Abstract
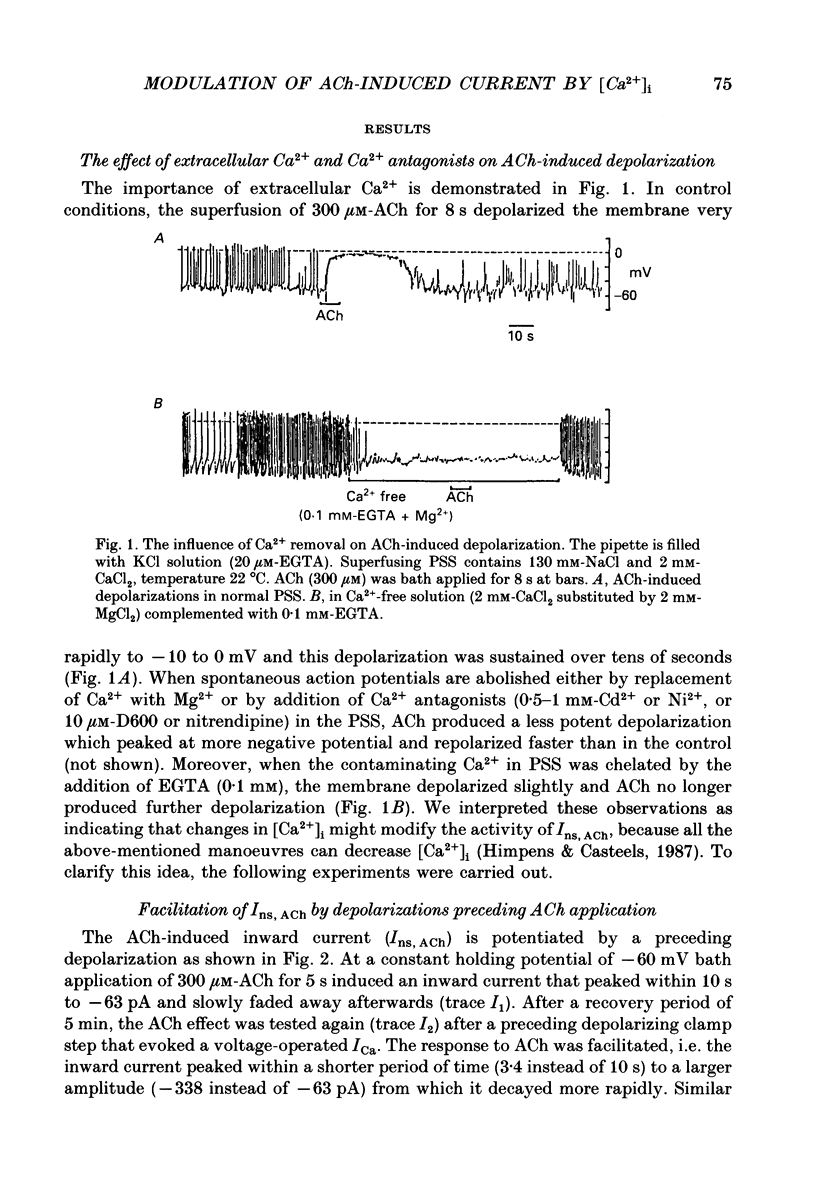
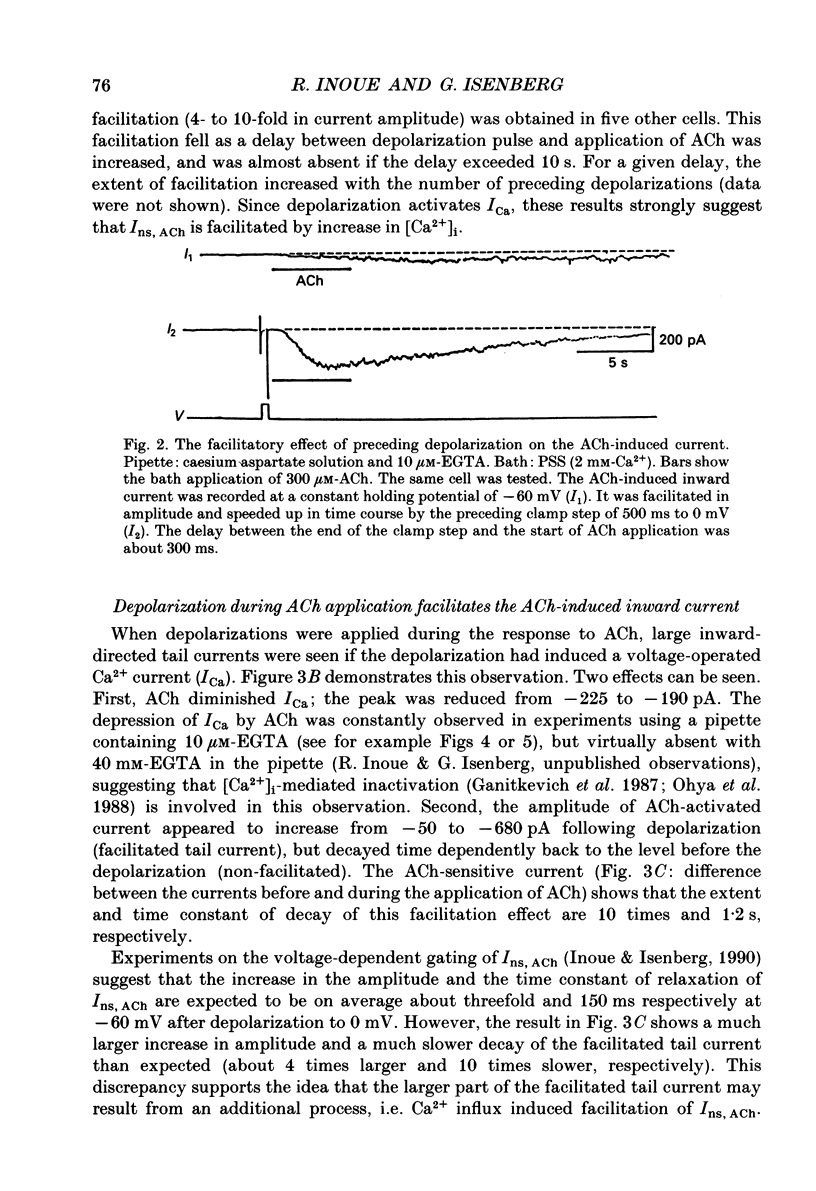
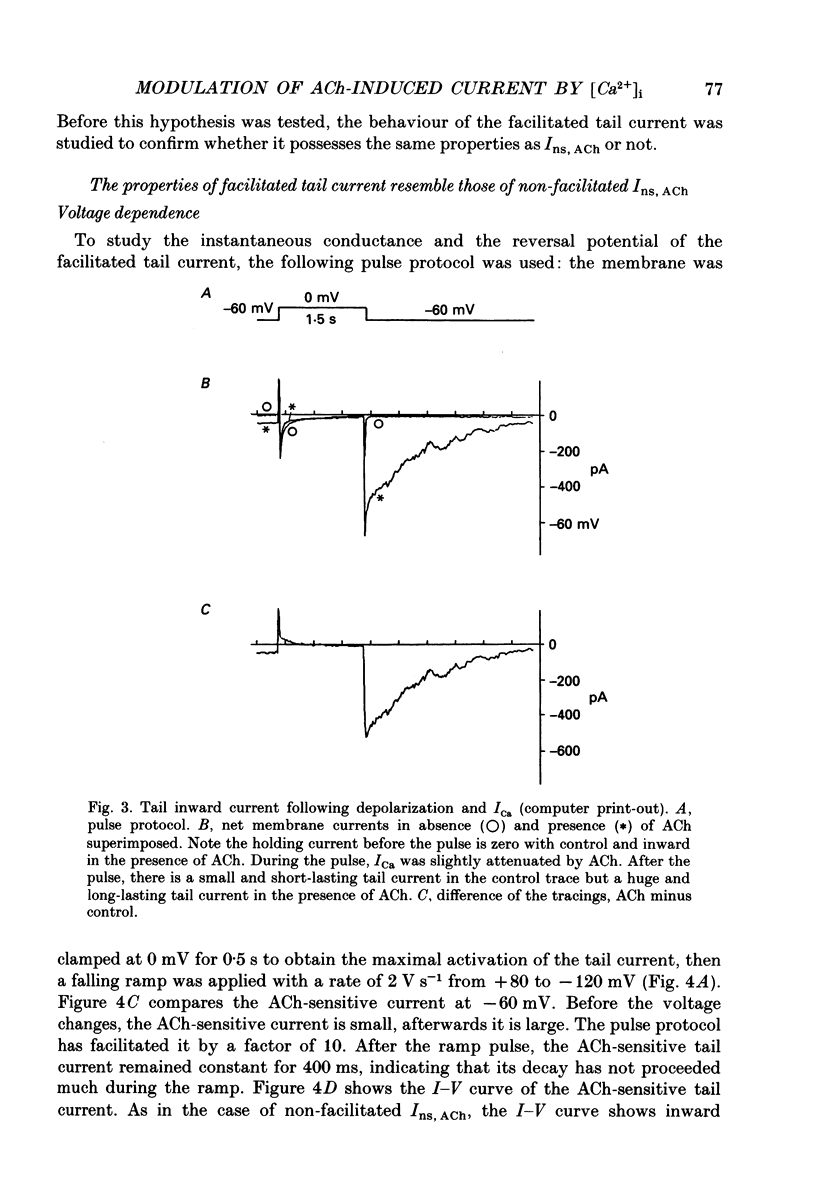
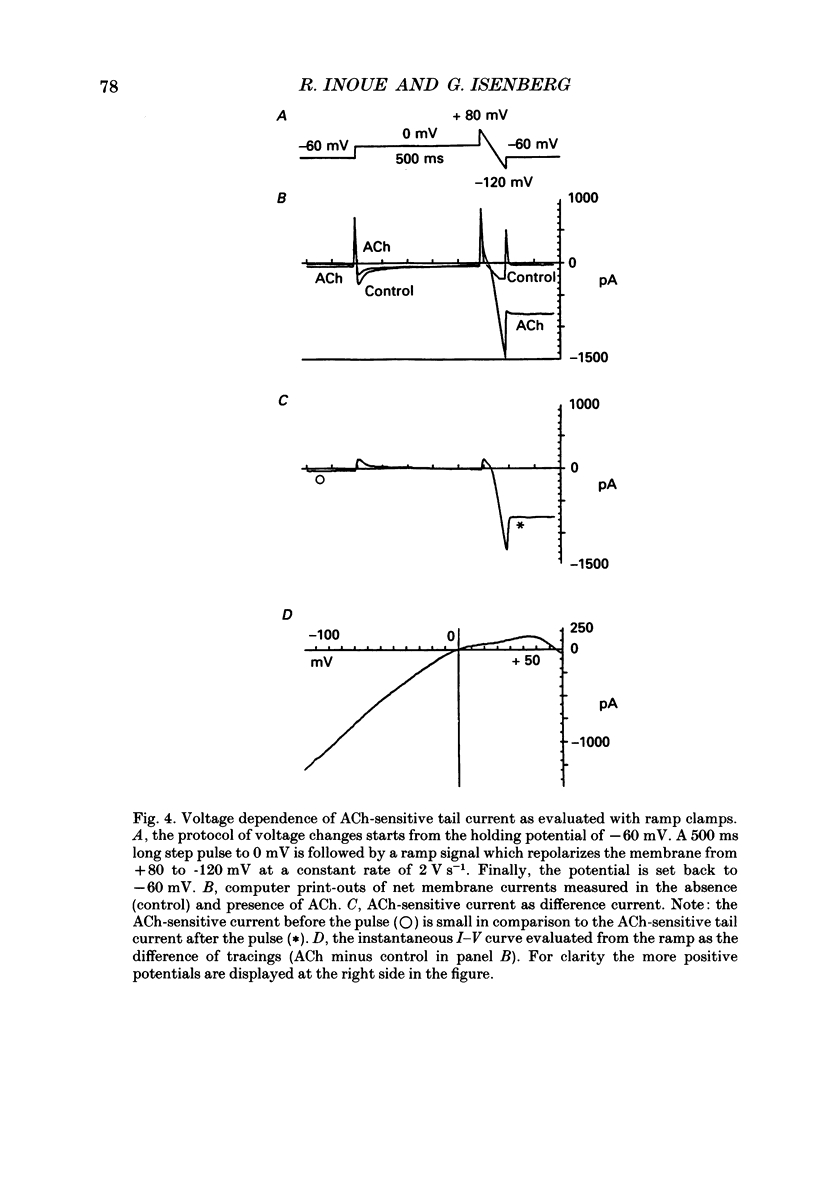
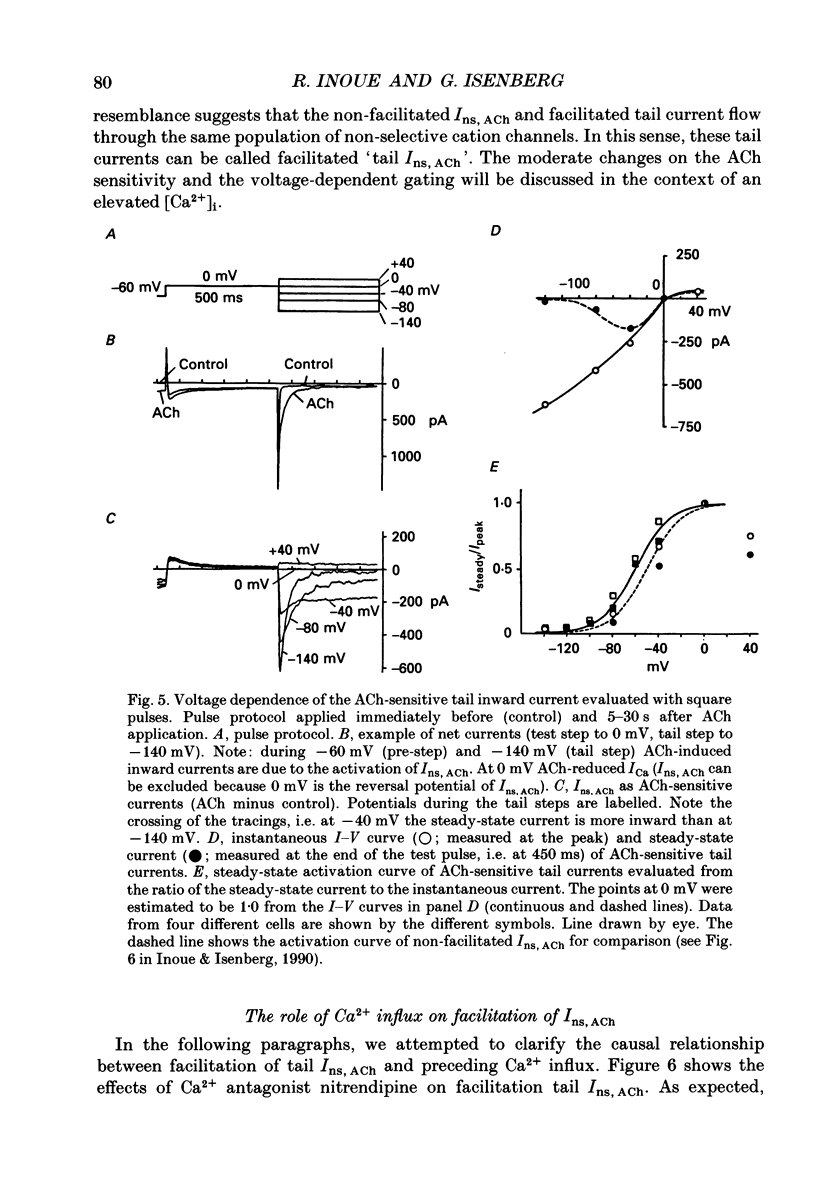
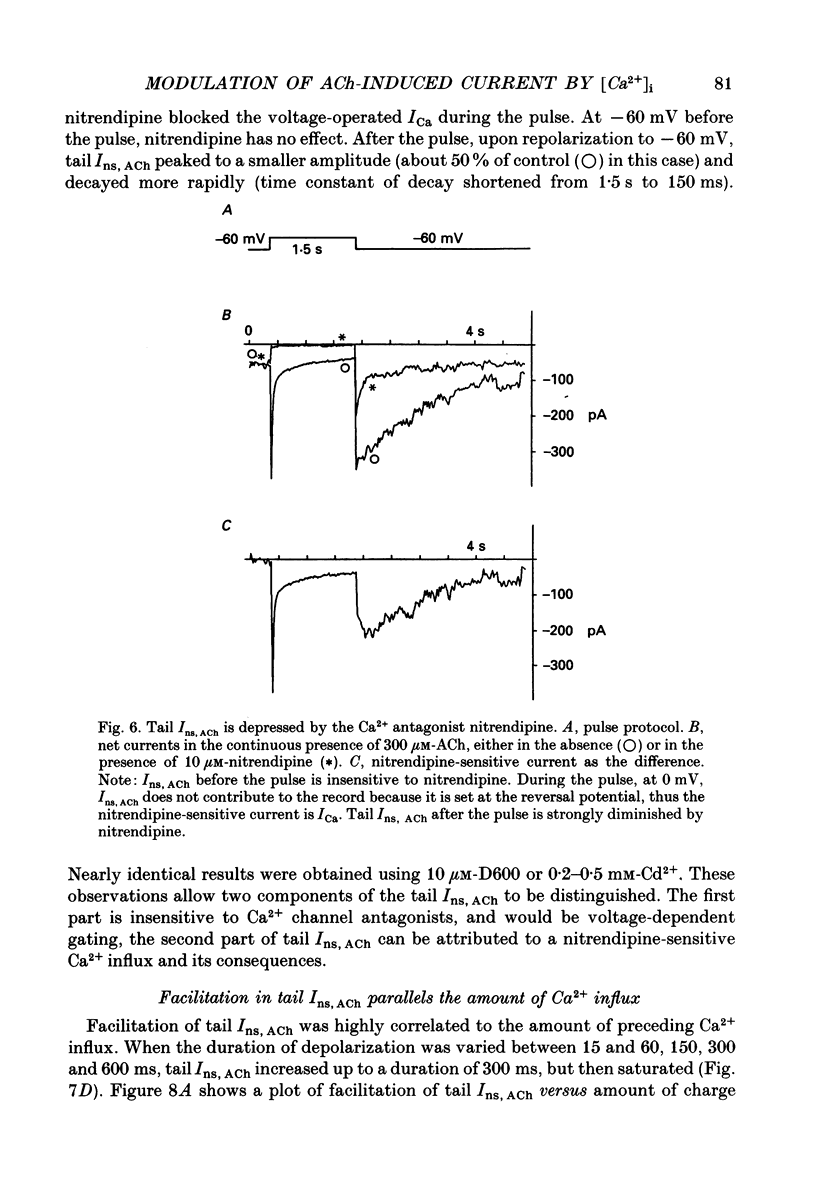
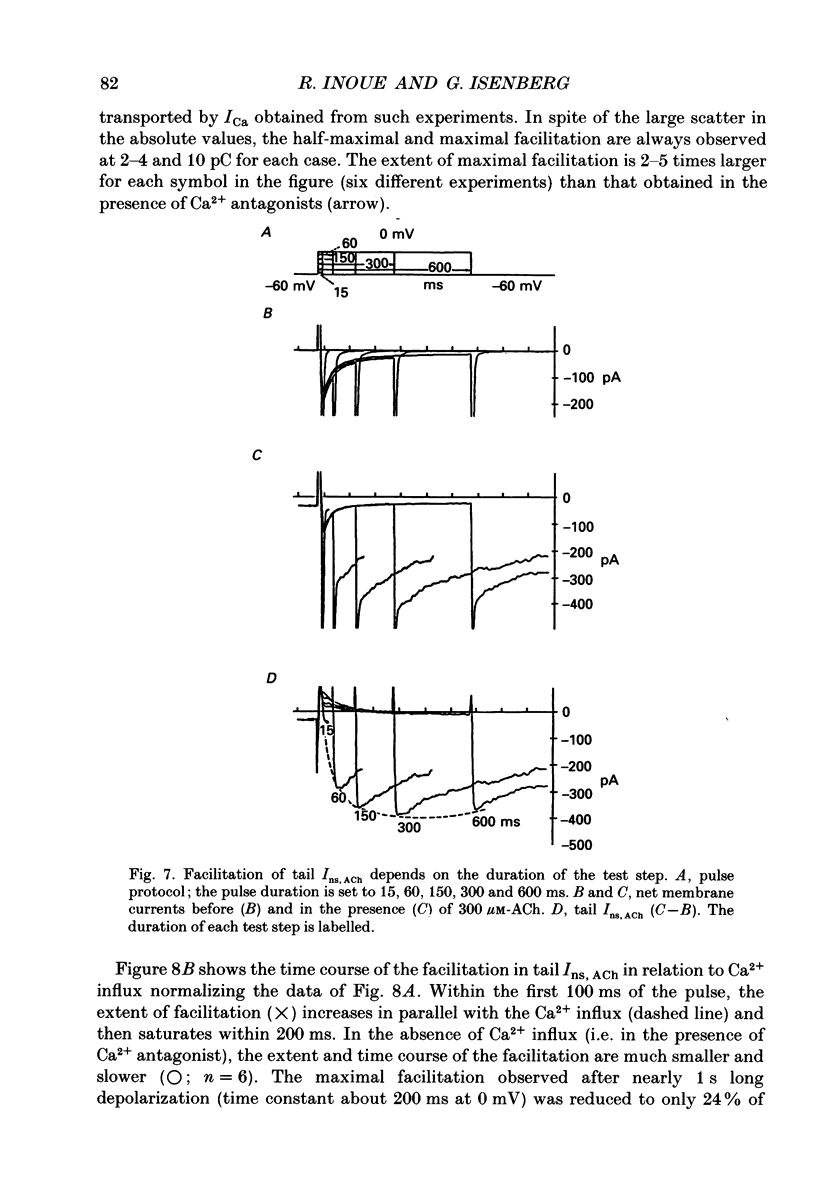
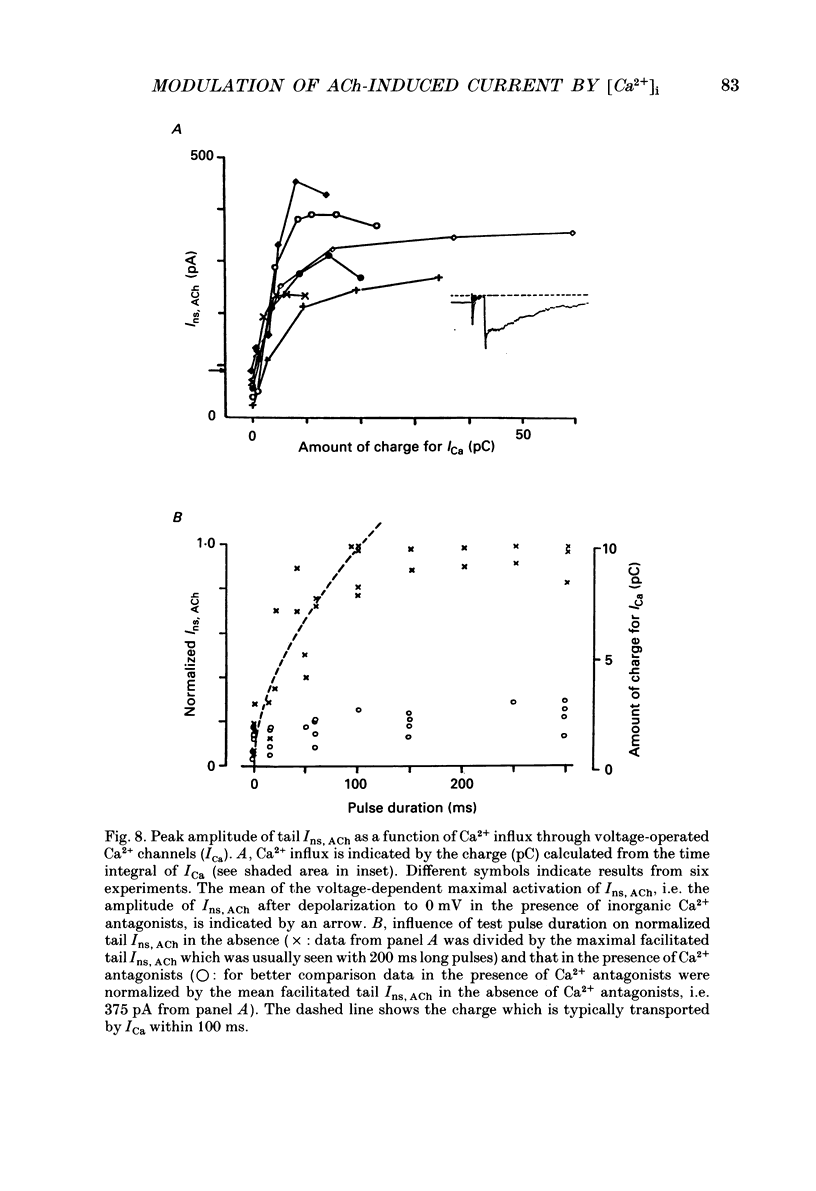
1. The modulatory effect of internal Ca2+ on the current through the ACh-activated non-selective cation channels (Ins, ACh) was investigated by the whole-cell patch clamp technique in single isolated cells of guinea-pig ileum. 2. Ins, ACh was isolated with caesium aspartate internal solution of low Ca2(+)-buffering capacity (10 microM-EGTA). With preceding depolarizations which evoked voltage-operated Ca2+ currents (ICa), Ins, ACh increased in amplitude and decayed more rapidly. The extent of this 'facilitating' effect depended on the number and duration of the depolarizations. 3. When depolarizing pulses were applied during the sustained phase of Ins, ACh, they were followed by large inward tail currents. These tail currents (tail Ins, ACh) resembled the non-facilitated Ins, ACh recorded without the depolarizing pulse, in regard to voltage-dependent gating and dependence on the extracellular Na+ concentration, thus suggesting that the currents are flowing through the same class of channels. 4. The tail Ins, ACh was apparently composed of two components distinguished by the insensitivity to organic Ca2+ antagonists. The minor component (about 20% of tail Ins, ACh) showed a rapid decay (about 150 ms at -60 mV) which could be attributed to voltage-dependent kinetics. The major component decayed slowly within 5 s and appeared to be related to changes in the intracellular Ca2+ concentration. The latter component was not recorded when Ba2+ or Sr2+ were used as a charge carrier for ICa and was blocked by 10 microM-D600 or nitrendipine, or Cd2+ 0.2-0.5 mM). 5. The tail Ins, ACh increased in proportion to Ca2+ influx when the duration of depolarizing pulses were prolonged from 15 to 200 ms, but this 'facilitating' effect was greatly suppressed when the cell was perfused with 40 mM-EGTA. 6. When the pCa in the pipette was varied using 40 mM-Ca-EGTA, the conductance through Ins, ACh increased in a manner dependent on intracellular Ca2+ concentration. Half-maximal and submaximal activation occurred at about 200 nM and 1 microM, respectively. 7. These results show that the activity of Ins, ACh is very sensitive to the intracellular Ca2+ concentration in the physiological range.
Full text
PDF


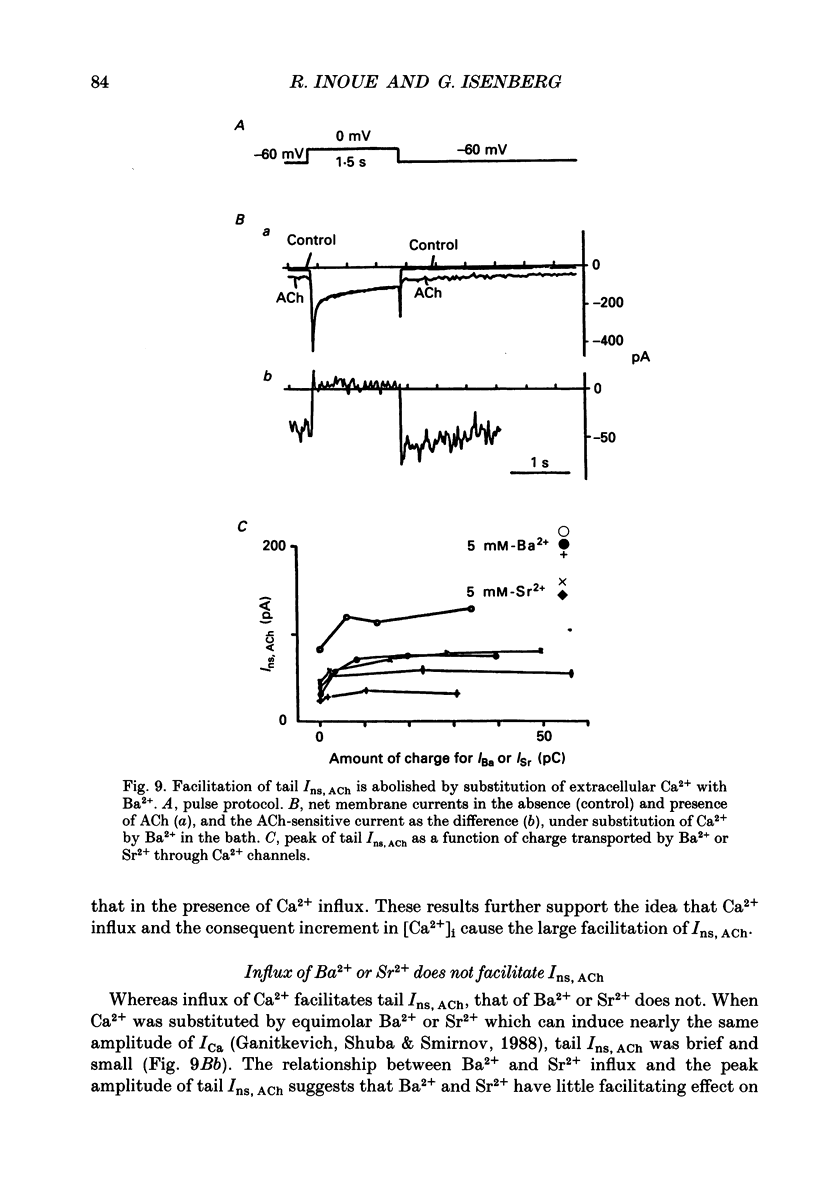
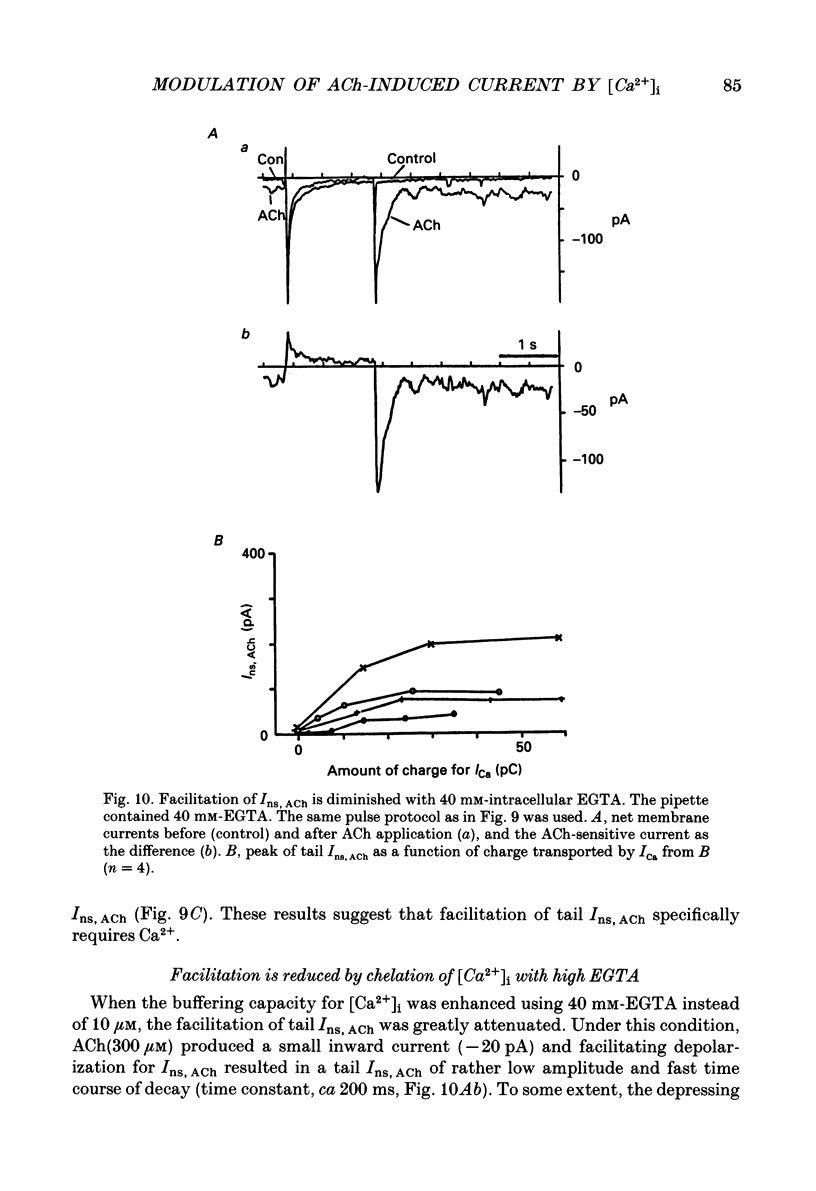

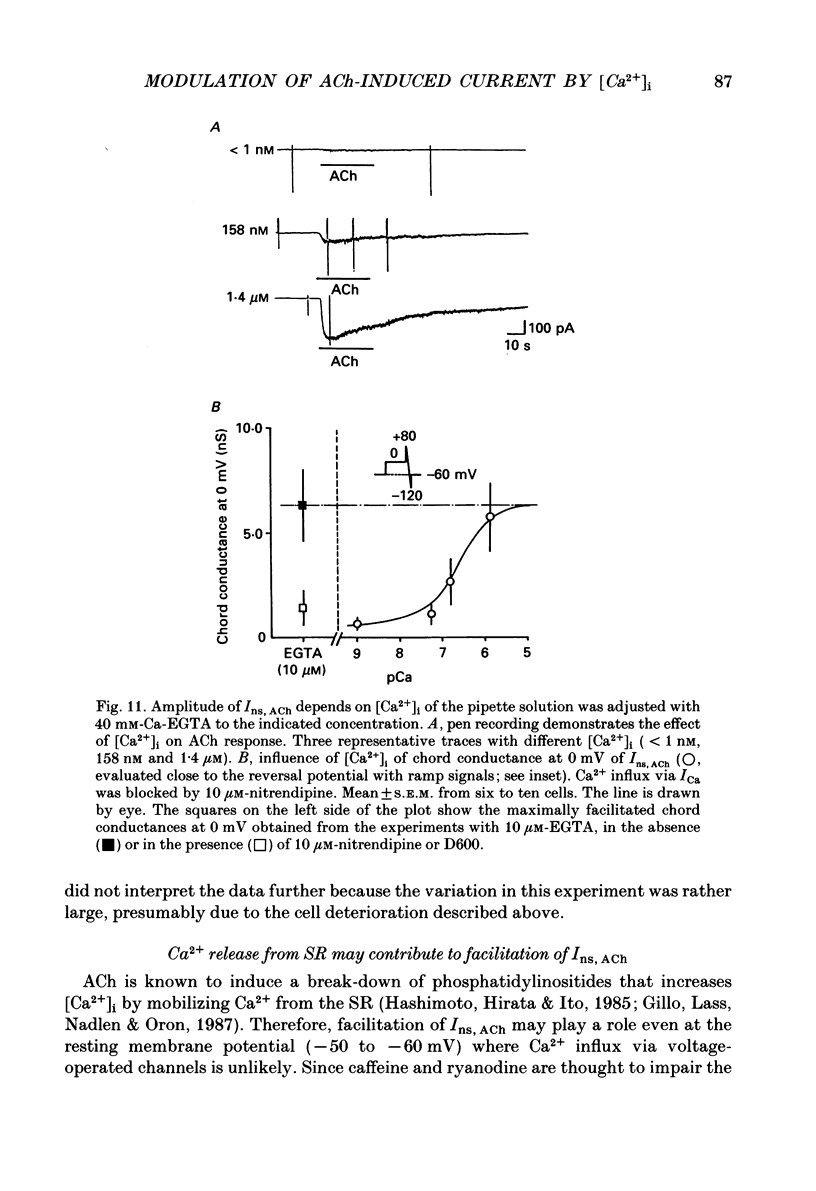





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belles B., Hescheler J., Trautwein W., Blomgren K., Karlsson J. O. A possible physiological role of the Ca-dependent protease calpain and its inhibitor calpastatin on the Ca current in guinea pig myocytes. Pflugers Arch. 1988 Oct;412(5):554–556. doi: 10.1007/BF00582548. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985 Jul 25;316(6026):345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Ehara T., Noma A., Ono K. Calcium-activated non-selective cation channel in ventricular cells isolated from adult guinea-pig hearts. J Physiol. 1988 Sep;403:117–133. doi: 10.1113/jphysiol.1988.sp017242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Findlay I., Petersen O. H. Acetylcholine stimulates a Ca2+-dependent C1- conductance in mouse lacrimal acinar cells. Pflugers Arch. 1985 Mar;403(3):328–330. doi: 10.1007/BF00583609. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Calcium-dependent inactivation of potential-dependent calcium inward current in an isolated guinea-pig smooth muscle cell. J Physiol. 1987 Nov;392:431–449. doi: 10.1113/jphysiol.1987.sp016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Shuba M. F., Smirnov S. V. Saturation of calcium channels in single isolated smooth muscle cells of guinea-pig taenia caeci. J Physiol. 1988 May;399:419–436. doi: 10.1113/jphysiol.1988.sp017089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillo B., Lass Y., Nadler E., Oron Y. The involvement of inositol 1,4,5-trisphosphate and calcium in the two-component response to acetylcholine in Xenopus oocytes. J Physiol. 1987 Nov;392:349–361. doi: 10.1113/jphysiol.1987.sp016784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. J., Gillette R. Regulation of cAMP-stimulated ion current by intracellular pH, Ca2+, and calmodulin blockers. J Neurophysiol. 1988 Jan;59(1):248–258. doi: 10.1152/jn.1988.59.1.248. [DOI] [PubMed] [Google Scholar]
- Grider J. R., Makhlouf G. M. Contraction mediated by Ca++ release in circular and Ca++ influx in longitudinal intestinal muscle cells. J Pharmacol Exp Ther. 1988 Feb;244(2):432–437. [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989 Feb;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Ito Y. A role for inositol 1,4,5-trisphosphate in the initiation of agonist-induced contractions of dog tracheal smooth muscle. Br J Pharmacol. 1985 Sep;86(1):191–199. doi: 10.1111/j.1476-5381.1985.tb09449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himpens B., Casteels R. Measurement by Quin2 of changes of the intracellular calcium concentration in strips of the rabbit ear artery and of the guinea-pig ileum. Pflugers Arch. 1987 Jan;408(1):32–37. doi: 10.1007/BF00581837. [DOI] [PubMed] [Google Scholar]
- Himpens B., Somlyo A. P. Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea-pig smooth muscle. J Physiol. 1988 Jan;395:507–530. doi: 10.1113/jphysiol.1988.sp016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Kobayashi T., Endo M. Use of ryanodine for functional removal of the calcium store in smooth muscle cells of the guinea-pig. Biochem Biophys Res Commun. 1988 Apr 15;152(1):417–422. doi: 10.1016/s0006-291x(88)80730-7. [DOI] [PubMed] [Google Scholar]
- Inoue M., Oomura Y., Yakushiji T., Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986 Nov 13;324(6093):156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990 May;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Acetylcholine activates single sodium channels in smooth muscle cells. Pflugers Arch. 1987 Sep;410(1-2):69–74. doi: 10.1007/BF00581898. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H., Parker I. Calcium transients evoked by electrical stimulation of smooth muscle from guinea-pig ileum recorded by the use of Fura-2. J Physiol. 1988 Dec;407:117–134. doi: 10.1113/jphysiol.1988.sp017406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. P., Bolton T. B. A calcium-dependent rather than a G-protein mechanism is involved in the inward current evoked by muscarinic receptor stimulation in dialysed single smooth muscle cells of small intestine. Br J Pharmacol. 1988 Oct;95(2):325–327. doi: 10.1111/j.1476-5381.1988.tb11649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Regulation of calcium current by intracellular calcium in smooth muscle cells of rabbit portal vein. Circ Res. 1988 Feb;62(2):375–383. doi: 10.1161/01.res.62.2.375. [DOI] [PubMed] [Google Scholar]
- Saida K. Intracellular Ca release in skinned smooth muscle. J Gen Physiol. 1982 Aug;80(2):191–202. doi: 10.1085/jgp.80.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V., Jr Characterization of calcium-activated potassium channels in single smooth muscle cells using the patch-clamp technique. Pflugers Arch. 1987 Feb;408(2):98–111. doi: 10.1007/BF00581337. [DOI] [PubMed] [Google Scholar]
- Tohse N., Kameyama M., Irisawa H. Intracellular Ca2+ and protein kinase C modulate K+ current in guinea pig heart cells. Am J Physiol. 1987 Nov;253(5 Pt 2):H1321–H1324. doi: 10.1152/ajpheart.1987.253.5.H1321. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986 Jul;18(7):691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Yagi S., Becker P. L., Fay F. S. Relationship between force and Ca2+ concentration in smooth muscle as revealed by measurements on single cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4109–4113. doi: 10.1073/pnas.85.11.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H. Recording of intracellular Ca2+ from smooth muscle cells by sub-micron tip, double-barrelled CA2+-selective microelectrodes. Cell Calcium. 1986 Aug;7(4):203–219. doi: 10.1016/0143-4160(86)90001-1. [DOI] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]



