Abstract
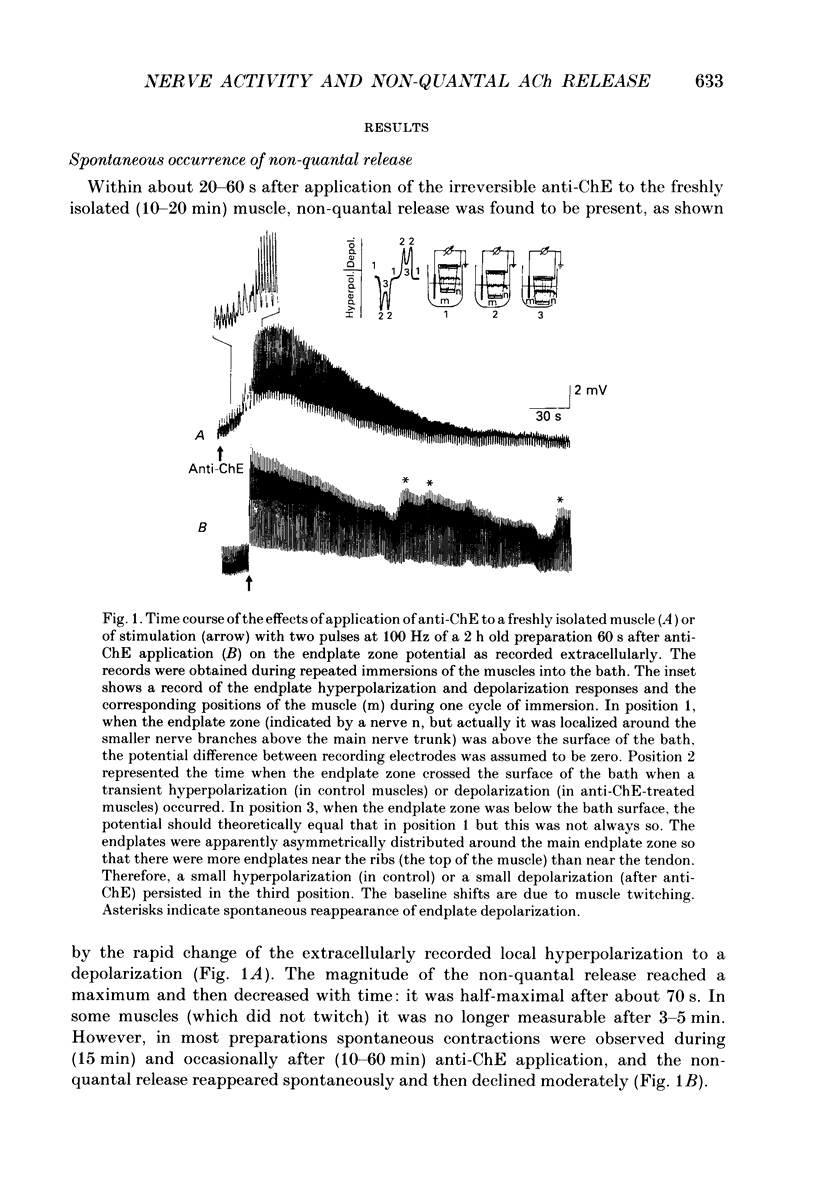
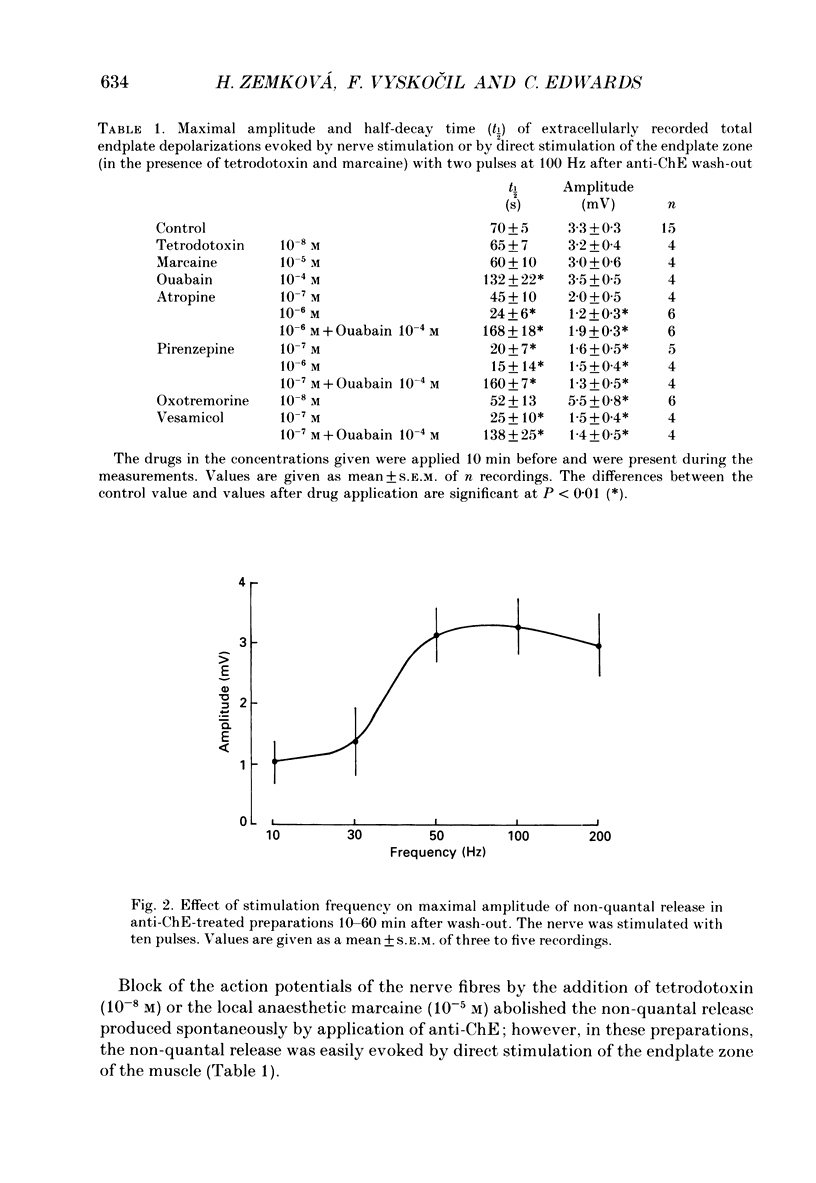
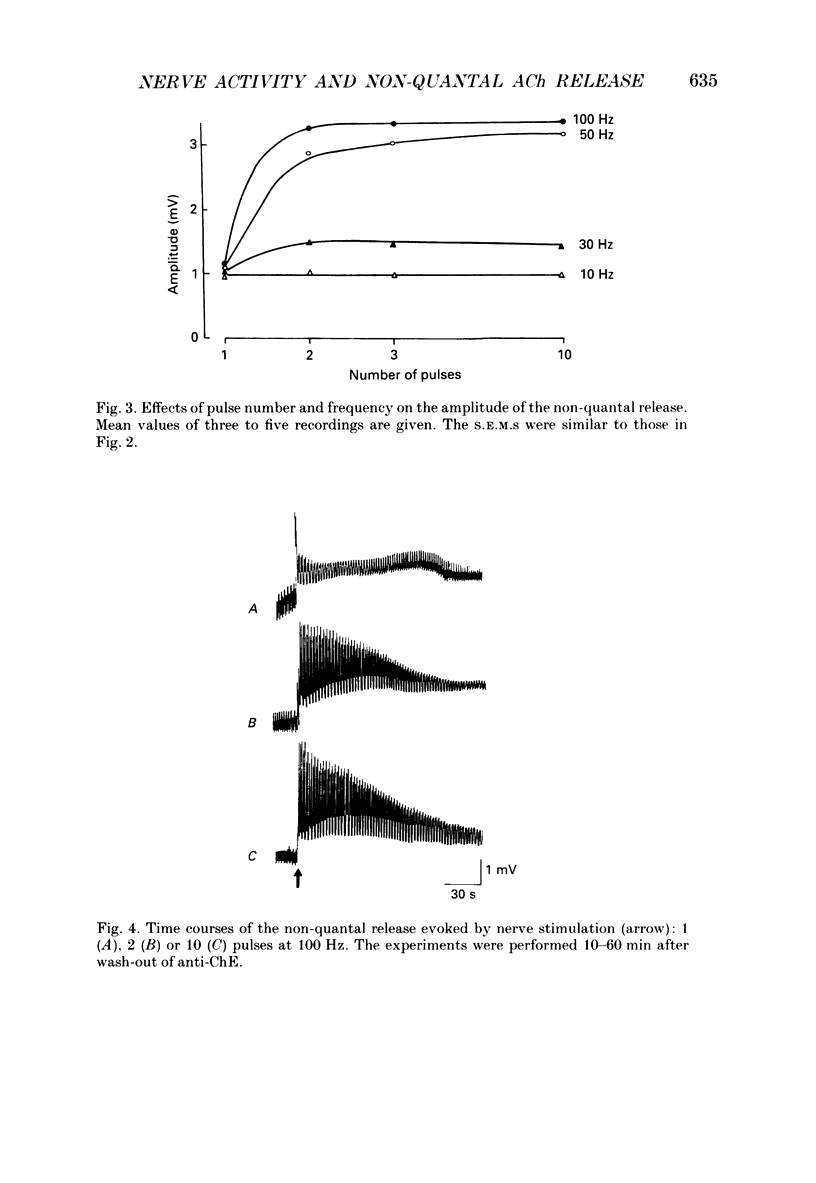
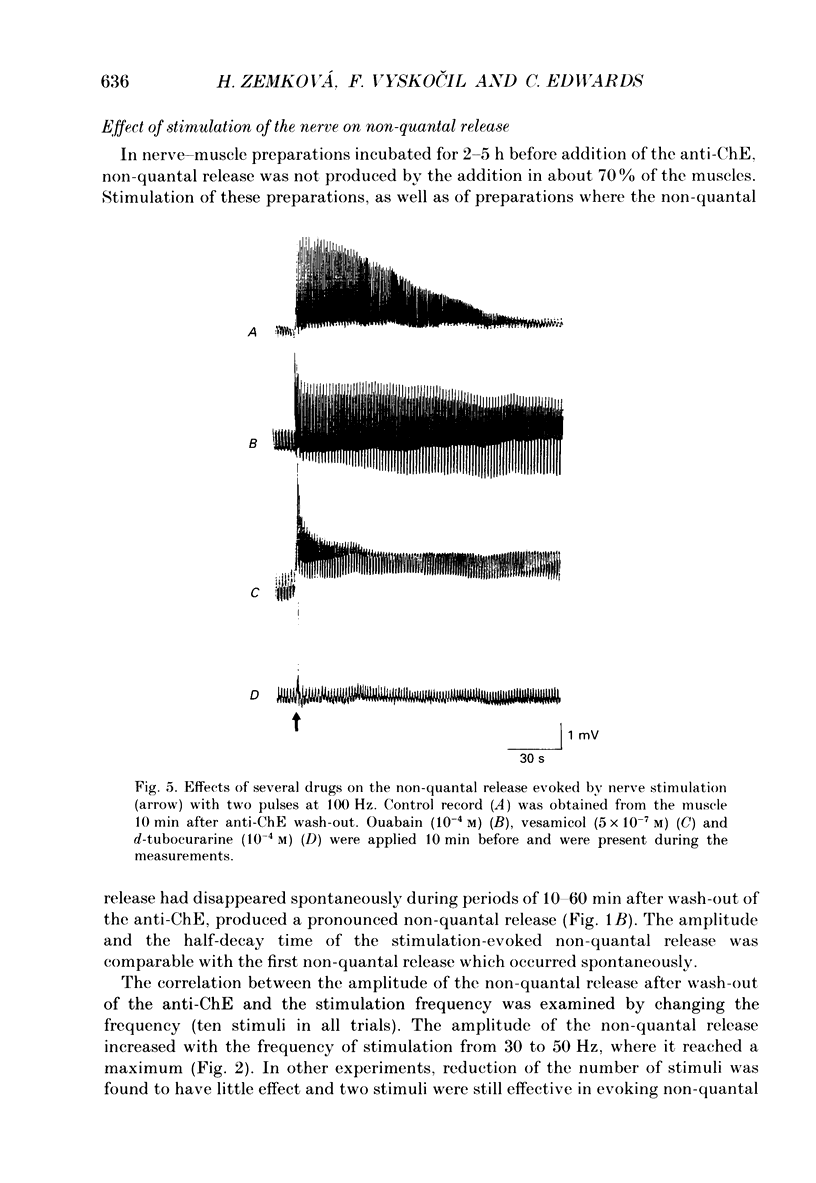
1. Local endplate depolarization induced by anticholinesterase application to mouse nerve-diaphragm preparations was taken as a measure of non-quantal release of acetylcholine. 2. Non-quantal acetylcholine release occurred within 20-60 s after anticholinesterase application, either spontaneously or evoked by nerve stimulation. Non-quantal release declined with time and disappeared after 3-5 min. 3. The amplitude of stimulation-evoked non-quantal release increased with the frequency of stimulation and was maximal at frequencies above 50 Hz. Two stimuli were sufficient to evoke the maximal effect. 4. Micromolar concentrations of atropine, pirenzepine and vesamicol reduced the amplitude and shortened the duration of non-quantal release. Oxotremorine (10(-8) M) enhanced the amplitude and ouabain (10(-4) M) prolonged the duration of non-quantal release. 5. Our results support the idea that the non-quantal release is due to the vesicular acetylcholine transport system which becomes transiently a part of the nerve terminal during exocytotic release of quantal acetylcholine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizenman E., Bierkamper G. G., Stanley E. F. Botulinum toxin prevents stimulus-induced backfiring produced by neostigmine in the mouse phrenic nerve-diaphragm. J Physiol. 1986 Mar;372:395–404. doi: 10.1113/jphysiol.1986.sp016015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., King S. C., Parsons S. M. Pharmacological characterization of the acetylcholine transport system in purified Torpedo electric organ synaptic vesicles. Mol Pharmacol. 1983 Jul;24(1):48–54. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal V., Vyskocil F., Tucek S. Decrease of the spontaneous non-quantal release of acetylcholine from the phrenic nerve in botulinum-poisoned rat diaphragm. Pflugers Arch. 1983 Jun 1;397(4):319–322. doi: 10.1007/BF00580268. [DOI] [PubMed] [Google Scholar]
- Dolly J. O., Black J., Williams R. S., Melling J. Acceptors for botulinum neurotoxin reside on motor nerve terminals and mediate its internalization. Nature. 1984 Feb 2;307(5950):457–460. doi: 10.1038/307457a0. [DOI] [PubMed] [Google Scholar]
- Edwards C., Dolezal V., Tucek S., Zemkova H., Vyskocil F. A possible role for the acetylcholine transport system in non-quantal release of acetylcholine at the rodent myoneural junction. P R Health Sci J. 1988 Aug;7(2):71–74. [PubMed] [Google Scholar]
- Edwards C., Dolezal V., Tucek S., Zemková H., Vyskocil F. Is an acetylcholine transport system responsible for nonquantal release of acetylcholine at the rodent myoneural junction? Proc Natl Acad Sci U S A. 1985 May;82(10):3514–3518. doi: 10.1073/pnas.82.10.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P. The electromotive action of acetylcholine at the motor end-plate. J Physiol. 1950 Oct 16;111(3-4):408–422. doi: 10.1113/jphysiol.1950.sp004492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimann C., Torri-Tarelli F., Fesce R., Ceccarelli B. Measurement of quantal secretion induced by ouabain and its correlation with depletion of synaptic vesicles. J Cell Biol. 1985 Nov;101(5 Pt 1):1953–1965. doi: 10.1083/jcb.101.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Does the motor nerve impulse evoke 'non-quantal' transmitter release? Proc R Soc Lond B Biol Sci. 1981 May 7;212(1186):131–137. doi: 10.1098/rspb.1981.0029. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar P. C., Oen B. S., Polak R. L., van der Laaken A. L. Surplus acetylcholine and acetylcholine release in the rat diaphragm. J Physiol. 1987 Apr;385:147–167. doi: 10.1113/jphysiol.1987.sp016489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi E. S., Vyskocil F. Changes in total and quantal release of acetylcholine in the mouse diaphragm during activation and inhibition of membrane ATPase. J Physiol. 1979 Jan;286:1–14. doi: 10.1113/jphysiol.1979.sp012603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskocil F., Illés P. Electrophysiological examination of transmitter release in non-quantal form in the mouse diaphragm and the activity of membrane ATP-ase. Physiol Bohemoslov. 1978;27(5):449–455. [PubMed] [Google Scholar]
- Vyskocil F., Illés P. Non-quantal release of transmitter at mouse neuromuscular junction and its dependence on the activity of Na+-K+ ATP-ase. Pflugers Arch. 1977 Sep 16;370(3):295–297. doi: 10.1007/BF00585542. [DOI] [PubMed] [Google Scholar]
- Vyskocil F. Inhibition of non-quantal acetylcholine leakage by 2(4-phenylpiperidine)cyclohexanol in the mouse diaphragm. Neurosci Lett. 1985 Sep 6;59(3):277–280. doi: 10.1016/0304-3940(85)90144-2. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Nikolsky E., Edwards C. An analysis of the mechanisms underlying the non-quantal release of acetylcholine at the mouse neuromuscular junction. Neuroscience. 1983 Jun;9(2):429–435. doi: 10.1016/0306-4522(83)90305-6. [DOI] [PubMed] [Google Scholar]
- Zemková H., Vyskocil F., Edwards C. A study on early post-denervation changes of non-quantal and quantal acetylcholine release in the rat diaphragm. Pflugers Arch. 1987 Aug;409(4-5):540–546. doi: 10.1007/BF00583813. [DOI] [PubMed] [Google Scholar]
- Zemková H., Vyskocil F. Effect of Mg2+ on non-quantal acetylcholine release at the mouse neuromuscular junction. Neurosci Lett. 1989 Sep 11;103(3):293–297. doi: 10.1016/0304-3940(89)90115-8. [DOI] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Changes in miniature endplate potential frequency during repetitive nerve stimulation in the presence of Ca2+, Ba2+, and Sr2+ at the frog neuromuscular junction. J Gen Physiol. 1981 May;77(5):503–529. doi: 10.1085/jgp.77.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]



