Abstract
外泌体是一种纳米级胞外囊泡,广泛存在于各种体液中,携带蛋白质、脂质、核酸等多种物质,通过参与免疫调节、细胞间信号转导、蛋白质及核酸的传输等多种方式在生物体内发挥重要作用。外泌体通过调控铁死亡介导肿瘤发展和肿瘤耐药等过程。利用外泌体运送载体的特性调控铁死亡为临床抗肿瘤治疗提供了新的思路和更多的可能性。
Keywords: 外泌体, 铁死亡, 肿瘤, 肿瘤微环境, 肿瘤耐药, 免疫调节
Abstract
Exosomes are nanoscale extracellular vesicles widely present in various body fluids. They carry a variety of substances, including proteins, lipids, and nucleic acids, and play significant roles in the body by participating in immune regulation, intercellular signal transduction, and the transport of proteins and nucleic acids. Exosomes can regulate tumor development and drug resistance by modulating ferroptosis. Leveraging the delivery capabilities of exosomes to modulate ferroptosis provides new ideas and greater possibilities for clinical anti-tumor therapies.
Keywords: exosomes, ferroptosis, tumor, tumor microenvironment, tumor drug resistance, immune regulation
外泌体是一种直径为40~160 nm的细胞外囊泡,可由多种细胞分泌,通过胞内的多泡体与细胞膜融合释放到细胞外[1]。外泌体内部可装载蛋白质、脂质、核酸等多种物质,并在血液、尿液、唾液和母乳等体液中循环[2]。巨噬细胞、肿瘤细胞、间充质干细胞(mesenchymal stem cells,MSCs)、上皮细胞、肥大细胞、内皮祖细胞和成纤维细胞等不同类型的细胞均可释放出外泌体[3]。外泌体携带与源细胞相似的脂质和蛋白质,可通过胞外作用从细胞释放到细胞外微环境中,与受体细胞相互作用,触发分子释放或诱导信号转导级联反应,最终导致细胞活性或功能的改变[4]。这些特性使其能够被多种肿瘤细胞利用并进行病理转运,参与肿瘤微环境(tumor microenvironment,TME)的建立,促进肿瘤发生;通过与肿瘤基质相互作用,促进肿瘤增殖,帮助肿瘤细胞逃避宿主免疫系统的检测和清除[5-7]。因此外泌体也是肿瘤发生和发展的重要影响因素。此外,外泌体可促进血管生成、抑制肿瘤细胞死亡、增强肿瘤细胞侵袭和转移能力[8-10]。外泌体还能增强肿瘤细胞对放射治疗和化学治疗的耐药性,从而降低肿瘤治疗效果[11]。基于在肿瘤发生和发展中的广泛作用,外泌体已成为寻找肿瘤新治疗方法的焦点[12]。
外泌体一方面促进肿瘤的发生和转移,另一方面可抑制肿瘤的进展,具有双重作用[13]。铁死亡作为一种细胞程序性死亡机制,主要特征为脂质代谢紊乱、谷胱甘肽(glutathione,GSH)失衡和铁代谢紊乱。铁死亡相关蛋白谷胱甘肽过氧化物酶4(glutathione peroxidase 4,GPX4)以GSH为底物,抑制有毒过氧化物形成无毒羟基组分,保护细胞膜的稳定性,使其正常生理功能不受过氧化物的干扰和破坏,从而抑制肿瘤细胞的铁死亡[14]。GPX4基因缺失可导致脂质过氧化,进而调控肿瘤发展中的铁死亡通路[15]。铁死亡的另一种特征是铁的积累,铁在生理过程中是必不可少的,但过量的铁对身体有害。增加细胞铁输出可以增强细胞对铁死亡的抵抗力[16-17]。此外,活性氧(reactive oxygen species,ROS)的聚集也与铁死亡密切相关。当GPX4和GSH被耗尽时,ROS的积累会产生细胞毒性,从而诱导细胞铁死亡[18]。酰基辅酶A合成酶长链家族成员4(acyl-CoA synthetase long-chain family member 4,ACSL4)在调节脂肪酸和脂质代谢中起关键作用。外泌体介导的微RNA(microRNA,miRNA)可调节ACSL4的表达,从而在铁死亡中发挥作用[19]。已有研究[20-22]表明:激活铁死亡相关通路可以有效地阻止肿瘤进展,增强化学治疗、靶向治疗甚至免疫治疗的效果。近年来,外泌体参与调控肿瘤细胞铁死亡的机制研究引起了广泛关注,外泌体与铁死亡之间的关系亟需探索,以期为肿瘤治疗提供新的策略。
1. 外泌体参与调控铁死亡介导的肿瘤发展
外泌体可通过诱导肿瘤细胞铁死亡,介导细胞免疫反应,调节TME,进而发挥抗肿瘤作用。肿瘤细胞来源外泌体(tumor cell-derived exosome,TEX)可通过将生物活性因子转运至外周血参与肿瘤的迁移,并促进肿瘤细胞逃离免疫系统的监视[23]。一些富含miRNA的TEX具有肿瘤标志物的潜力[24]。Jiang等[25]从骨肉瘤组织中提取外泌体,并通过体内、体外实验探讨外泌体中的miRNA-144-3p和E盒结合锌指蛋白1(E-box-binding zinc finger protein 1,ZEB1)对骨肉瘤的影响,结果表明miRNA-144-3p可通过负调控ZEB1的表达,诱导骨肉瘤细胞发生铁死亡,从而抑制骨肉瘤细胞的增殖、迁移和侵袭。
外泌体还可通过抑制铁死亡的发生来发挥作用。膀胱癌组织来源的外泌体通过转运miRNA-217,下调ACSL4的表达,上调GPX4、溶质载体家族7成员11(solute carrier family 7 member 11,SLC7A11)的表达,从而抑制膀胱癌T24细胞的铁死亡[26]。胰腺癌细胞分泌的外泌体中富集的天门冬氨酸转氨酶1(aspartate transaminase 1,GOT1)可抑制细胞铁死亡,促进肿瘤细胞增殖、侵袭和迁移。另有研究[27]结果显示外泌体分泌的GOT1通过上调C-C趋化因子受体2(C-C chemokine receptor 2,CCR2)表达激活核因子E2相关因子2(nuclear factor E2-realated factor 2,Nrf2)/血红素氧合酶1(heme oxygenase-1,HO-1)信号通路,抑制胰腺癌细胞铁死亡,促进胰腺癌进展。Yuan等[28]通过体外实验发现心肌细胞分泌的外泌体可抑制肿瘤细胞对铁死亡的敏感性。研究[28]进一步表明,心肌梗死可通过释放源自心肌细胞的富集miRNA-22-3p外泌体,抑制肿瘤细胞中ACSL4的表达,进而抑制铁死亡,从而促进肿瘤的生长。
TME由细胞外基质和多种间充质细胞组成[29]。肿瘤相关成纤维细胞(cancer-associated fibroblasts,CAFs)是TME中主要的基质细胞类型,可通过传递核酸、蛋白质等信号分子,在肿瘤生长、转移、免疫抑制和耐药中发挥重要作用[30-31]。赵军等[32]研究发现从CAFs提取出来的外泌体CAFs-exo可通过促进GPX4的表达,抑制Nrf2和ACSL4的表达,从而抑制铁死亡,促进前列腺癌细胞的增殖、迁移和上皮-间充质转化(epithelial-mesenchymal transition,EMT)。此外,肿瘤相关巨噬细胞(tumor associated macrophage,TAM)在促进肿瘤恶性进展和治疗抵抗中发挥关键作用。Luo等[33]发现TAM可抑制宫颈癌细胞铁死亡,其具体机制为巨噬细胞来源的miRNA-660-5p被包装在外泌体中并运输到宫颈癌细胞中,下调花生四烯酸15-脂氧合酶(arachidonate acid 15-lipoxygenase,ALOX15)的表达以抑制宫颈癌细胞铁死亡。由此可见,外泌体通过参与调控脂质代谢途径来抑制肿瘤细胞铁死亡。此外,巨噬细胞中miRNA-660-5p的上调依赖于自分泌白细胞介素(interleukin-4,IL)-4/IL-13激活的信号转导及转录激活因子6(signal transducers and activators of transcription 6,STAT6)通路。鼻咽癌细胞源性外泌体通过巨噬细胞移动抑制因子(macrophage migration inhibition factor,MIF)增加GPX4的表达,且GPX4的表达与MIF呈正相关。MIF在鼻咽癌细胞中高表达,且由鼻咽癌细胞分泌的外泌体可被巨噬细胞摄取,从而抑制巨噬细胞的铁死亡,促进鼻咽癌的转移[34]。MSCs是存在于人体组织中的多能基质细胞。研究[13]表明:MSCs分泌的外泌体作为TME中的介质,在肿瘤发生、血管生成和转移中发挥作用。例如,来自MSCs的外泌体可将miRNA-424转移到肺癌细胞中,从而下调ACSL4的表达并抑制铁死亡[19]。以上研究提示,靶向外泌体介导的肿瘤病理通讯可能为基于铁死亡的抗肿瘤治疗提供一条新的途径。
有研究[35]发现:肺腺癌患者血浆中的外泌体cir93可特异地降低脂质过氧化水平,使肺腺癌细胞对铁死亡敏感性下降。其机制为外泌体cir93与脂肪酸结合蛋白3(fatty acid binding protein 3,FABP3)相互作用,促进花生四烯酸(arachidonic acid,AA)与牛磺酸反应,进而诱导N-花生四烯酸牛磺酸(N-arachidonoyl taurine,NAT)的产生,使肺腺癌细胞对铁死亡脱敏。因此外泌体cir93是抑制肺腺癌细胞铁死亡敏感性的关键,阻断外泌体可能有助于未来肺腺癌的治疗。
2. 外泌体参与调控铁死亡介导的肿瘤耐药
化学治疗是治疗晚期肿瘤的主要方法,其耐药性一直是肿瘤治疗的主要挑战。对化学治疗耐药通常与DNA损伤修复、调节细胞凋亡的分子突变和GSH表达水平升高有关[36-38]。肝细胞癌靶向外泌体(ExoSP94-Lamp2b-RRM)可以特异性地将多种干扰小RNA传递到肝细胞癌组织,通过下调GPX4和二氢乳清酸脱氢酶(dihydroorotate dehydrogenase,DHODH)表达,促进索拉非尼诱导的铁死亡,从而提高肝细胞癌对索拉非尼的敏感性。这一发现在铁死亡的角度上为临床克服索拉非尼耐药开辟了新的途径[39]。
源自CAFs的外泌体介导的miRNA可促进肿瘤转移并增加肿瘤细胞对化学治疗的耐药性[40]。在探究外泌体介导的miRNA调节胃癌细胞铁死亡的实验研究[41]中发现:顺铂和紫杉醇通过激活泛素特异性蛋白酶7(ubiquitin-specific protease 7,USP7)/异质性核糖核蛋白A1(heterogeneous nuclear ribonucleoprotein A1,hnRNPA1)轴促进CAFs中miRNA-522的分泌,抑制ALOX15的表达,并减少胃癌细胞中脂质ROS积累,进而导致胃癌细胞对化学治疗敏感性降低。研究[41]结果表明:CAFs通过分泌外泌体miRNA-522,靶向ALOX15并阻断脂质ROS积累,从而抑制胃癌细胞铁死亡。外泌体可通过影响脂质ROS水平调控铁死亡的发生。Qu等[42]研究发现外泌体DACT3-AS1通过靶向miRNA-181a-5p/sirtuin 1(SIRT1)轴抑制胃癌细胞增殖、迁移和侵袭。此外,DACT3-AS1主要通过外泌体从CAFs传递到胃癌细胞[42]。体外和体内实验结果均表明,DACT3-AS1通过SIRT1介导的铁死亡使胃癌细胞对奥沙利铂敏感[42]。在肺癌中,外泌体miRNA-4443的高表达可通过负调控甲基转移酶样蛋白3(methyltransferase-like 3,METTL3)诱导的铁死亡抑制蛋白1(ferroptosis suppressor protein 1,FSP1)的m6A修饰,抑制顺铂诱导的铁死亡,导致肺癌对顺铂的耐药[43]。体外研究[43]亦表明,miRNA-4443的过表达抑制了顺铂治疗诱导的FSP1介导的铁死亡,并促进了体内肿瘤的生长。Qi等[44]研究发现:胰腺导管腺癌中CAFs来源的外泌体miRNA在基线时不表现出先天的吉西他滨抗性;但在吉西他滨治疗后,CAFs通过分泌外泌体miRNA和与肿瘤细胞的信号交流,促进胰腺导管腺癌细胞对化学治疗的耐药。具体机制涉及CAFs来源的外泌体miRNA-3173-5p通过海绵化ACSL4抑制肿瘤细胞铁死亡[44]。
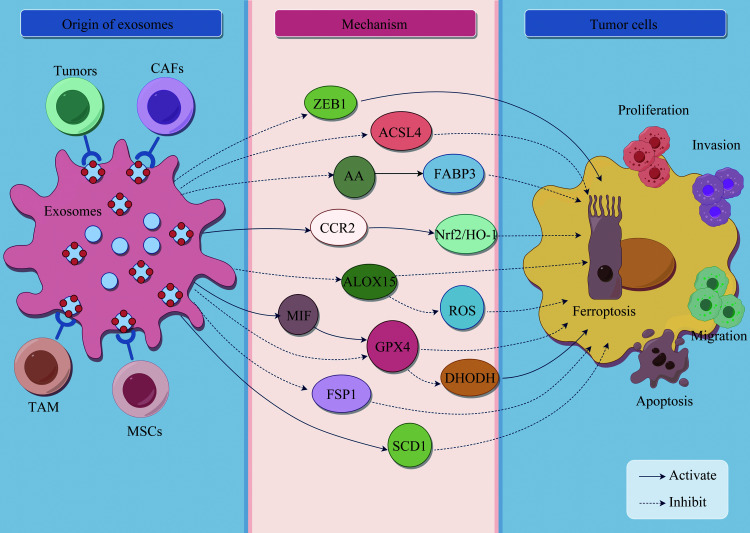
肿瘤干细胞(cancer stem cells,CSCs)是一种具有自我更新和不对称分裂特性的肿瘤细胞亚群,与胃癌患者高病死率和治疗后肿瘤复发有关,是肿瘤复发和耐药的重要原因[45-46]。来自胃癌细胞的外泌体lncFERO与硬脂酰-辅酶A去饱和酶1(stearoyl-CoA desaturase 1,SCD1)的表达呈正相关,并与脂质过氧化物呈负相关[47]。研究[47]结果表明,外泌体lncFERO的分泌可促进SCD1的表达,从而抑制CSCs铁死亡,降低胃癌对化学治疗的敏感性。外泌体调控肿瘤细胞铁死亡的作用及其机制总结见图1、表1。
图1.
外泌体调控肿瘤细胞铁死亡的机制
Figure 1 Mechanism of exosome regulation of tumor ferroptosis This figure was drawn by Figdraw. ZEB1: E-box-binding zinc finger protein 1; ACSL4: Acyl-CoA synthetase long-chain family member 4; AA: Arachidonic acid; FABP3: Fatty acid binding protein 3; CCR2: C-C chemokine receptor 2; Nrf2: Nuclear factor-E2-related factor 2; HO-1: Heme oxygenase-1; ALOX15: Arachidonate acid 15-lipoxygenase; MIF: Macrophage migration inhibition factor; ROS: Reactive oxygen species; GPX4: Glutathione peroxidase 4; DHODH: Dihydroorotate dehydrogenase; FSP1: Ferroptosis suppressor protein 1; TAM: Tumor associated macrophages; MSCs: Mesenchymal stem cells; SCD1: Stearoyl-CoA desaturase 1.
表1.
外泌体调控肿瘤细胞铁死亡的作用及其机制总结
Table 1 Summary of the role of exosomes in regulating ferroptosis in tumor cells and its mechanisms
| 外泌体 | 肿瘤类型 | 作用机制 |
调控 铁死亡 |
对肿瘤的影响 | 文献 |
|---|---|---|---|---|---|
| MiRNA-144-3p | 骨肉瘤 | ZEB1↓ | 诱导 | 抑制细胞增殖、迁移和侵袭 | [25] |
| MiRNA-217 | 膀胱癌 |
ACSL4↓,GPX4↑, SLC7A11↑ |
抑制 | 抑制细胞凋亡 | [26] |
| GOT1 | 胰腺癌 | CCR2↑,Nrf2/HO-1轴↑ | 抑制 | 促进肿瘤生长 | [27] |
| MiRNA-3173-5p | 胰腺癌 | ACSL4↓ | 抑制 | 增强对吉西他滨的耐药性 | [44] |
| MiRNA-22-3p | 肺癌、骨肉瘤 | ACSL4↓ | 抑制 | 促进肿瘤生长 | [28] |
| CAFs-exo | 前列腺癌 | GPX4↑,Nrf2↓,ACSL4↓ | 抑制 | 促进细胞增殖、迁移和EMT | [32] |
| MiRNA-660-5p | 宫颈癌 | ALOX15↓ | 抑制 | 抑制细胞死亡 | [33] |
| Cir93 | 肺腺癌 | AA↓,NAT↑ | 抑制 | 使细胞对铁死亡脱敏 | [35] |
| ExoSP94-Lamp2b-RRM | 肝细胞癌 | GPX4↓,DHODH↓ | 促进 | 增强对索拉非尼的敏感性 | [39] |
| MiRNA-522 | 胃癌 | ALOX15↓,ROS↓ | 抑制 | 降低对顺铂和紫杉醇化学治疗的敏感性 | [41] |
| DACT3-AS1 | 胃癌 | SIRT1↑ | 诱导 | 增强对奥沙利铂的敏感性 | [42] |
| LncFERO | 胃癌 | SCD1↑ | 抑制 | 降低化学治疗敏感性 | [47] |
| MiRNA-4443 | 肺癌 | FSP1的m6A修饰↓ | 抑制 | 促进肿瘤生长,增强对顺铂的耐药性 | [43] |
↑:上调或激活;↓:下调或抑制。ZEB1:E盒结合锌指蛋白1;ACSL4:酰基辅酶A合成酶长链家族成员4;GPX4:谷胱甘肽过氧化物酶4;SLC7A11:溶质载体家族7成员11;GOT1:天门冬氨酸转氨酶1;CCR2:C-C趋化因子受体2;Nrf2:核因子E2相关因子2;HO-1:血红素氧合酶1;EMT:上皮-间充质转化;ALOX15:花生四烯酸15-脂氧合酶;AA:花生四烯酸;NAT:N-花生四烯酸牛磺酸;DHODH:二氢乳清酸脱氢酶;ROS:活性氧;SIRT1:沉默调节蛋白1;SCD1:硬脂酰-辅酶A去饱和酶1;FSP1:铁死亡抑制蛋白1。
3. 外泌体调控铁死亡在肿瘤中的应用
外泌体作为药物传递载体可与细胞膜相互作用并将药物输送到细胞中[48-49]。工程化外泌体具有巨大的治疗潜力。Du等[50]研究发现由CD47、铁死亡诱导剂erastin和Rose Bengal组成的工程化外泌体在肝细胞癌中可诱导显著的铁死亡,且对肝、肾的毒性很小。针对铁死亡的叶酸(folic acid,FA)修饰外泌体可用于临床应用。Yu等[51]通过超声波将erastin负载到FA标志的外泌体中,形成装载erastin的FA矢量外泌体(erastin@FA‐exo),以靶向FA受体过表达的三阴性乳腺癌细胞。结果表明:erastin@FA‐exo可比erastin@exo和free erastin更有效地抑制GPX4和上调半胱氨酸双加氧酶1(cysteine dioxygenase type 1,CDO1)的表达,同时erastin@FA‐exo可明显降低三阴性乳腺癌细胞MDA-MB-231的线粒体膜电位,抑制肿瘤Warburg现象,促进肿瘤细胞中氧自由基的生成[51]。总之,erastin@FA‐exo可以主动和选择性地靶向MDA-MB-231细胞,并有效地诱导肿瘤细胞铁死亡。这为外泌体用于治疗类型的恶性肿瘤提供了一种有希望的策略。
在胶质母细胞瘤中,血脑屏障(blood brain barrier,BBB)药物渗透性差一直是影响肿瘤治疗效果的主要障碍。通过基因工程化手段修饰,携带angiopep-2多肽的外泌体不仅具有穿透BBB的能力,还可通过识别低密度脂蛋白受体相关蛋白1(low density lipoprotein receptor related protein 1,LRP1)受体并靶向胶质母细胞瘤细胞,从而增强铁死亡[52]。Hu等[31]开发了一种可同时靶向肿瘤实质细胞和基质细胞且易于大量制备的外泌体样肿瘤疫苗,即成纤维细胞活化蛋白(fibroblast activation protein,FAP)基因工程化肿瘤细胞源性外泌体样纳米囊泡(eNVs-FAP)。该疫苗在结肠癌、黑色素瘤、肺癌、乳腺癌等多种荷瘤模型小鼠中均有抗肿瘤作用[31]。实验结果表明,针对肿瘤细胞和CAFs的eNVs-FAP通过募集效应T细胞和减少免疫抑制细胞来抑制肿瘤生长,而且eNVs-FAP诱导的抗肿瘤免疫可促进肿瘤细胞铁死亡[31]。肿瘤疫苗与铁死亡诱导剂联合使用可增强抗肿瘤的效果。外泌体样肿瘤疫苗与铁死亡诱导剂联合用于肿瘤治疗具有良好的临床应用潜力。
免疫抑制性肿瘤微环境(immunosuppressive tumor microenvironment,ITM)是影响临床免疫治疗的一个障碍。程序性死亡受体配体1(programmed death-ligand 1,PD-L1)分泌过多可导致程序性死亡受体1(programmed death-1,PD-1)/PD-L1免疫治疗的耐药和临床失败。抗PD-L1治疗可恢复T细胞功能,诱导恶性黑色素瘤细胞铁死亡,从而改善ITM[53]。Wang等[54]基于PD-L1与肿瘤细胞铁死亡之间的关系,开发了HACAFe@GW4869纳米颗粒,该纳米颗粒将外泌体抑制剂(GW4869)与铁死亡诱导剂(Fe3+)结合在一起,以刺激黑色素瘤细胞的抗肿瘤反应。研究[54]结果表明:GW4869可抑制PD-L1的分泌,从而触发T细胞活化并促进干扰素γ(interferon-γ,IFN-γ)的分泌。IFN-γ通过抑制肿瘤细胞中的SLC7A11和SLC3A2的表达,降低胱氨酸和GSH水平,抑制GPX4表达,从而促进黑色素瘤细胞铁死亡。
纳米囊泡可能会遭受挤压而引起结构损伤并导致部分物质和功能损失,与之相比,工程化的巨噬细胞外泌体可保留更多的功能和遗传物质。Wang等[55]成功构建装载铁死亡诱导剂RSL3的M1型巨噬细胞来源性外泌体RSL3-Exo,RSL3-Exo中的遗传物质来自M1型巨噬细胞。体内和体外实验[55]结果表明:RSL3-Exo可自发归巢到肿瘤并在肿瘤中蓄积,且能显著促进致瘤的M2型巨噬细胞向抗肿瘤的M1型巨噬细胞转化,减少调节性T细胞数目,以减轻ITM。RSL3-Exo通过增强系统免疫反应和调节铁死亡相关蛋白的表达促进铁死亡,抑制肿瘤进展。生物相容性M1-Exo工程策略可以成功地消除肿瘤免疫抑制,加速免疫激活的乳腺肿瘤细胞的铁死亡。外泌体调控铁死亡在肿瘤中的应用见表2。
表2.
外泌体调控铁死亡在肿瘤中的应用研究
Table 2 Application study of exosome-regulated ferroptosis in tumor diseases
| 外泌体应用方式 | 肿瘤类型 | 作用机制 | 优势 | 文献 |
|---|---|---|---|---|
| Erastin@FA‐exo | 乳腺癌 | GPX4↓,CDO1↑ |
主动和选择性地靶向肿瘤细胞, 有效地诱导细胞铁死亡 |
[51] |
| 携带angiopep-2多肽的外泌体 | 胶质母细胞瘤 | 穿透BBB,识别LRP1 | 精准定位,靶向胶质母细胞瘤细胞,增强铁死亡 | [52] |
| eNVs-FAP | 肠癌、黑色素瘤、肺癌、乳腺癌等 |
募集效应T细胞和降低TME中 免疫抑制细胞的数目 |
同时靶向肿瘤实质细胞和间质细胞,诱导抗肿瘤免疫,促进肿瘤细胞 铁死亡 |
[31] |
|
HACAFe@GW4869 纳米颗粒 |
黑色素瘤 |
IFN-γ↑,SLC7A11↓、SLC3A2↓、 胱氨酸↓、GSH↓、GPX4↓ |
弥补游离抗体局限性,增强全身 免疫反应 |
[54] |
| RSL3-Exo | 乳腺癌 | 减少调节性T细胞数目、ITM↓ | 生物安全性较高,改善ITM,加速激活免疫反应 | [55] |
↑:上调或激活;↓:下调或抑制。FA:叶酸;GPX4:谷胱甘肽过氧化物酶4;CDO1:半胱氨酸双加氧酶1;IFN-γ:干扰素γ;SLC7A11:溶质载体家族7成员11;SLC3A2:溶质载体家族3成员2;GSH:谷胱甘肽;GPX4:谷胱甘肽过氧化物酶4;RSL3-Exo:铁死亡诱导剂RSL3的M1型巨噬细胞来源性外泌体;ITM:免疫抑制肿瘤微环境;BBB:血脑屏障;LRP1:低密度脂蛋白受体相关蛋白1;FAP:成纤维细胞活化蛋白。
综上所述,不同的组织源性的外泌体通过不同途径实现对铁死亡的诱导或抑制。外泌体通过传递不同的生物活性物质促进或抑制肿瘤细胞的铁死亡,进而调节肿瘤细胞增殖、肿瘤免疫反应和TME。外泌体作为一种药物传递系统已成为当前研究的热门课题。目前对装载抗癌药物靶向铁死亡的外泌体的研究有限,未来可能会开发出针对不同铁死亡途径的其他外泌体介导的抗肿瘤药物。
4. 结语与展望
目前关于外泌体参与调控铁死亡在肿瘤中的研究仍有不足之处。例如,部分实验样本量相对较小且多是动物模型和细胞实验,需要进一步临床试验来验证研究结果的准确性;一些临床特征(如肿瘤分期)对铁死亡调控的影响尚未得到证实;将外泌体应用于疾病的不同阶段尚需要更全面、更深层次的基础和临床研究支持。此外,外泌体参与调控铁死亡的上游、下游机制尚未完全明确,并且外泌体应用于肿瘤的相关文献报道较少,其优势与缺点也尚未完全明确。尽管如此,利用外泌体的运送载体特性调控铁死亡为临床抗肿瘤治疗提供了新的思路和更多的可能性,该研究方向具有较大的研究价值和应用潜力。未来需要开展更多的深入研究,进一步揭示和阐述外泌体、铁死亡与肿瘤之间的关系,以获得更全面的外泌体参与铁死亡的分子机制,为其更好地应用于临床提供研究基础。
基金资助
国家自然科学基金(81402344);陕西省自然科学基础研究计划项目(2023-JC-YB-745)。This work was supported by the National Natural Science Foundation (81402344) and the Natural Science Basic Research Program of Shaanxi Province (2023-JC-YB-745), China.
利益冲突声明
作者声称无任何利益冲突。
作者贡献
徐瑞雪 论文撰写与修改;王宇 论文指导与修改。所有作者阅读并同意最终的文本。
Footnotes
http://dx.chinadoi.cn/
原文网址
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/2024101683.pdf
参考文献
- 1. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes[J/OL]. Science, 2020, 367(6478): eaau6977[2023-12-20]. 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang YX, Liu JB, Ma JF, et al. Exosomal circRNAs: biogenesis, effect and application in human diseases[J]. Mol Cancer, 2019, 18(1): 116. 10.1186/s12943-019-1041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song H, Liu B, Dong B, et al. Exosome-based delivery of natural products in cancer therapy[J]. Front Cell Dev Biol, 2021, 9: 650426. 10.3389/fcell.2021.650426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munich S, Sobo-Vujanovic A, Buchser WJ, et al. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands[J]. Oncoimmunology, 2012, 1(7): 1074-1083. 10.4161/onci.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raimondi L, de Luca A, Gallo A, et al. Osteosarcoma cell-derived exosomes affect tumor microenvironment by specific packaging of microRNAs[J]. Carcinogenesis, 2020, 41(5): 666-677. 10.1093/carcin/bgz130. [DOI] [PubMed] [Google Scholar]
- 6. Endo-Munoz L, Cai N, Cumming A, et al. Progression of osteosarcoma from a non-metastatic to a metastatic phenotype is causally associated with activation of an autocrine and paracrine uPA axis[J/OL]. PLoS One, 2015, 10(8): e0133592[2023-12-20]. 10.1371/journal.pone.0133592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Milane L, Singh A, Mattheolabakis G, et al. Exosome mediated communication within the tumor microenvironment[J]. J Control Release, 2015, 219: 278-294. 10.1016/j.jconrel.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 8. Li S, Li JL, Zhang HY, et al. Gastric cancer derived exosomes mediate the delivery of circRNA to promote angiogenesis by targeting miR-29a/VEGF axis in endothelial cells[J]. Biochem Biophys Res Commun, 2021, 560: 37-44. 10.1016/j.bbrc.2021.04.099. [DOI] [PubMed] [Google Scholar]
- 9. Zeng QX, Zhu ZY, Song LJ, et al. Transferred by exosomes-derived miR-19b-3p targets PTEN to regulate esophageal cancer cell apoptosis, migration and invasion[J/OL]. Biosci Rep, 2020, 40(11): BSR20201858[2023-12-20]. 10.1042/BSR20201858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang SS, Ma S, Dou H, et al. Breast cancer-derived exosomes regulate cell invasion and metastasis in breast cancer via miR-146a to activate cancer associated fibroblasts in tumor microenvironment[J]. Exp Cell Res, 2020, 391(2): 111983. 10.1016/j.yexcr.2020.111983. [DOI] [PubMed] [Google Scholar]
- 11. Hu JL, Wang W, Lan XL, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer[J]. Mol Cancer, 2019, 18(1): 91. 10.1186/s12943-019-1019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou YJ, Zhang Y, Gong H, et al. The role of exosomes and their applications in cancer[J]. Int J Mol Sci, 2021, 22(22): 12204. 10.3390/ijms222212204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vakhshiteh F, Atyabi F, Ostad SN. Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy[J]. Int J Nanomedicine, 2019, 14: 2847-2859. 10.2147/IJN.S200036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4[J]. Cell, 2014, 156(1/2): 317-331. 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Angeli JPF, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice[J]. Nat Cell Biol, 2014, 16(12): 1180-1191. 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao MH, Monian P, Quadri N, et al. Glutaminolysis and transferrin regulate ferroptosis[J]. Mol Cell, 2015, 59(2): 298-308. 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tuo QZ, Lei P, Jackman KA, et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke[J]. Mol Psychiatry, 2017, 22(11): 1520-1530. 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- 18. Zuo YB, Zhang YF, Zhang R, et al. Ferroptosis in cancer progression: role of noncoding RNAs[J]. Int J Biol Sci, 2022, 18(5): 1829-1843. 10.7150/ijbs.66917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dai SM, Li FJ, Long HZ, et al. Relationship between miRNA and ferroptosis in tumors[J]. Front Pharmacol, 2022, 13: 977062. 10.3389/fphar.2022.977062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo JP, Xu BF, Han Q, et al. Ferroptosis: a novel anti-tumor action for cisplatin[J]. Cancer Res Treat, 2018, 50(2): 445-460. 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun XF, Niu XH, Chen RC, et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis[J]. Hepatology, 2016, 64(2): 488-500. 10.1002/hep.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang WM, Green M, Choi JE, et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy[J]. Nature, 2019, 569(7755): 270-274. 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stefanius K, Servage K, Orth K. Exosomes in cancer development[J]. Curr Opin Genet Dev, 2021, 66: 83-92. 10.1016/j.gde.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 24. Kumar D, Gupta D, Shankar S, et al. Biomolecular characterization of exosomes released from cancer stem cells: possible implications for biomarker and treatment of cancer[J]. Oncotarget, 2015, 6(5): 3280-3291. 10.18632/oncotarget.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang MY, Jike YJ, Liu KC, et al. Exosome-mediated miR-144-3p promotes ferroptosis to inhibit osteosarcoma proliferation, migration, and invasion through regulating ZEB1[J]. Mol Cancer, 2023, 22(1): 113. 10.1186/s12943-023-01804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang ZM, Wang H, Ji ZG. Bladder cancer tissue-derived exosomes suppress ferroptosis of T24 bladder cancer cells by transporting miR-217[J]. Environ Mol Mutagen, 2023, 64(1): 39-49. 10.1002/em.22520. [DOI] [PubMed] [Google Scholar]
- 27. Guo Y, Chen TY, Liang XY, et al. Tumor cell derived exosomal GOT1 suppresses tumor cell ferroptosis to accelerate pancreatic cancer progression by activating Nrf2/HO-1 axis via upregulating CCR2 expression[J]. Cells, 2022, 11(23): 3893. 10.3390/cells11233893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan Y, Mei ZT, Qu ZZ, et al. Exosomes secreted from cardiomyocytes suppress the sensitivity of tumor ferroptosis in ischemic heart failure[J]. Signal Transduct Target Ther, 2023, 8(1): 121. 10.1038/s41392-023-01336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson ARA, Weaver AM, Cummings PT, et al. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment[J]. Cell, 2006, 127(5): 905-915. 10.1016/j.cell.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 30. Ligorio M, Sil S, Malagon-Lopez J, et al. Stromal microenvironment shapes the intratumoral architecture of pancreatic cancer[J/OL]. Cell, 2019, 178(1): 160-175. e27[2023-12-20]. 10.1016/j.cell.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu SC, Ma JH, Su C, et al. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis[J]. Acta Biomater, 2021, 135: 567-581. 10.1016/j.actbio.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 32. 赵军, 毛亮, 沈继杰, 等. 癌相关成纤维细胞外泌体通过抑制铁死亡促进前列癌恶性侵袭[J]. 中国肿瘤外科杂志, 2023, 15(2): 133-140. 10.3969/j.issn.1674-4136.2023.02.006. [DOI] [Google Scholar]; ZHAO Jun, MAO Liang, SHEN Jijie, et al. Cancer-associated fibroblast-derived exosomes promote malignant invasion of prostate cancer through inhibition of ferroptosis[J]. Chinese Journal of Surgical Oncology, 2023, 15(2): 133-140. 10.3969/j.issn.1674-4136.2023.02.006. [DOI] [Google Scholar]
- 33. Luo YL, Chen YB, Jin H, et al. The suppression of cervical cancer ferroptosis by macrophages: the attenuation of ALOX15 in cancer cells by macrophages-derived exosomes[J]. Acta Pharm Sin B, 2023, 13(6): 2645-2662. 10.1016/j.apsb.2023.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen WH, Zuo F, Zhang KW, et al. Exosomal MIF derived from nasopharyngeal carcinoma promotes metastasis by repressing ferroptosis of macrophages[J]. Front Cell Dev Biol, 2021, 9: 791187. 10.3389/fcell.2021.791187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. 张骁, 徐云华, 马丽芳, 等. 外泌体和circRNA_101093在抑制肺腺癌铁死亡敏感性中的重要作用[J]. 癌症, 2023, 42(2): 63-89. [Google Scholar]; ZHANG Xiao, XU Yunhua, MA Lifang, et al. The important role of exosomes and circRNA_101093 in inhibiting iron death sensitivity of lung adenocarcinoma[J]. Chinese Journal of Cancer, 2023, 42(2): 63-89. [Google Scholar]
- 36. Rebollido-Rios R, Venton G, Sánchez-Redondo S, et al. Dual disruption of aldehyde dehydrogenases 1 and 3 promotes functional changes in the glutathione redox system and enhances chemosensitivity in nonsmall cell lung cancer[J]. Oncogene, 2020, 39(13): 2756-2771. 10.1038/s41388-020-1184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Silva MM, Rocha CRR, Kinker GS, et al. The balance between NRF2/GSH antioxidant mediated pathway and DNA repair modulates cisplatin resistance in lung cancer cells[J]. Sci Rep, 2019, 9(1): 17639. 10.1038/s41598-019-54065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. 李欣, 杨作成. 外源性外泌体在细胞凋亡中的调控作用[J]. 中南大学学报(医学版), 2017, 42(2): 215-220. 10.11817/j.issn.1672-7347.2017.02.016. [DOI] [PubMed] [Google Scholar]; LI Xin, YANG Zuocheng. Regulatory effect of exosome on cell apoptosis[J]. Journal of Central South University. Medical Science, 2017, 42(2): 215-220. 10.11817/j.issn.1672-7347.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 39. Li XJ, Yu QQ, Zhao RZ, et al. Designer exosomes for targeted delivery of a novel therapeutic cargo to enhance sorafenib-mediated ferroptosis in hepatocellular carcinoma[J]. Front Oncol, 2022, 12: 898156. 10.3389/fonc.2022.898156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qin X, Guo HY, Wang XN, et al. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5[J]. Genome Biol, 2019, 20(1): 12. 10.1186/s13059-018-1604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang HY, Deng T, Liu R, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer[J]. Mol Cancer, 2020, 19(1): 43. 10.1186/s12943-020-01168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qu XL, Liu B, Wang LG, et al. Loss of cancer-associated fibroblast-derived exosomal DACT3-AS1 promotes malignant transformation and ferroptosis-mediated oxaliplatin resistance in gastric cancer[J]. Drug Resist Updat, 2023, 68: 100936. 10.1016/j.drup.2023.100936. [DOI] [PubMed] [Google Scholar]
- 43. Song ZY, Jia G, Ma PZ, et al. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis[J]. Life Sci, 2021, 276: 119399. 10.1016/j.lfs.2021.119399. [DOI] [PubMed] [Google Scholar]
- 44. Qi R, Bai YX, Li K, et al. Cancer-associated fibroblasts suppress ferroptosis and induce gemcitabine resistance in pancreatic cancer cells by secreting exosome-derived ACSL4-targeting miRNAs[J]. Drug Resist Updat, 2023, 68: 100960. 10.1016/j.drup.2023.100960. [DOI] [PubMed] [Google Scholar]
- 45. Giraud J, Molina-Castro S, Seeneevassen L, et al. Verteporfin targeting YAP1/TAZ-TEAD transcriptional activity inhibits the tumorigenic properties of gastric cancer stem cells[J]. Int J Cancer, 2020, 146(8): 2255-2267. 10.1002/ijc.32667. [DOI] [PubMed] [Google Scholar]
- 46. Yang LQ, Shi PF, Zhao GC, et al. Targeting cancer stem cell pathways for cancer therapy[J]. Signal Transduct Target Ther, 2020, 5(1): 8. 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang HY, Wang M, He Y, et al. Chemotoxicity-induced exosomal lncFERO regulates ferroptosis and stemness in gastric cancer stem cells[J]. Cell Death Dis, 2021, 12(12): 1116. 10.1038/s41419-021-04406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles[J]. Trends Cell Biol, 2015, 25(6): 364-372. 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 49. Yu DD, Wu Y, Shen HY, et al. Exosomes in development, metastasis and drug resistance of breast cancer[J]. Cancer Sci, 2015, 106(8): 959-964. 10.1111/cas.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Du JB, Wan Z, Wang C, et al. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy[J]. Theranostics, 2021, 11(17): 8185-8196. 10.7150/thno.59121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yu MY, Gai CC, Li Z, et al. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells[J]. Cancer Sci, 2019, 110(10): 3173-3182. 10.1111/cas.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li BY, Chen X, Qiu W, et al. Synchronous disintegration of ferroptosis defense axis via engineered exosome-conjugated magnetic nanoparticles for glioblastoma therapy[J/OL]. Adv Sci, 2022, 9(17): e2105451[2023-12-20]. 10.1002/advs.202105451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xie LS, Li J, Wang GH, et al. Phototheranostic metal-phenolic networks with antiexosomal PD-L1 enhanced ferroptosis for synergistic immunotherapy[J]. J Am Chem Soc, 2022, 144(2): 787-797. 10.1021/jacs.1c09753. [DOI] [PubMed] [Google Scholar]
- 54. Wang GH, Xie LS, Li B, et al. A nanounit strategy reverses immune suppression of exosomal PD-L1 and is associated with enhanced ferroptosis[J]. Nat Commun, 2021, 12(1): 5733. 10.1038/s41467-021-25990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang D, Qiu GH, Zhu XQ, et al. Macrophage-inherited exosome excise tumor immunosuppression to expedite immune-activated ferroptosis[J/OL]. J Immunother Cancer, 2023, 11(5): e006516[2023-12-20]. 10.1136/jitc-2022-006516. [DOI] [PMC free article] [PubMed] [Google Scholar]