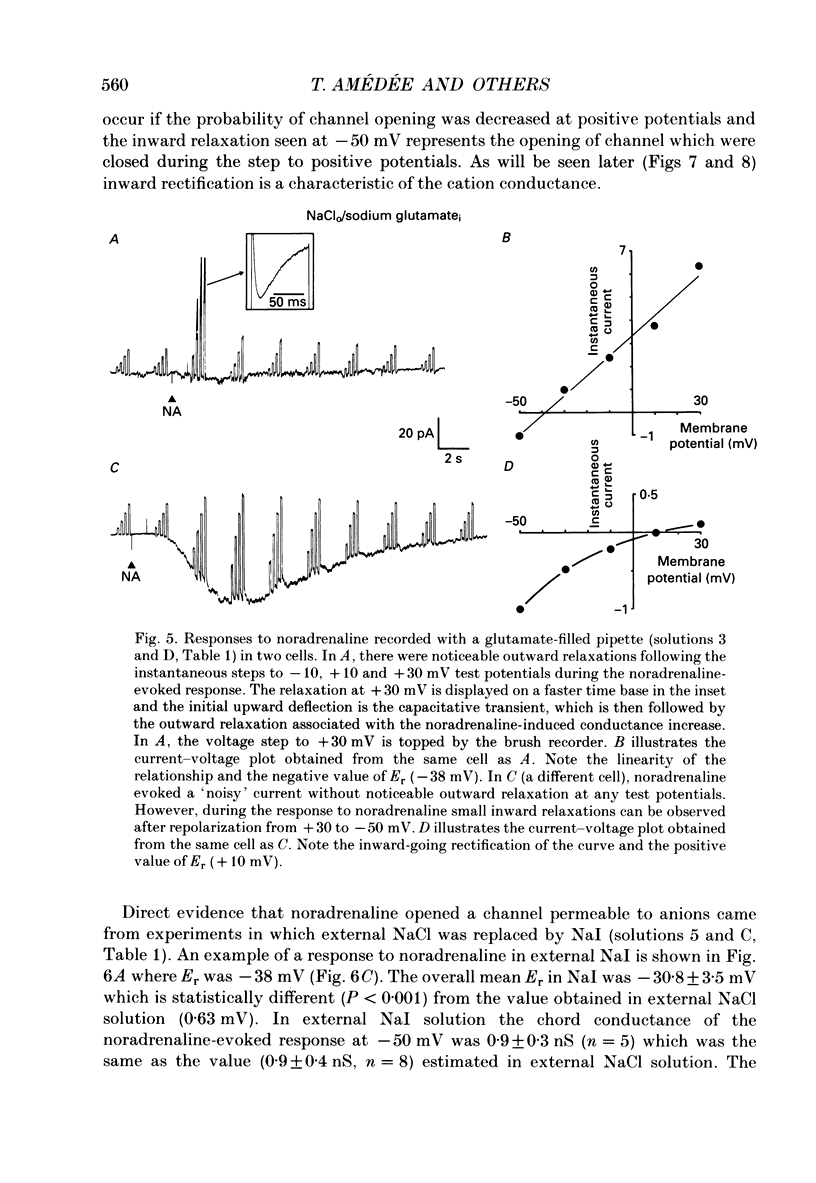
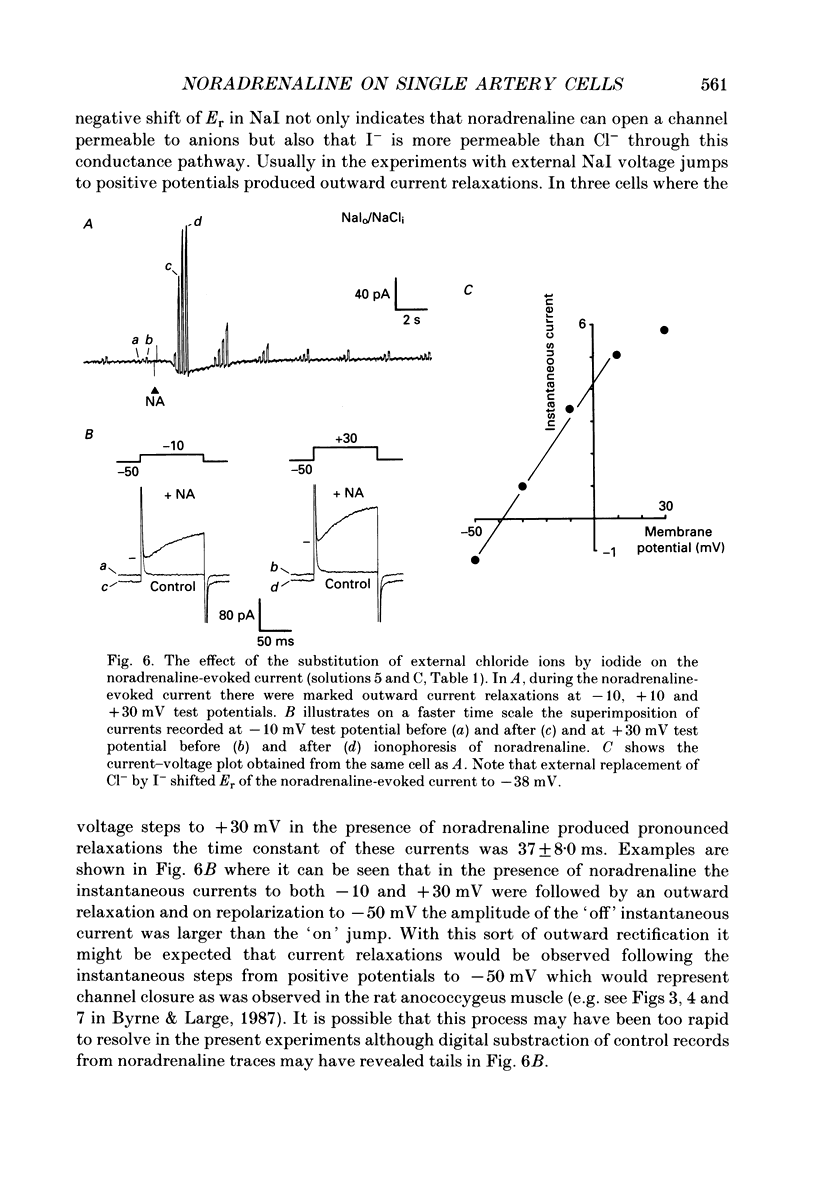
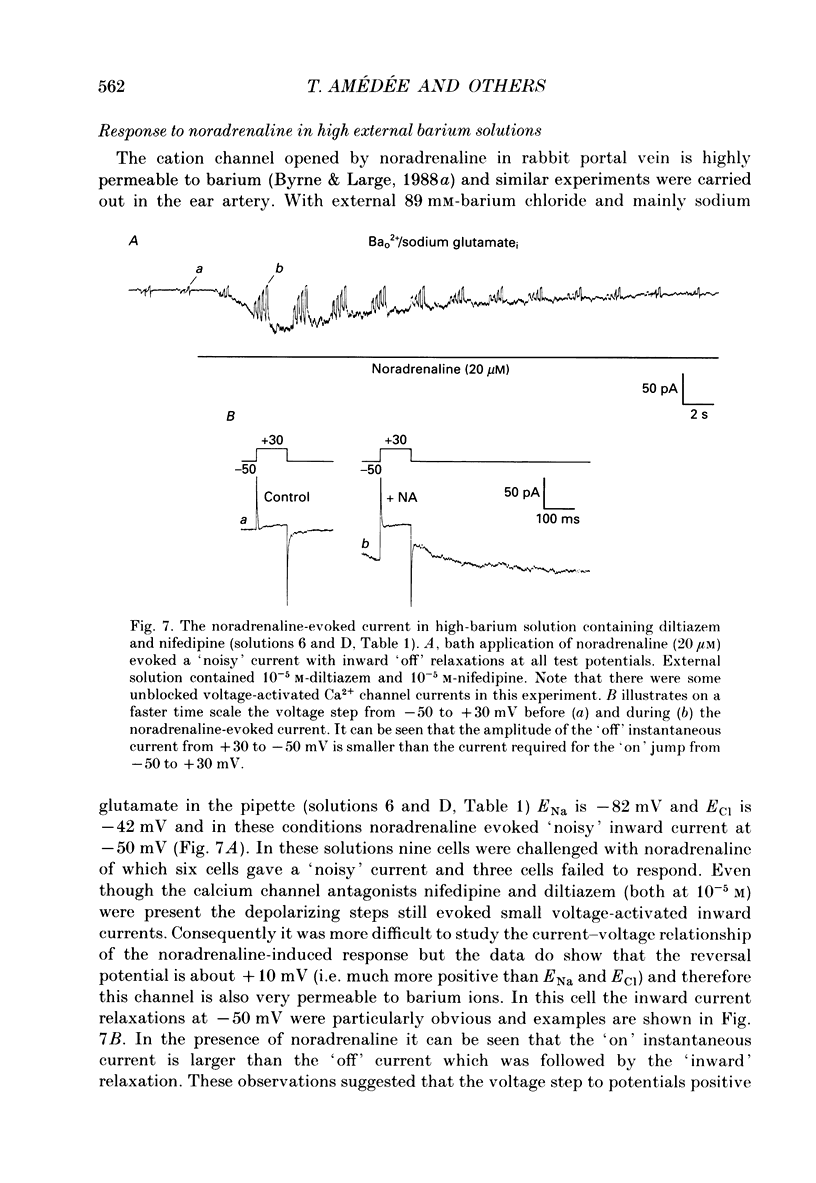
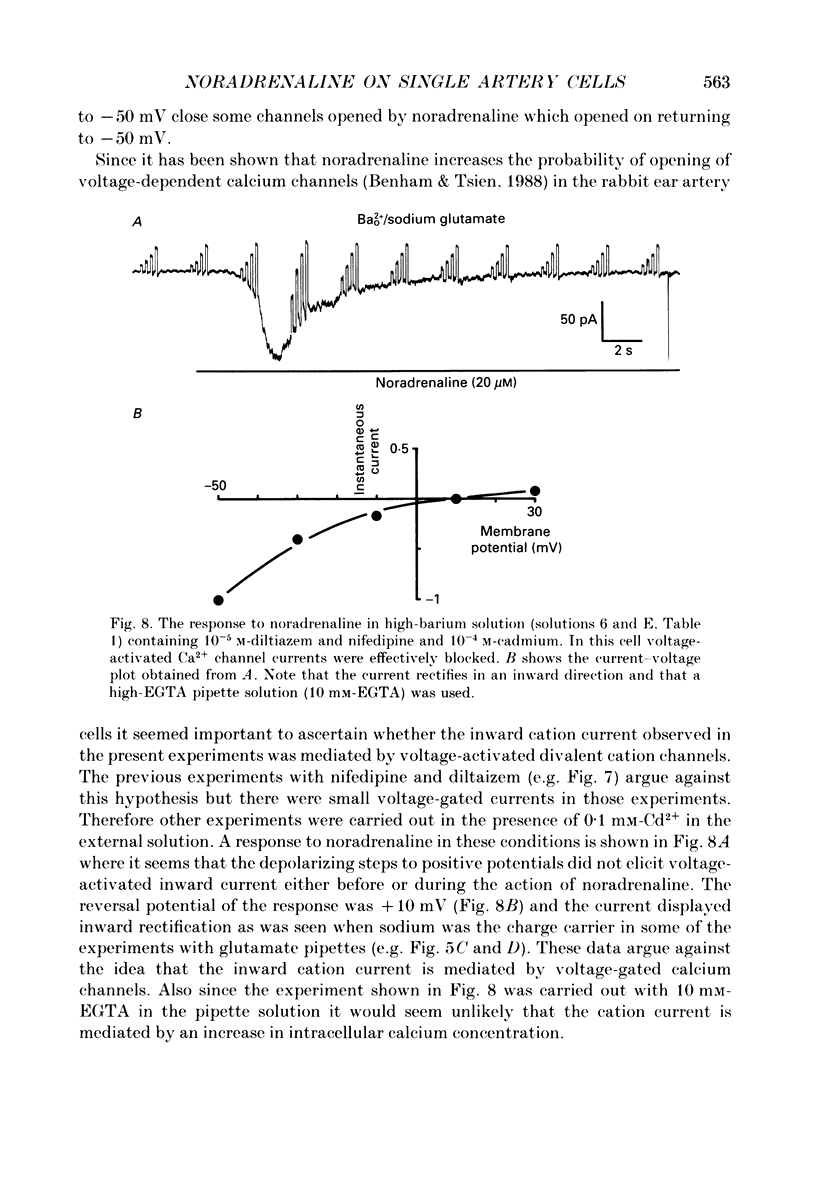
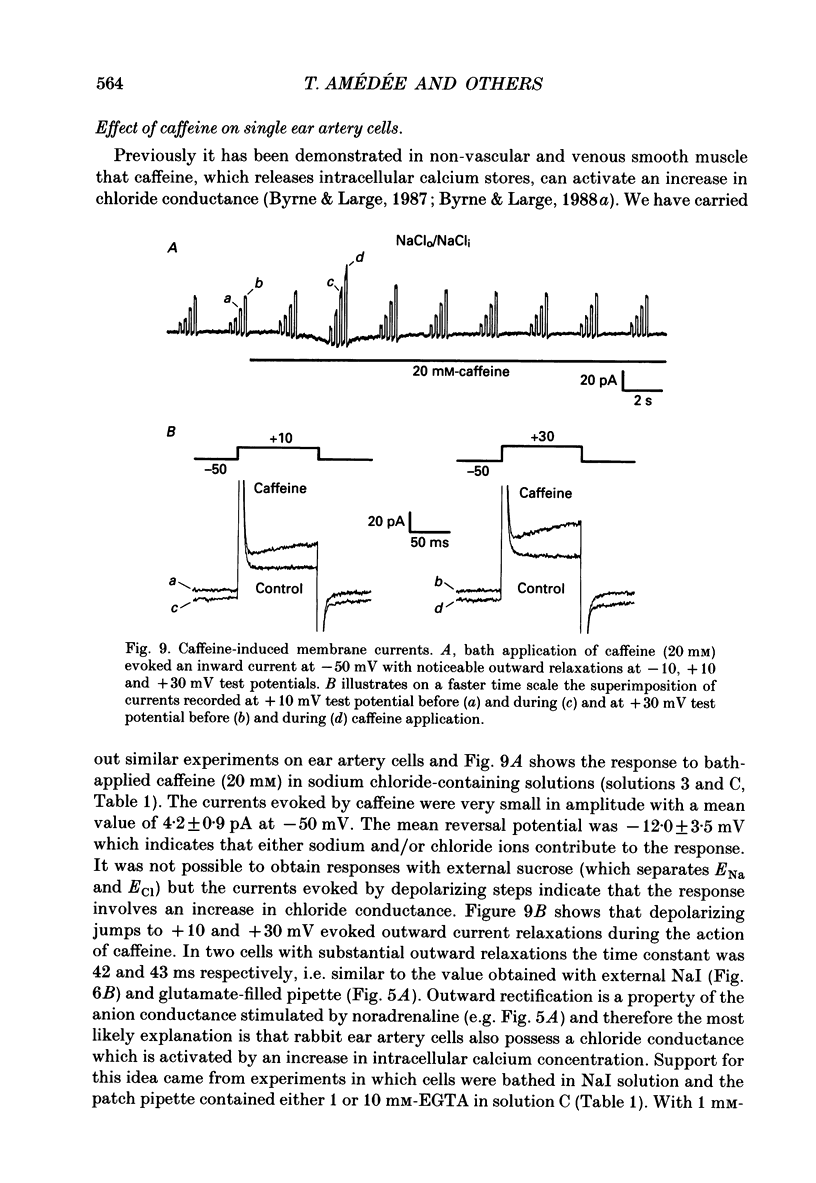
1. The action of noradrenaline on cells isolated from the rabbit ear artery was studied with the whole-cell configuration of the patch clamp technique. In normal potassium-containing solutions at a holding potential of -50 mV noradrenaline elicited either outward, inward or mixed outward and inward currents. These responses were blocked by the alpha-adrenoceptor antagonist, phentolamine (10(-6) M). 2. The outward current occurred as a consequence of an increase in membrane conductance and the reversal potential was close to the potassium equilibrium potential (EK). It was possible to record outward currents without external calcium but not when the concentration of EGTA in the pipette was increased to 10 mM or when potassium was absent from the pipette solution. It is concluded that the outward current evoked by alpha-adrenoceptor stimulation is produced by a calcium mediated increase in potassium conductance. 3. The ionic basis of the inward current was investigated in potassium-free external and pipette solutions. When sodium chloride was the major constituent of the external and pipette solutions the reversal potential (Er) of the noradrenaline-induced current was 0.63 mV, close to ENa and ECl. 4. When most of the external sodium chloride was replaced by sucrose Er was intermediate between ENa and ECl but had shifted significantly towards ENa. Further ion substitution experiments suggest that noradrenaline increased the membrane conductance to both anions and cations. 5. When the current was carried predominantly by anions, depolarizing steps (from -50 mV) produced outward current relaxations with a time constant of about 40 ms. Bath-applied caffeine also produced a membrane current which rectified in the outward direction. 6. When the response to noradrenaline was mediated mainly by cations, the relationship between the membrane current and clamp potential was non-linear and the amplitude of the currents at potentials positive to -50 mV became disproportionately smaller. In addition on repolarization to -50 mV the instantaneous current was followed by an inward relaxation. 7. In high external barium solution noradrenaline evoked a membrane current with a reversal potential much more positive than ENa or ECl. This current was recorded in the presence of 10(-5) M-nifedipine and diltiazem and in 10(-4) M-cadmium which suggests that the voltage-dependent calcium channel is not implicated in the generation of the non-selective cation current to noradrenaline.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Action of externally applied adenosine triphosphate on single smooth muscle cells dispersed from rabbit ear artery. J Physiol. 1987 Jun;387:473–488. doi: 10.1113/jphysiol.1987.sp016585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. Noradrenaline modulation of calcium channels in single smooth muscle cells from rabbit ear artery. J Physiol. 1988 Oct;404:767–784. doi: 10.1113/jphysiol.1988.sp017318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Large W. A. Are junction potentials essential? Dual mechanism of smooth muscle cell activation by transmitter released from autonomic nerves. Q J Exp Physiol. 1986 Jan;71(1):1–28. doi: 10.1113/expphysiol.1986.sp002960. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Action of noradrenaline on single smooth muscle cells freshly dispersed from the rat anococcygeus muscle. J Physiol. 1987 Aug;389:513–525. doi: 10.1113/jphysiol.1987.sp016669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Comparison of the biphasic excitatory junction potential with membrane responses to adenosine triphosphate and noradrenaline in the rat anococcygeus muscle. Br J Pharmacol. 1984 Nov;83(3):751–758. doi: 10.1111/j.1476-5381.1984.tb16229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Mechanism of action of alpha-adrenoceptor activation in single cells freshly dissociated from the rabbit portal vein. Br J Pharmacol. 1988 Jun;94(2):475–482. doi: 10.1111/j.1476-5381.1988.tb11550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Membrane ionic mechanisms activated by noradrenaline in cells isolated from the rabbit portal vein. J Physiol. 1988 Oct;404:557–573. doi: 10.1113/jphysiol.1988.sp017306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Declerck I., Casteels R. Effect of adrenergic agonists on Ca2+-channel currents in single vascular smooth muscle cells. Pflugers Arch. 1987 Jun;409(1-2):7–12. doi: 10.1007/BF00584744. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Standen N. B., Brayden J. E., Worley J. F., 3rd Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988 Nov 24;336(6197):382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kou K. Electrical components contributing to the nerve-mediated contractions in the smooth muscles of the rabbit ear artery. Jpn J Physiol. 1983;33(5):743–756. doi: 10.2170/jjphysiol.33.743. [DOI] [PubMed] [Google Scholar]
- Trapani A., Matsuki N., Abel P. W., Hermsmeyer K. Norepinephrine produces tension through electromechanical coupling in rabbit ear artery. Eur J Pharmacol. 1981 Jun 10;72(1):87–91. doi: 10.1016/0014-2999(81)90301-0. [DOI] [PubMed] [Google Scholar]


