Abstract
Transgene expression in stem cells is a powerful means of regulating cellular properties and differentiation into various cell types. However, existing vectors for transgene expression in stem cells suffer from limitations such as the need for genomic integration, the transient nature of gene expression, and the inability to temporally regulate transgene expression, which hinder biomedical and clinical applications. Here we report a new class of RNA virus-based vectors for scalable and integration-free transgene expression in mouse embryonic stem cells (mESCs). The vector is equipped with a small molecule-regulated riboswitch and a drug selection marker that allow temporal regulation of transgene expression and stable maintenance of the vector in proliferating stem cells. We demonstrated the utility of the vector by maintaining the pluripotency of mESCs in a differentiation induction medium by expressing Nanog and inducing myogenic differentiation by triggering Myod1 expression, without altering the mESC genome.
Keywords: riboswitches, RNA virus, vesicular stomatitis virus, embryonic stem cells, aptamers, ribozymes
Graphical abstract

Kim and Yokobayashi developed an RNA viral vector that allows chemically regulated transgene expression in mESCs to control cell fate without chromosomal integration in a scalable manner.
Introduction
Exogenous gene expression in stem cells is a powerful means to regulate their differentiation into various cell types or to maintain their properties. Currently, there are two major approaches to express exogenous genes in mammalian stem cells: (1) use a viral vector (e.g., lentivirus), a transposon-based vector (e.g., piggyBac), or genome editing to stably integrate a gene expression cassette into the chromosome of the stem cells, and (2) transiently transfect plasmid or synthetic mRNA encoding differentiation factor into the stem cells.1,2,3 Chromosomal integration allows long-term and heritable expression of transgenes, and it also allows chemically inducible expression from an engineered transcription factor-regulated promoter such as the doxycycline-inducible Tet-ON system.4,5,6 It is also highly scalable because the transduced stem cells can be expanded indefinitely.7 However, permanent alteration of the chromosome severely limits clinical applications. Transient expression using non-integrating and non-replicative vectors such as plasmids and mRNAs is considered safer for clinical applications, but it is not highly scalable. It also suffers from limited transfection efficiency, high cell-to-cell variability in expression level, and a limited duration of gene expression.
The regulation of exogenous gene expression by RNA viral vectors would significantly expand their potential biomedical applications. Canonical chemical gene regulation systems based on engineered transcription factors such as the Tet-ON system cannot be adapted to RNA viral vectors that do not undergo nuclear double-stranded DNA intermediates. We recently developed a vesicular stomatitis virus (VSV) vector whose replication and transgene expression can be regulated by a small molecule (guanine).8,9 VSV is a negative-strand RNA virus that replicates in the cytoplasm without a DNA intermediate. By inserting a guanine-activated self-cleaving ribozyme (GuaM8HDV)10 in the 3′ UTR of VSV L (involved in viral RNA polymerization and processing) and transgene transcripts, transgene expression in baby hamster kidney (BHK-21) cells could be strongly suppressed in the presence of guanine added to the culture medium.8 The vector lacks the glycoprotein (G) coding gene; thus, it is fully replicable in the cytoplasm of the infected cells but incapable of producing new infectious viral particles, making it safer and easier to handle. However, there are several technical obstacles to the use of chemically regulated VSV vectors for stem cell applications. First, although the vector is replicable and can be passed onto daughter cells upon cell division, it cannot be maintained stably because uninfected cells replicate faster. Second, VSV—and most other replicable vectors—exhibit too much cytotoxicity in stem cells. This work aims to address these key issues by (1) integrating a drug selection marker into the VSV vector to allow stable expansion of vector-carrying stem cells and (2) selecting new VSV mutants from infected mouse embryonic stem cells (mESCs) that exhibit lower cytotoxicity. We demonstrate that mESCs infected with our vector can stably replicate for an extended period of time without inducing transgene expression under drug selection and in the presence of guanine, which suppresses transgene expression. Upon sufficient amplification of vector-infected ESCs, transgene expression can be induced in the entire cell population for efficient differentiation by removing guanine from the culture medium. Alternatively, we demonstrated stable overexpression of Nanog from our vector in mESCs, which maintained pluripotency in the absence of the key additives in the culture medium and subsequent removal of the vector by chemical treatment. Overall, we established a new class of transgene expression vectors for stem cells that are chemically regulated, drug selectable, replicative, and non-integrating.
Results
Stable and long-term maintenance of riboswitch-equipped VSV in mESCs
We recently identified two novel mutations in the VSV vector genome that support its stable replication in mESCs with reduced toxicity and riboswitch-regulated DsRedExpress2 (DsRedEx2) expression by guanine.9 Subsequently, we studied additional VSV mutants that showed strong DsRedEx2 expression in the infected mESCs and discovered a mutant with five mutations in addition to our previous VSV variant8 (Table S2 and Figure S7). This new VSV variant was used throughout this study. Similar to our previous vector,8 the new VSV vector contained the GuaM8HDV aptazyme (riboswitch) in the 3′ UTR of DsRedEx2 and VSV-L mRNAs (Figure 1A), which are negatively regulated by guanine. In addition, we inserted a puromycin selection marker (PuroR) downstream of DsRedEx2 to allow drug selection for VSV-infected cells (Figures 1A and 2A).
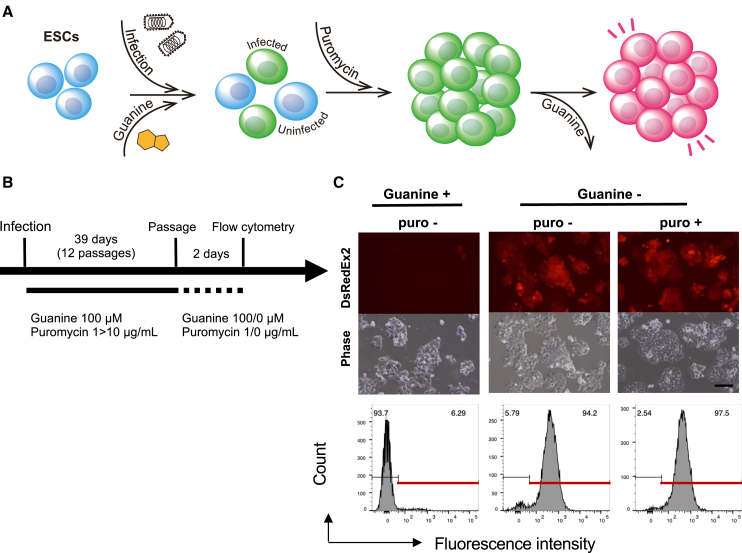
Figure 1.
Propagation of mESCs infected with DsRedEx2-VSV
(A) RNA genome structure of DsRedEx2-VSV and schematic illustration of the mechanism of guanine-regulated vector replication and transgene expression. Guanine activates self-cleavage of the GuaM8HDV ribozyme located in the 3′ UTR of VSV-L and DsRedEx2 mRNAs, resulting in downregulation of the corresponding proteins. Because VSV-L is responsible for both genomic RNA replication and mRNA production, all vector-encoded proteins (including PuroR) downregulated in the presence of guanine, but replication of the genomic RNA and the expression of PuroR are maintained at functional levels. (B) Schedule of VSV infection and maintenance of mESCs. The cells were infected at an MOI of 1 based on the titer determined using BHK21 cells. (C) Total accumulated cell numbers of infected mESCs under different puromycin concentrations. Accumulated cell numbers were calculated as follows: A(d) = A(d − 3) × C(d)/(2 × 104), where A is the accumulated cell number; C, the cell count; and d, day. (D) Fraction of DsRedEx2-positive cells at each passage under different puromycin concentrations. (E) Images of DsRedEx2-VSV-infected mESCs under different puromycin concentrations at passage 10. (F) Immunofluorescence staining of mESCs for OCT4, NANOG, and SSEA-1. Infected, DsRedEx2-VSV infected-mESCs at passage 11; PB-DsRedEx2, an mESC clone with a chromosomally integrated CAG-DsRedEx2-GuaM8HDV cassette generated by a piggyBac transposon system; Uninfected, Uninfected mESCs.
Scale bars, 100 μm.
Figure 2.
Transgene regulation in mESCs infected with DsRedEx2-VSV
(A) Schematic illustration of the scalable transgene induction strategy for mESCs using the riboswitch-controlled VSV vector. Initial small-scale infection of mESCs with the VSV vector and subsequent cell culture under drug selection and guanine addition allows mESC amplification without transgene induction. Removal of guanine after cell expansion allows large-scale transgene induction in the entire cell population. (B) Schedule of long-term mESC culture without transgene expression followed by transgene induction by guanine removal. The time course of cell proliferation in the presence of guanine is shown in Figure S1C. (C) DsRedEx2 expression of the infected mESCs imaged and measured at day 39 + 2. The red bars indicate DsRedEx2-positive cells. Results of a similar experiment with a different number of passages and another experiment using an alternative mESC strain (ES-R1) are shown in Figures S1A and S1B.
Scale bars, 100 μm.
mESCs were infected with the VSV vector carrying DsRedEx2 and PuroR (DsRedEx2-VSV, Figure 1A) and cultured for 33 days under various concentrations of puromycin while passaging every 3 days (Figure 1B). The cells were cultured in the absence of guanine, which allows unhindered vector replication and transgene expression. mESCs showed constant cell growth at moderately reduced replication rates with higher puromycin concentrations (Figure 1C). However, at least 5 μg/mL puromycin was necessary to maintain DsRedEx2 expression in the cell population in long-term cultures (Figures 1D and 1E). The appearance of the cell colonies after long-term culture shows that the infected cells display somewhat looser cell-cell contact compared with the uninfected mESC colonies, as we observed previously.9 Nevertheless, the cells infected with DsRedEx2-VSV cultured under 10 μg/mL puromycin for 33 days showed expression of pluripotency markers comparable with that of the uninfected cells (Figure 1F).
Next, we sought to determine whether our VSV vector could be maintained in the presence of guanine, which suppresses vector replication and transgene expression (Figure 2A). mESCs infected with DsRedEx2-VSV were cultured for 39 days with 100 μM guanine and 1–10 μg/mL puromycin (Figures 2B, 2C, and S1C). Although PuroR expression was expected to be suppressed as well, mESCs showed constant growth with minimal DsRedEx2 expression (Figures 2C, S1C, and S1D). After 39 days, the cells were split and cultured with and without guanine and puromycin for 2 days, and the cells were analyzed by flow cytometry (Figures 2C, S1A, and S1B). Almost all cells cultured without guanine showed robust DsRedEx2 expression, whereas those cultured with guanine remained nonfluorescent (Figures 2C, S1A, and S1B). Similar results were obtained with another mESC strain, ES-R1 (Figure S1B). These results demonstrate that our riboswitch-equipped VSV vector can be stably maintained in mESCs in long-term cultures, even with suppressed transgene expression under puromycin selection in the presence of guanine. This allows large-scale amplification of VSV-infected mESCs without transgene activation. Transgene expression can be induced after long-term culture by removing guanine from the medium.
mESCs infected with Nanog-VSV maintain pluripotency under differentiation induction condition
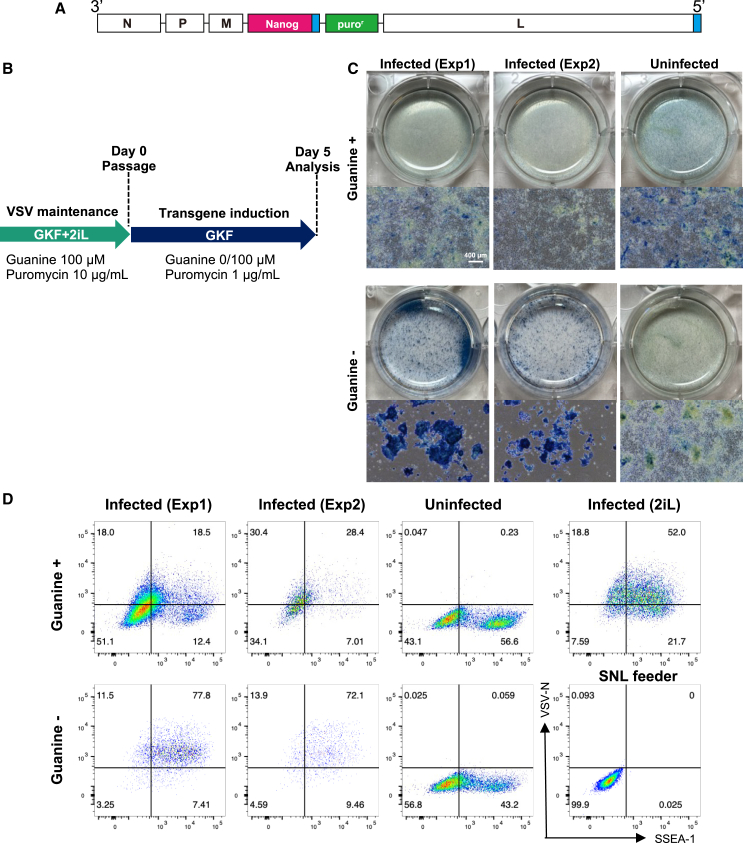
To demonstrate the functional manipulation of mESCs with our VSV vector, we inserted the Nanog gene in place of DsRedEx2 (Nanog-VSV) (Figure 3A). Nanog is a transcription factor required to maintain pluripotency in mESCs.11,12 Overexpression of Nanog has been shown to allow pluripotency maintenance even in the absence of leukemia inhibitory factor (LIF), which is necessary for regular mESC maintenance.11,12 mESCs infected with Nanog-VSV were cultured with guanine and puromycin for a few passages in the presence of LIF, an MEK inhibitor (PD0325901), and a GSK3β inhibitor (CHIR99021) (2iL) for maintenance of robust pluripotency.13 The cells were then passaged under -2iL and puromycin (1 μg/mL) condition with and without guanine to induce differentiation for 5 days (Figure 3B). The alkaline phosphatase activity assay showed strong and homogeneous activity in the absence of guanine while forming compact colonies, suggesting that Nanog expression prevents differentiation (Figure 3C). However, the majority of the infected mESCs cultured with guanine clearly lost alkaline phosphatase activity and failed to form compact colonies (Figure 3C). Immunofluorescence staining and flow cytometric analysis also showed that the transgene-induced cells (-guanine) maintained the cell surface marker for pluripotency (SSEA-1) and viral VSV-N expression (Figure 3D).
Figure 3.
mESCs infected with Nanog-VSV maintained the endogenous pluripotency marker SSEA-1 in a differentiation induction medium
(A) RNA genome structure of Nanog-VSV. (B) Schedule of infection with Nanog-VSV and maintenance of mESCs in -2iL medium. The culture medium was replaced daily. Two independently infected mESCs were examined for replicates as Exp1 and Exp2. (C) Alkaline phosphatase activities of mESCs infected with Nanog-VSV cultured with guanine (100 μM) or without guanine on day 5. Scale bar, 400 μm. (D) Flow cytometric analysis of immunofluorescence staining of mESCs infected with Nanog-VSV cultured for 5 days with or without guanine in differentiation induction medium (-2iL). The x and y axes indicate SSEA-1 and VSV-N, respectively. Gates were set according to the SNL feeder data. Infected (2iL), Nanog-VSV-infected mESCs (same cell line as Exp1) cultured under a pluripotency maintenance condition with guanine (100 μM) and puromycin (10 μg/mL); Infected Exp1 and Exp2; Nanog-VSV-infected mESCs cultured under differentiation induction conditions with puromycin (1 μg/mL) for 5 days; Uninfected, uninfected mESCs cultured without 2iL for 5 days.
Removal of VSV vector from mESCs by guanine and ribavirin
Removal of the non-integrating VSV vector from the infected cells would be desirable for some applications, such as regenerative medicine.14 Therefore, we explored the possibility of efficient removal of the VSV vector from the mESCs infected with Nanog-VSV described above. The Nanog-VSV-infected cells in -2iL medium in Figure 3 were transferred to 2iL medium without puromycin (Figure 4A). The cells were subsequently treated with guanine, which suppresses vector replication, and/or ribavirin, a small molecule known to have antiviral activity against RNA viruses, including VSV.15
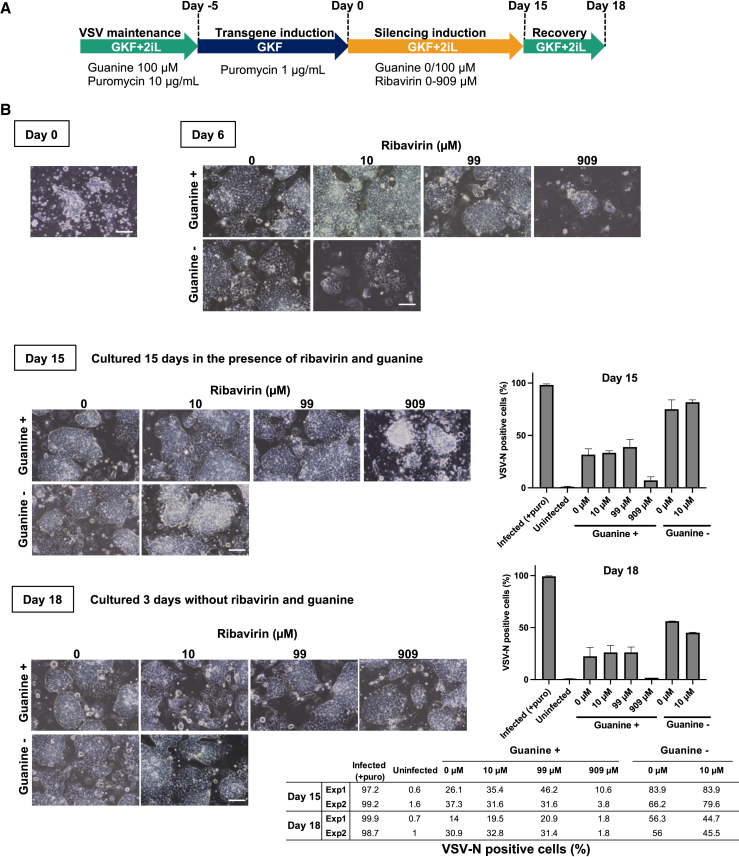
Figure 4.
Removal of Nanog-VSV from infected mESCs by ribavirin and guanine
(A) Schedule of the culture of mESCs infected with Nanog-VSV to remove the vector. The cells from Exp1 and Exp2 in Figure 3 after 5 days of transgene induction were used. The cells were passaged every 3 days during vector removal. (B) Images of the infected mESCs on days 0, 6, 15, and 18 under different concentrations of guanine and ribavirin (Exp1). The bar graphs indicate the means of fractions of cells expressing VSV-N protein as measured by flow cytometric analysis on days 15 and 18. The error bars indicate the ranges of Exp1 and Exp2. The numerical data are presented in the table. The flow cytometry data and qPCR data are shown in Figures S3 and S4 shows another set of experiments using independently infected mESCs (Exp2).
Scale bars, 100 μm.
Initially, up to 10 μM ribavirin was added to treat the infected mESCs because the uninfected mESCs did not survive more than 10 μM ribavirin (Figure S2). However, we coincidentally discovered that mESCs tolerated up to 909 μM ribavirin in the presence of 100 μM guanine (Figure S2). Therefore, ribavirin concentrations of 10, 99, and 909 μM were tested for the cells treated with guanine (Figures 4, S3, and S4).
According to the flow cytometric analysis of the mESCs infected with Nanog-VSV cultured in -2iL medium with puromycin, nearly 90% of the cells expressed the viral protein VSV-N in this experiment (Figure 3D, Guanine-, Infected (Exp1) and (Exp2)). The cells were then transferred to 2iL condition without puromycin (day 0) (Figure 4A). We observed that the cell growth was significantly slower in 10 μM ribavirin without guanine and with 909 μM ribavirin and 100 μM guanine. Therefore, the cells were passaged every 3 days and cultured for 15 days to obtain enough cells for flow cytometric analyses under all conditions. Most colonies of the cells treated with guanine and/or ribavirin still displayed loose cell-cell contact, except for the cells treated with 909 μM ribavirin and 100 μM guanine, which seemed to form small colonies at day 6. On day 15, however, some or most of the cells in all conditions grew in morphologically compact colonies, suggesting that the viral effects on colony appearance diminished after 15 days (Figures 4B and S4, day 15).
After mESCs infected with Nanog-VSV were cultured in different ribavirin and guanine concentrations for 15 days, the cells were further cultured for 3 days without ribavirin and guanine. VSV-N expression was detected to evaluate the remaining VSV vector in the cells, and SSEA-1 expression was also detected to confirm that the cells maintained pluripotency at a level similar to that of the uninfected mESCs (Figures 4, S3, and S4). The percentage of cells expressing VSV-N on day 15 was lower in cells treated with guanine (10.6%–46.2%) than in those that were not (83.9%) (Figure 4B, day 15, Exp1). Notably, percentage of the cells expressing VSV-N treated with 909 μM ribavirin and 100 μM guanine (10.6%) was significantly lower among the cells treated with guanine. An additional 3 days of culture (day 18) without ribavirin and guanine in all conditions resulted in an overall decrease in the percentage of VSV-N-positive cells, which indicated that VSV replication was not restored in the cells after the removal of guanine and ribavirin. The relative viral RNA levels measured by qRT-PCR were consistent with the corresponding protein levels (Figures S3 and S4). The cells treated with 909 μM ribavirin and 100 μM guanine showed 1.8% VSV-N-positive cells, which is comparable with that of the uninfected mESCs (0.7%). In summary, 15 days of VSV-infected mESCs cultured with ribavirin and guanine followed by 3 days of culture without ribavirin and guanine successfully attenuated VSV-N expression. Another experiment that was carried out using independently infected mESCs (Exp2) also showed the same trends (Figures 4 and S4).
Induction of myogenic differentiation by Myod1 expression
Overexpression of a specific transcription factor is an efficient way to induce stem cell differentiation. However, transient transfection of a plasmid or an mRNA vector is limited by transfection efficiency and is not very scalable. Genomic integration of an inducible expression cassette requires permanent alteration of the cellular genome. Therefore, we sought to demonstrate transcription factor-directed differentiation of mESCs using the VSV vector. We inserted the Myod1 (MyoD) gene into our VSV vector (MyoD-VSV) (Figure 5A), which efficiently induces myogenic differentiation.16 mESCs infected with MyoD-VSV were cultured in the presence of guanine and puromycin without inducing differentiation (Figures S1C and S6). Expression of pluripotency markers in infected mESCs was confirmed under the pluripotency maintenance condition (2iL) (Figure S6). The cells were then passaged on a laminin-coated plate under the same medium condition (2iL) (Figure 5B). Guanine was removed the next day to induce exogenous Myod1 expression (day 0). One day after Myod1 induction, the medium was changed to a B27 supplement-containing medium without 2iL (-2iL). Puromycin was removed on day 5 because further treatment resulted in cell loss.
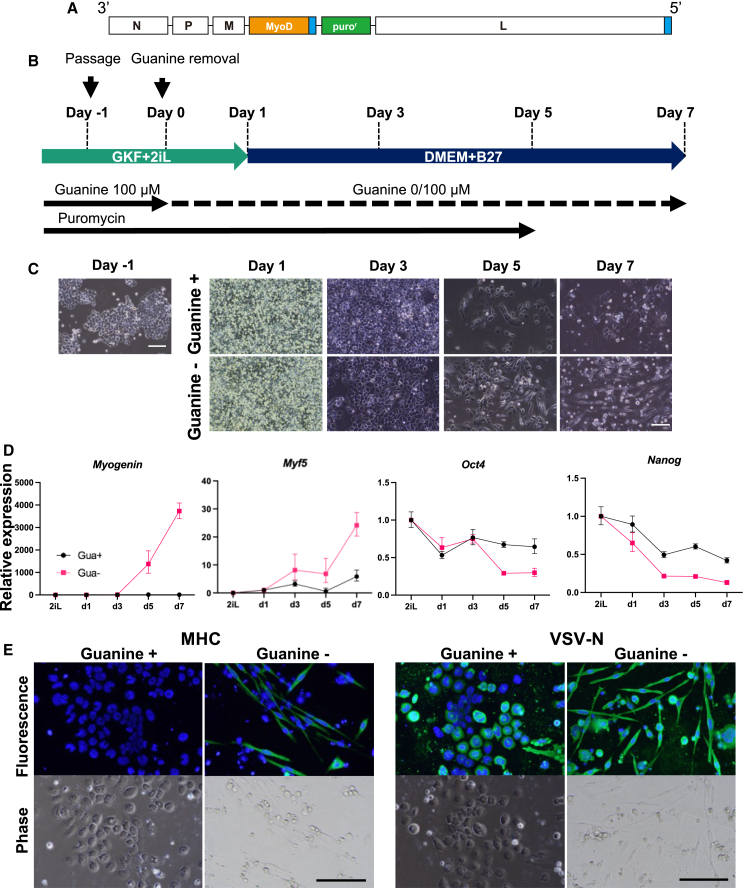
Figure 5.
Induction of myogenic differentiation by MyoD-VSV
(A) RNA genome structure of MyoD-VSV. (B) Schedule of myogenic differentiation of mESCs infected with MyoD-VSV. (C and D) Images and relative mRNA levels of mESCs infected with MyoD-VSV on days −1, 1, 3, 5, and 7. The y axis values indicate relative RNA levels normalized against GAPDH (RQ). Each data point indicates the mean of technical duplicates or triplicates. Error bars indicate the maximum and minimum RQ. Another set of repeated experiments is shown in Figures S5A and S5B. (E) Immunofluorescence staining of differentiation-induced cells with or without guanine on day 7. The experiment was carried out independently from the experiment of (C and D). Blue, Hoechst green, MHC or VSV-N. Images from two additional experiments are shown in Figures S5C and S5D.
Scale bars, 100 μm.
The infected mESCs maintained their colonies with rounded cell morphology before MyoD induction (Figures 5C and S5). On days 3 and 5, few spindle-like cells emerged in the cells cultured without guanine (Figures 5C and S5). Analyses of the endogenous gene expression by qPCR of the total RNAs showed reduced expression levels of the pluripotency markers Oct4 and Nanog in the cells cultured with and without guanine, although the cells in which Myod1 expression was induced by removing guanine showed more pronounced reduction at days 5 and 7 (Figures 5D and S5B). Myogenic cell-specific mRNA levels (Myogenin and Myf5) sharply increased in the absence of guanine from day 3 to day 5, but they remained suppressed in the presence of guanine (Figures 5D and S5B). Upregulation of myogenic cell-specific mRNA levels matches the emergence of spindle-like cells in the absence of guanine (Figures 5C, 5D, S5A, and S5B). Immunofluorescence staining of the cells on day 7 showed myosin heavy chain (MHC:MyHC) expression in the spindle-like cells found in the cells without guanine, but not in the rounded cells cultured with guanine (Figures 5E, S5C, and S5D). VSV-N expression was observed in cells cultured with and without guanine, indicating that the VSV vector is stably maintained under these conditions. Thus, these results demonstrate that a transgene differentiation factor can be stably maintained in mESCs infected with the VSV vector without inducing differentiation in the presence of guanine and puromycin, and its expression can be induced by removing guanine to efficiently differentiate mESCs to a specific cell type.
Discussion
RNA viral vectors or self-replicating RNA vectors based on RNA viruses allow stable and long-term expression of exogenous genes even in proliferating host cells, such as stem cells, without genomic integration. These characteristics make RNA viral vectors attractive for biomedical applications, such as gene therapy and regenerative medicine. However, there are a number of limitations with the existing vectors, such as cytotoxicity, difficulty of vector removal, and regulation of vector replication and transgene expression. Recently, engineered vectors based on the Sendai virus have gained popularity for reprogramming somatic cells into induced pluripotent stem cells (iPSCs) because they can strongly and efficiently express reprogramming factors without chromosomal integration.17 However, the use of Sendai virus vectors and other RNA viral vectors in stem cells has been rare. Tan et al.18 recently used a Sendai virus vector to overexpress mouse Myod1 in human ESCs and iPSCs to induce myogenic differentiation. Similarly, the same group used a Sendai virus vector to produce spinal motor neurons.19 A Borna disease virus-based vector was also used to drive myogenic differentiation by human MYOD1.20 These approaches yield differentiated cells without chromosomal modifications, but they do not take advantage of the replicative nature of the vector because transgene expression is not regulated. Using these RNA viral vectors, stem cells differentiate immediately upon vector transduction, but the transduced cells cannot be expanded while suppressing differentiation for scalable production of differentiated cells. We recognized that riboswitches are suitable for chemical regulation of transgene expression from RNA viral vectors and demonstrated reversible and long-term regulation of reporter gene expression using a VSV vector equipped with a guanine-responsive riboswitch in BHK-21 cells.8 However, we observed strong cytotoxicity when we attempted to transduce mESCs with the VSV vector. This cytotoxicity was circumvented by a mutant isolated from surviving mESCs.9
This work is based on these previous efforts with several important technical advances. First, through further characterization of the VSV mutants isolated from infected mESCs, we identified an improved VSV mutant that exhibited diminished cytotoxicity and improved transgene expression in mESCs. Second, we added a puromycin selectable marker (PuroR) that allows stable selection of infected mESCs while maintaining guanine riboswitch function. Finally, we demonstrated the chemical regulation of functional transgenes (Nanog and Myod1) in mESCs with practical implications. Importantly, our VSV vector does not seem to compromise the endogenous protein and RNA expression levels of the key pluripotency markers tested in the infected mESCs in the presence of guanine, and withdrawal of guanine induced the endogenous myogenic differentiation markers in MyoD-VSV-infected mESCs. However, further investigations such as transcriptome analysis would be required to conclusively determine if the infected cells maintain pluripotency.
We demonstrated continuous proliferation of the infected mESCs up to 1 month or 10 passages in the presence of guanine and puromycin (Figure 1), during which the inserted transgene expression was efficiently suppressed while maintaining infection. As shown in Figure 1A, PuroR is not directly regulated by the GuaM8HDV aptazyme. While guanine is expected to downregulate PuroR due to suppression of the VSV genome replication (through downregulation of VSV-L expression), it is not suppressed as much as DsRedEx2, which is further downregulated by the aptazyme in its mRNA. Therefore, puromycin can select for VSV-infected cells in the presence of guanine, while DsRedEx2 expression is strongly suppressed. Remarkably, transgene expression was activated in almost all cells in the population upon removing guanine even after such a long-term culture (Figures 2, S1A, and S1B). For regenerative medicine applications, this feature allows large-scale culture of stem cells infected with an inducible differentiation factor, allowing efficient and scalable production of various cell types.
For clinical applications, removal of the VSV vector upon completion of the intended task is desirable. Active removal of the vector by a combination of ribavirin and guanine was demonstrated for Nanog-VSV in mESCs (Figures 4, S3, and S4). However, a similar experiment with MyoD-VSV was hindered by the low viability of differentiated myogenic cells. Ribavirin is known as a broad-spectrum, guanosine analog antiviral agent that acts against RNA and DNA viruses, and it has been used to treat infections with hepatitis C virus, respiratory syncytial virus, and Lassa fever virus.15,21 Ribavirin was previously used in an attempt to remove a GFP-expressing Sendai virus vector from primate ESCs.22 While a 3-day treatment with 4 mM ribavirin effectively suppressed GFP expression, the cells could not be propagated further, and treatment at lower concentrations (0.5–0.75 mM) resulted in partial vector suppression and near-complete recovery of vector replication after removing ribavirin. In our case, the synergistic effects of ribavirin and guanine allowed near-complete removal of Nanog-VSV from the infected mESCs (Figures 4, S3, and S4).
An alternative approach to chemically regulate an RNA viral vector is to control viral protein function using a small molecule. Heilmann et al.23 strategically inserted HIV protease into VSV-P or VSV-L and demonstrated that they could control the vector replication and transgene expression using a clinically approved protease inhibitor. The VSV vectors exhibited excellent regulation in response to the small molecule both in cell culture and in mice, although no functional transgenes were incorporated. However, protein-based switches based on exogenous factors may trigger immunological complications for in vivo applications. Because this strategy requires direct engineering of a viral protein involved in virus replication, substantial efforts would be necessary to adapt the strategy to other RNA viral vectors. It should also be noted that the VSV vector developed by Heilmann et al. contains all viral genes, including VSV-G, which makes it capable of producing infectious progeny viruses. Such vectors would be useful for oncolytic virus application, but they need to be handled carefully to avoid unintended infections and viral escape. It remains to be seen whether protein-based regulation in the ΔVSV-G format (incapable of producing infectious progenies) enables comparable transgene regulation and vector stability without a drug selection marker.
In summary, this work describes the first non-integrating and replicable vector that can be stably maintained and chemically regulate transgene expression in stem cells. We used this vector to demonstrate pluripotency maintenance by Nanog expression and myogenic differentiation by Myod1 expression in mESCs. Our riboswitch approach may be easily transferrable to other RNA viral vectors such as Sendai virus vectors.24
Materials and methods
Cell culture and small molecules
mESC strains BRC6 (AES0010, RIKEN BRC) and ES-R1 (07072001, ECACC) were used in these experiments and maintained as described previously.9 mESCs were cultured with mitomycin C (catalog no. M0503, Sigma-Aldrich)-treated SNL feeder cells (CBA-316, Cell Biolabs) in 5% CO2 at 37°C in GKF medium (GKF ESM): Glasgow Minimum Essential Medium (catalog no. G6148, Sigma-Aldrich), 15% KnockOut Serum Replacement (catalog no. 10828-028, Thermo Fisher Scientific), 0.3% fetal bovine serum (FBS) (catalog no. 10437-028, Thermo Fisher Scientific), 2 mM L-glutamine (catalog no. 25030-081, Thermo Fisher Scientific), 1 mM sodium pyruvate (catalog no. 11360-070, Thermo Fisher Scientific), 1% MEM Non-essential Amino Acid Solution (catalog no. 11140-050, Thermo Fisher Scientific), 0.1 mM 2-mercaptoethanol (catalog no. 21985-023, Thermo Fisher Scientific), and 1% penicillin-streptomycin (catalog no. 15140-122, Thermo Fisher Scientific). For pluripotency maintenance, 1 μM MEK inhibitor (PD0325901) (catalog no. 162–25291, Wako), 3 μM GSK3β inhibitor (CHIR99021) (catalog no. SML1046, Sigma-Aldrich), and laboratory-prepared human LIF (1,000× dilution) were added to GKF ESM (2iL). Human LIF was produced in 293T cells by transfecting the pCAGGS-hLIF plasmid (RDB09182, RIKEN BRC DNA Bank).25 The titer of the supernatant containing human LIF was confirmed using BRC6 mESCs. Mitomycin C-treated SNL feeder cells were prepared a few days before passaging mESCs in DMEM (catalog no. 10566-016, Thermo Fisher Scientific) containing 10% FBS and 1% penicillin-streptomycin on a gelatin (catalog no. G1890, Sigma-Aldrich)-coated plate/dish (1 × 106 cells/10-cm dish). Guanine stock solution (20 mM) was prepared in 0.2 N NaOH.
To maintain mESCs, the culture medium was replaced with fresh medium daily. mESCs were dissociated by TrypLE Express (catalog no. 12604-013, Thermo Fisher Scientific) and counted using a counting chamber and Trypan Blue Solution (catalog no. T8154, Sigma-Aldrich) to passage the cells. The cells were passaged every 3 days at the density of 5 × 104 (uninfected mESCs) or 1 × 105 (infected mESCs cultured with puromycin) cells per well in 6-well plates (catalog no. 3516, Corning). To observe the cell growth as shown in Figure 1C, DsRedEx2-VSV-infected mESCs were passaged at the density of 2 × 104 cells/well in 12-well plates (catalog no.3513, Corning). For Figures 2B and 2C, the DsRedEx2-VSV-infected mESCs after 39 days of culture with guanine and puromycin were passaged on a gelatin-coated 6-well plate at 2 × 105 cells/well with or without guanine and puromycin. For the immunofluorescence staining images in Figure 1F, the infected and uninfected mESCs in pluripotent stem cell medium were passaged at 1×104 cells/well on a gelatin-coated μ-Slide 8 well (catalog no. 80826, ibidi) and cultured for 3 days.
Nanog-VSV-infected mESCs (Figure 3B) were maintained in the presence of puromycin (10 μg/mL) and guanine (100 μM). For the transgene expression experiment, the cells were dissociated using TrypLE Express and washed with GKF ESM (-2iL) by centrifugation (200×g, 3 min) three times. The cells were then plated on a gelatin-coated 10 cm dish (catalog no. 3020-100, IWAKI) (6 × 106 cells/dish) for flow cytometry analysis and a 6-well plate (1 × 106 cells/well) for the detection of alkaline phosphatase activity in GFK ESM containing puromycin (1 μg/mL) with or without guanine (100 μM). Uninfected mESCs were cultured in parallel with the Nanog-VSV infected mESCs, which were plated on a gelatin-coated 10 cm dish (1.5 × 106 cells/dish) for flow cytometry, and a 6-well plate (2.5 × 105 cells/well) for the detection of alkaline phosphatase activity and cultured in GKF ESM (-2iL) without puromycin. The medium was changed daily during the 5 days of transgene induction period. The VSV vector removal experiments (Figures 4, S3, and S4) were performed using the mESCs infected with Nanog-VSV cultured in the differentiation induction medium after 5 days of transgene induction (Figure 3D, Guanine -, Infected (Exp1) and (Exp2)). Initially, the cells in each condition were passaged on mitomycin C-treated SNL feeder cells in a 6-well plate at 1 × 105 cells/well in GKF ESM with 2iL and/or guanine (100 μM) and ribavirin (catalog no. S2504, Selleckchem) without puromycin. Ribavirin stock solution in water (10 mM) was added directly to GKF ESM with 2iL to the final concentrations of 10, 99, and 909 μM when the medium was replaced. The culture medium in each condition was replaced daily. Depending on the cell growth for each condition after the initial passage, the cells were passaged every 3 days at a cell density of 1 × 105, 5 × 104, or all cells per well in a 6-well plate and subsequently 3–4 × 105, 2.5 × 106, or all cells in a 10-cm dish.
Myogenic differentiation was performed following the schedule shown in Figure 5B. BRC6 mESCs infected with MyoD-VSV were maintained in GKF ESM containing 2iL, guanine (100 μM), and puromycin (10 μg/mL). The cells were dissociated by TrypLE Express and 1×105 cells/well were passaged on a Biolaminin 521 LN (catalog no.BLA-LN521-05, BioLamina)-coated 12-well plate (1 × 105 cells/well) (catalog no. 3513, Corning) for RNA extraction (day −1). For immunofluorescence staining, cells infected with MyoD-VSV were passaged on Biolamina 521-coated μ-Plate 24-well black (catalog no. 82406, ibidi) at 5 × 104 cells/well (Figures 5E and S5C), or 24-well plates (catalog no. 3526, Corning) at 2 × 104 cells/well (Figure S5D). The next day, the medium was replaced with fresh medium with or without guanine (100 μM) (day 0). The following day (day 1), the medium was replaced from GKF ESM to the serum-free differentiation induction medium containing DMEM (catalog no.12100-046, Thermo Fisher Scientific), 2% B-27 supplement (catalog no. 17504-044, Thermo Fisher Scientific), 2 mM L-glutamine, 1 mM sodium pyruvate, 1% MEM Non-essential Amino Acid Solution, 0.1 mM 2-mercaptoethanol, and 1% penicillin-streptomycin. The medium was adjusted to pH 7.6 under 5% CO2 at 37°C using 1 M NaHCO3. During myogenic differentiation induction, the culture medium was replaced daily until day 7. Puromycin (10 μg/mL) was added until day 5, and then the cells were cultured without puromycin to avoid the loss of differentiated cells.
For virus production, 293T (12022001, ECACC) and BHK-21 cells were maintained in DMEM (catalog no. 10566-016, Thermo Fisher Scientific) containing 1% penicillin-streptomycin and 10% FBS.
Plasmid construction
The mutated viral RNA genome derived from the VSV vector in long-term cultured VSV-infected mESCs was reverse transcribed to cDNA and used to replace the whole VSV genome sequence in the VSV vectors described in our previous paper.9 The DsRedEx2 gene in the plasmid was removed and the restriction enzyme sites for MluI and NotI between VSV-M and VSV-L were used to yield the parental vector for generating DsRedEx2-VSV, MyoD-VSV and Nanog-VSV used in this article. The vector sequence of DsRedEx2-VSV is provided in Figure S7. The vectors were prepared by ligating appropriate fragments into the MluI and NotI restriction sites, transforming them into NEB Stable Competent E. coli (catalog no. C3040, NEB), and culturing the transformed cells at 30°C in LB medium with ampicillin. The mouse Nanog sequence was cloned from the total RNA extracted from BRC6 mESCs, and the mouse Myod1 gene was obtained from LV-TRE-WT mouse MyoD-T2A-dsRedExpress2 (Addgene #60624) by PCR. The plasmid vectors were purified using QIAGEN Plasmid Maxi Kit (catalog no. 12163, QIAGEN) for transfection into 293T cells. The plasmid sequences were verified using Sanger sequencing and whole plasmid sequencing (Azenta).
Viral particle production
VSV vector particles were prepared as described before.8,9 293T cells were passaged the day before transfection in a 6-well plate (1 × 106 cells/well) at 37°C in 5% CO2. For transfection, 250 μL of OPTI-MEM (catalog no. 31985062, Thermo Fisher Scientific) and plasmids for viral production were mixed in a 1.5-mL tube: VSV vector plasmid (5 μg, DsRedEx2-VSV/MyoD-VSV/Nanog-VSV), pCAG-VSV-N (1.5 μg, Addgene #64087), pCAG-VSVP (2.5 μg, Addgene #64088), pCAG-VSVL (0.5 μg, Addgene #64085), pCAG-VSVG (4 μg, Addgene #35616), and pCAG-T7pol (5 μg Addgene #59926). Thirty seven microliters of TransIT-293 Reagent (catalog no. MIR2705, Mirus Bio) were added to the plasmid solution and incubated 20 min at room temperature. Each plasmid mixture was added to the 293T cells, and the medium was replaced after 4 h of incubation at 37°C under 5% CO2. After 4–5 days of culture, the supernatants were harvested and used to infect BHK-21 cells transfected with pCAG-VSVG to further increase the viral titer. After 2 days of culture of BHK-21 cells, the supernatants were harvested and passed through a syringe filter (0.22 μm). The supernatants were concentrated using PEG-it (catalog no. LV825A-1, System Biosciences) following the manufacturer’s instructions and the aliquots were stored at −80°C. The virus solution was then used in each experiment after checking the titer (multiplicity of infection [MOI]) with BHK-21 cells as previously described.9 Instead of observing the red fluorescence of the infected BHK-21 cells for DsRedEx2-VSV, immunofluorescence staining with anti-VSV-N antibody was performed to titer the preparations of Nanog-VSV and MyoD-VSV particles.
VSV-infected mESC preparation
Dissociated mESCs for viral infection (1 × 105 cells/well in a 12-well plate for the experiments shown in Figures 1, 1 × 106 or 2 × 105 cells/well in a 6-well plate for the other experiments) were suspended in 1 mL GKF with VSV supernatant (with a MOI of 1 for the experiments shown in Figure 1 and a MOI of ≤1 for the other experiments) and polybrene (10 μg/mL) (catalog no. 12996-81, Nacalai Tesque) in a 1.5-mL microtube. The cells were then incubated at room temperature with rotation for 1 h. To wash the infected cells, the cells in the tube were centrifuged (200×g, 3 min), and the supernatant was removed. Fresh GKF ESM (1 mL) was then added to the tube to wash the cells by centrifugation again. The cells were then seeded on SNL feeder cells with/without guanine (100 μM) in pluripotency maintenance medium. The next day, the medium was replaced with fresh medium and puromycin treatment was initiated. DsRedEx2-VSV-infected mESCs were used directly for the experiments in Figures 1 and 2 without freezing and thawing unless otherwise specified in Figure S1. mESCs infected with Nanog-VSV and MyoD-VSV were cultured with guanine (100 μM) and puromycin (10 μg/mL) for a few passages and preserved in STEM-CELLBANKER (catalog no. CB045, Nippon Zenyaku Kogyo) as stocks. The thawed stocks were then cultured for a few passages, and cells with up to nine passages after infection were used in the experiments.
Alkaline phosphatase staining
Adherent cells were fixed with 4% paraformaldehyde (PFA) (catalog no. 50-980-495, Electron Microscopy Sciences) in PBS (D-PBS (−), catalog no. 045–29795, Wako) for 5 min at room temperature. After washing with PBS three times, alkaline phosphatase activity in cells was detected using Vector Blue Substrate Kit (catalog no. SK-5300, Vector Laboratories) according to the manufacturer’s instructions.
Immunofluorescence staining
Cells were fixed with 4% PFA in PBS for 10 min at room temperature. After washing with PBS, the fixed cells were incubated for 45 min at room temperature with a blocking solution containing 5% goat serum (catalog no. 31872, Thermo Fisher Scientific) and 0.1% TrirtonX-100 (excluded for SSEA-1 staining) in PBS followed by another wash with PBS. A solution including the primary antibody was added to each well and incubated at 4°C overnight at the following dilutions in PBS containing 1% goat serum: 1:50 of Oct3/4 (C-10) (catalog no. sc-5279, Santa Cruz Biotechnology), 1:300 of Anti-Nanog (catalog no. ab80892, Abcam), 1:50 of fluorescence-conjugated SSEA-1 antibody (SSEA1 Monoclonal Antibody (eBioMC-480 (MC-480)), eFluor 488, catalog no. 53-8813-42, eBioscience), 1:125 of Anti-MHC, Mouse-Mono (MF20) (MHC) (catalog no. MAB4470, R&D Systems), 1:1,000 of Anti-VSVN (10G4) (catalog no. EB0009, Kerafast), and 1:200 of Anti-ki67 antibody [SP6] (catalog no. ab16667, Abcam). The fixed cells were washed with PBS three times (first wash, rinsed immediately; second and third washes, 10 min), and the secondary antibody was applied at the following dilutions in PBS: 1:500 of Alexa Fluor 488 goat anti-mouse IgG (H + L) (catalog no. A11001, Thermo Fisher Scientific) for Oct3/4, Anti-VSVN and Anti-MHC, 1:500 of Alexa Fluor 488 goat anti-rabbit IgG (H + L) (catalog no. A11008, Thermo Fisher Scientific) for Anti-Nanog and Anti-ki67. After incubation for 1 h at room temperature, the fixed cells were washed three times with PBS (first wash, rinsed immediately; second and third washes, 10 min). To stain the cell nuclei, Hoechst 33342 (catalog no. H1399, Thermo Fisher Scientific) was diluted in PBS (1 μg/mL), added to each well, and incubated for 10 min at room temperature. The fixed cells were washed with PBS three times (first wash, rinsed immediately; second and third washes, 5 min) and observed using an inverted microscope (ECLIPSE Ts2, Nikon). For Alexa 488-conjugated SSEA-1, the secondary antibodies and Hoechst staining steps were omitted. Cell images were acquired using a Nikon 7500.
For flow cytometry, 1 × 107–1.2 × 107 dissociated living cells were prepared in a 1.5-mL tube for immunofluorescence staining. Cells were fixed with 400 μL of 2% PFA in PBS for 15 min with rotation. PFA was removed from the cells by centrifugation (600×g, 5 min), and the cells were washed with 1 mL FACS buffer containing 2% FBS and 0.05% sodium azide in PBS. This washing procedure was repeated two more times. The cells were then used for staining or preserved at 4°C in FACS buffer containing 5% FBS overnight.
For staining, the cells were permeabilized with 200 μL of 0.1% Triton X-100 in 5% goat serum in PBS for 20 min with rotation. The cells were then immediately washed with 1 mL FACS buffer three times by centrifugation (600×g, 5 min). At the last washing step, each sample was divided into two tubes, one of which was used as a negative control without the primary antibodies and was subjected to the same staining steps with the secondary antibody only. After centrifugation to remove FACS buffer from each tube, 100 μL of VSV-N antibody (1:100) in FACS buffer was added to the cells in a tube and incubated at room temperature for 30 min with rotation. The cells were washed three times with 1 mL FACS buffer by centrifugation (600×g, 5 min). The secondary antibody solution (100 μL) in FACS buffer (Alexa Fluor 488 goat anti-mouse IgG (H + L), 1:500 dilution) was then added and incubated for 30 min at room temperature with rotation. After the same washing procedure as with the primary antibody, 100 μL of FACS buffer containing 1:20 dilution of fluorescence-conjugated SSEA-1 antibody (SSEA1 Monoclonal Antibody (eBioMC-480 (MC-480)), eFluor 660, catalog no. 50-8813-42, eBioscience) was added to the tube to resuspend the cells. The cells were incubated for 30 min at room temperature with rotation, washed three times with 1 mL FACS buffer by centrifugation. Finally, the cells were resuspended in 200 μL FACS buffer for flow cytometric analysis.
Flow cytometry
All flow cytometry experiments were performed on a BD FACS Aria III (BD Biosciences). The data were analyzed using FlowJo (BD Biosciences). For the detection of DsRedEx2 in living cells, dissociated cells were suspended in PBS and analyzed by flow cytometry using the DsRed channel. Uninfected mESCs and a clonal mESC cell line that stably expresses DsRedExpress2-GuaM8HDV from the CAG promoter prepared by piggyBac transposition were used in each experiment as negative and positive controls, respectively.
For the detection of VSV-N and SSEA-1 in the fixed and stained cells, the cells were suspended in FACS buffer and analyzed by flow cytometry using Alexa 488 and Alexa 647 channels. Uninfected mESCs, mESCs infected with Nanog-VSV cultured with puromycin, guanine, and SNL feeder cells were used as controls for each antibody. Cells processed only with the secondary antibody (Alexa 488) were prepared for the control samples in each experiment. The gate settings used are described in the figure legends.
Quantitative PCR
RNeasy Mini Kit (catalog no.74104, QIAGEN) was used for RNA extraction following the manufacturer’s instructions to observe gene expression levels. For myogenic differentiation, the lysis solution was added directly to the adherent cells during differentiation (days 1, 3, 5, and 7) in a 12-well plate after removing the medium from each sample. For the detection of the viral RNA levels (Figures S3 and S4), some of the dissociated cells used for flow cytometry analysis (1 × 106 cells each) were applied. The extracted RNAs were reverse transcribed to generate cDNAs using Hi Capacity cDNA Reverse Transcription Kit (catalog no.4368814, Thermo Fisher Scientific). RNA expression levels were then analyzed using the cDNAs by real-time qPCR with gene-specific primers (Table S1) and PowerUP SYBR Green Master Mix (A25742, Thermo Fisher Scientific) using StepOnePlus (Thermo Fisher Scientific). The data were analyzed using the instrument software with comparative Ct (ΔΔCt) method as was described previously.9 VSV-infected mESCs cultured in the presence of puromycin and guanine were used as the reference sample (Figures 5D and S5B, MyoD-VSV infected mESCs; Figures S3 and S4, Nanog-VSV infected mESCs). For Myf5 data in Figure 5D, the cells from d1 cultured with guanine were set as the reference, as the Ct was undetermined in 2iL. GAPDH was used as the endogenous control.
Data and code availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplemental information.
Acknowledgments
This research was funded by Okinawa Institute of Science and Technology Graduate University (OIST) and JSPS KAKENHI grants (20K15669 and 22K15000) awarded to N.K.
Author contributions
Y.Y. managed the project, analyzed the results, and wrote the manuscript. N.K. designed and executed the experiments, analyzed the results, and wrote the manuscript.
Declaration of interests
None declared.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2025.01.005.
Supplemental information
References
- 1.Nayerossadat N., Maedeh T., Ali P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012;1:27. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W., Lin C., Lu D., Ning Z., Cox T., Melvin D., Wang X., Bradley A., Liu P. Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2008;105:9290–9295. doi: 10.1073/pnas.0801017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiyama T., Sato S., Chikazawa-Nohtomi N., Soma A., Kimura H., Wakabayashi S., Ko S.B.H., Ko M.S.H. Efficient differentiation of human pluripotent stem cells into skeletal muscle cells by combining RNA-based MYOD1-expression and POU5F1-silencing. Sci. Rep. 2018;8:1189. doi: 10.1038/s41598-017-19114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urlinger S., Baron U., Thellmann M., Hasan M.T., Bujard H., Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: Novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. USA. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gossen M., Freundlieb S., Bender G., Müller G., Hillen W., Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 6.Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martello G., Smith A. The Nature of Embryonic Stem Cells. Annu. Rev. Cell Dev. Biol. 2014;30:647–675. doi: 10.1146/annurev-cellbio-100913-013116. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K., Yokobayashi Y. Reversible Gene Regulation in Mammalian Cells Using Riboswitch-Engineered Vesicular Stomatitis Virus Vector. ACS Synth. Biol. 2019;8:1976–1982. doi: 10.1021/acssynbio.9b00177. [DOI] [PubMed] [Google Scholar]
- 9.Kim N., Yokobayashi Y. Novel RNA Viral Vectors for Chemically Regulated Gene Expression in Embryonic Stem Cells. ACS Synth. Biol. 2021;10:2959–2967. doi: 10.1021/acssynbio.1c00214. [DOI] [PubMed] [Google Scholar]
- 10.Nomura Y., Zhou L., Miu A., Yokobayashi Y. Controlling mammalian gene expression by allosteric hepatitis delta virus ribozymes. ACS Synth. Biol. 2013;2:684–689. doi: 10.1021/sb400037a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsui K., Tokuzawa Y., Itoh H., Segawa K., Murakami M., Takahashi K., Maruyama M., Maeda M., Yamanaka S. The homeoprotein nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/S0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 12.Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/S0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 13.Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabrera A., Edelstein H.I., Glykofrydis F., Love K.S., Palacios S., Tycko J., Zhang M., Lensch S., Shields C.E., Livingston M., et al. The sound of silence: Transgene silencing in mammalian cell engineering. Cell Syst. 2022;13:950–973. doi: 10.1016/j.cels.2022.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crotty S., Cameron C., Andino R. Ribavirin’s antiviral mechanism of action: Lethal mutagenesis? J. Mol. Med. 2002;80:86–95. doi: 10.1007/s00109-001-0308-0. [DOI] [PubMed] [Google Scholar]
- 16.Davis R.L., Weintraub H., Lassar A.B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-X. [DOI] [PubMed] [Google Scholar]
- 17.Kunitomi A., Hirohata R., Arreola V., Osawa M., Kato T.M., Nomura M., Kawaguchi J., Hara H., Kusano K., Takashima Y., et al. Improved Sendai viral system for reprogramming to naive pluripotency. Cell Rep. Methods. 2022;2 doi: 10.1016/j.crmeth.2022.100317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan G.W., Kondo T., Imamura K., Suga M., Enami T., Nagahashi A., Tsukita K., Inoue I., Kawaguchi J., Shu T., Inoue H. Simple derivation of skeletal muscle from human pluripotent stem cells using temperature-sensitive Sendai virus vector. J. Cell. Mol. Med. 2021;25:9586–9596. doi: 10.1111/jcmm.16899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goto K., Imamura K., Komatsu K., Mitani K., Aiba K., Nakatsuji N., Inoue M., Kawata A., Yamashita H., Takahashi R., Inoue H. Simple Derivation of Spinal Motor Neurons from ESCs/iPSCs Using Sendai Virus Vectors. Mol. Ther. Methods Clin. Dev. 2017;4:115–125. doi: 10.1016/j.omtm.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komatsu Y., Takeuchi D., Tokunaga T., Sakurai H., Makino A., Honda T., Ikeda Y., Tomonaga K. RNA Virus-Based Episomal Vector with a Fail-Safe Switch Facilitating Efficient Genetic Modification and Differentiation of iPSCs. Mol. Ther. Methods Clin. Dev. 2019;14:47–55. doi: 10.1016/j.omtm.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graci J.D., Cameron C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasaki K., Inoue M., Shibata H., Ueda Y., Muramatsu S.I., Okada T., Hasegawa M., Ozawa K., Hanazono Y. Efficient and stable Sendai virus-mediated gene transfer into primate embryonic stem cells with pluripotency preserved. Gene Ther. 2005;12:203–210. doi: 10.1038/sj.gt.3302409. [DOI] [PubMed] [Google Scholar]
- 23.Heilmann E., Kimpel J., Hofer B., Rössler A., Blaas I., Egerer L., Nolden T., Urbiola C., Kräusslich H.G., Wollmann G., von Laer D. Chemogenetic ON and OFF switches for RNA virus replication. Nat. Commun. 2021;12:1362. doi: 10.1038/s41467-021-21630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakanishi M., Otsu M. Development of Sendai Virus Vectors and their Potential Applications in Gene Therapy and Regenerative Medicine. Curr. Gene Ther. 2012;12:410–416. doi: 10.2174/156652312802762518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hitoshi N., Ken-ichi Y., Jun-ichi M. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplemental information.