Austin G. Kulasekararaj, MD PhD MPH
Department of Haematological Medicine, King's College Hospital; National Institute for Health Research and Wellcome King's Clinical Research Facility and King's College London, London, UK
Disclosures
A.G.K. has received honoraria from Agios Pharmaceuticals, Alexion AstraZeneca Rare Disease, Amgen, Celgene/Bristol Myers Squibb, Novartis, and Ra Pharma/UCB; is on the board of directors or is an advisory board member for Agios Pharmaceuticals, Alexion AstraZeneca Rare Disease, Amgen, Celgene/Bristol Myers Squibb, Geron, Novartis, F. Hoffmann-La Roche Ltd, and Ra Pharma; and has received consulting fees from Achillion, Agios Pharmaceuticals, Akari Therapeutics, Alexion AstraZeneca Rare Disease, BioCryst, Celgene/Bristol Myers Squibb, Novo Nordisk, Janssen Pharmaceuticals, F. Hoffmann-La Roche Ltd, Samsung, and Novartis.Title: Phase III COMMODORE 1 AND COMMODORE 2 Trials: Treatment Satisfaction and preference in patients with paroxysmal nocturnal hemoglobinuria (PNH) treated with Crovalimab and Eculizumab or Ravulizumab.
Email: austin.kulasekararaj@nhs.net
Fernando Ataulfo Gonzalez Fernandez, MD; Hematology Service, Hospital Clínico San Carlos, Madrid, Spain
Disclosures
F.A.G.F. is a consultant for Alexion AstraZeneca Rare Disease, F. Hoffmann-La Roche Ltd, Novartis, and Sobi; received honoraria from Alexion AstraZeneca Rare Disease, F. Hoffmann-La Roche Ltd, Novartis, and Sobi; and participated in speaker’s bureau for Alexion AstraZeneca Rare Disease, F. Hoffmann-La Roche Ltd, Novartis, and Sobi.
Email: fernandoataulfo.gonzalez@salud.madrid.org
Phillip Scheinberg, MD; Division of Hematology, Hospital A Beneficência Portuguesa de São Paulo, São Paulo, Brazil
Disclosures
P.S. is a consultant for AbbVie, Alexion AstraZeneca Rare Disease, AstraZeneca, BioCryst, Janssen Pharmaceuticals, Novartis, Pfizer, and F. Hoffmann-La Roche Ltd; has given scientific presentations for AbbVie, Alexion AstraZeneca Rare Disease, Amgen, AstraZeneca, Bristol Myers Squibb, Janssen Pharmaceuticals, and F. Hoffmann-La Roche Ltd; received research funding from Alnylam, AstraZeneca, BioCryst, Pfizer, and Viracta; and participated in speaker’s bureau for Novartis.
Email: scheinbp@bp.org.br
Nicole Straetmans, MD PhD; Department of Haematology, University Hospital Saint-Luc, Brussels, Belgium
Disclosures
N.S. is a consultant for Amgen, Novartis, and Sobi.
Email: nicole.straetmans@saintluc.uclouvain.be
Yasutaka Ueda, MD PhD; Department of Hematology and Oncology, Graduate School of Medicine, Faculty of Medicine, Osaka University, Suita, Japan
Disclosures
Y.U. is a consultant for Alexion AstraZeneca Rare Disease, Asahi Kase, Chugai Pharmaceutical, Janssen Pharmaceuticals, Novartis, Sanofi, and Sobi; is on the board of directors or is an advisory board member for Alexion AstraZeneca Rare Disease, Novartis, and Sanofi; has received honoraria from Alexion AstraZeneca Rare Disease, Chugai Pharmaceutical, and Sobi; received research funding from Chugai Pharmaceutical; and has participated in speaker’s bureau for Alexion AstraZeneca Rare Disease, Novartis, and Sanofi.
Email: yueda@bldon.med.osaka-u.ac.jp
Brittany Gentile, PhD; Genentech, Inc., South San Francisco, CA, USA
Disclosures
B.G. is a former employee of Genentech Inc.
Email: bgentile@gmail.com
Jennifer Stefani, PhD; F. Hoffmann-La Roche Ltd, Basel, Switzerland
Disclosures
J.S. is a current employee of F. Hoffmann-La Roche Ltd.
Email: jennifer.stefani@roche.com
Marianne Uguen, MS; F. Hoffmann-La Roche Ltd, Basel, Switzerland
Disclosures
M.U. is a current employee of F. Hoffmann-La Roche Ltd.
Email: marianne.uguen@roche.com
Alexander Röth, MD; Department of Hematology and Stem Cell Transplantation, University Hospital Essen, West German Cancer Center, University of Duisburg-Essen, Essen, Germany
Disclosures
A.R. is a consultant for Alexion AstraZeneca Rare Disease, Amgen, Apellis, Bioverativ, BioCryst, F. Hoffmann-La Roche Ltd, Novartis, Sanofi, and Sobi; is on the board of directors or is an advisory board member for Alexion AstraZeneca Rare Disease, Apellis, BioCryst, F. Hoffmann-La Roche Ltd, and Sanofi; and has received honoraria from Alexion AstraZeneca Rare Disease, Bioverativ, F. Hoffmann-La Roche Ltd, Grifols, Sanofi, and Sobi.
Email: alexander.roeth@uk-essen.de
Danièle Marceau, MD; Université Laval; CISSSCA
Disclosures
D.M. is a consultant for Sobi Canada, Alexion AstraZeneca Rare Disease, and Hoffmann-La Roche Ltd; received research funding from Sobi Canada and Alexion AstraZeneca Rare Disease; and has participated in speaker’s bureau for Alexion AstraZeneca Rare Disease.
Email: daniele.marceau@fmed.ulaval.ca
Submitted on behalf of Dr. Danièle Marceau by Naomi Eterman (Roche Canada) naomi.eterman@roche.com
All authors declare third-party medical writing assistance, furnished by Akshaya Srinivasan, PhD, of Nucleus Global, an Inizio Company, and funded by F. Hoffmann-La Roche Ltd.
Purpose: To report patient preferences and treatment satisfaction data from COMMODORE 1 (NCT04432584) and 2 (NCT04434092)1,2.
Methods: In COMMODORE 1 and 2, patients with PNH aged ≥18y were randomized to receive crovalimab (weight-based tiered regimen consisting of loading doses followed by SC Q4W maintenance3) or eculizumab (900mg IV Q2W) over 24 weeks. For the randomized arms, COMMODORE 1 enrolled C5i-experienced patients who were receiving eculizumab for ≥24 weeks and COMMODORE 2 enrolled C5i-naive patients. After Week 25, all patients in both studies could receive crovalimab if continuing in the study. Patients with the C5 R885H polymorphism, previously treated with ravulizumab or high-dose eculizumab, and ≤18 years old were enrolled in the COMMODORE 1 non-randomized arm. Adult patients in all arms of both studies completed the Treatment Satisfaction and Medication Questionnaire-9 (TSQM-9) at Weeks 13 and 25. Treatment preference was assessed in patients ≥18y using a validated Patient Preference Questionnaire after 17 weeks of crovalimab treatment in C5i-experienced patients in all COMMODORE 1 arms and in C5i–naive patients initially randomized to eculizumab who switched to crovalimab after 24 weeks of treatment in COMMODORE 2. Preference was also assessed in patients ≤18y enrolled in the COMMODORE 1 non-randomized arm. All analyses are descriptive.
Results: At clinical data cutoff (November 16, 2022) for primary analysis, 89 C5i-experienced patients (crovalimab: 45; eculizumab: 44) were randomized in COMMODORE 1 and 204 C5i-naive patients (crovalimab: 135; eculizumab: 69) were randomized in COMMODORE 2. 38 C5i-experienced patients were enrolled into the COMMODORE 1 non-randomized arm. Absolute TSQM-9 scores for perceived efficacy and global satisfaction were similar between arms; however, crovalimab was rated as more convenient at Week 25 in both COMMODORE 1 and 2. Among COMMODORE 1 and 2 patients randomized to eculizumab and then switched to crovalimab at the end of the primary treatment period (35 and 68 patients, respectively), TSQM-9 scores for perceived efficacy and overall satisfaction were generally maintained at Week 25, and convenience scores improved. In COMMODORE 1, 33 of 39 (85%) patients assessed in the crovalimab arm preferred it to eculizumab2. Of patients randomized to eculizumab who switched to crovalimab after 24 weeks, 27 of 28 (96%) assessed in COMMODORE 1 and 32 of 38 (84%) in COMMODORE 21 preferred crovalimab. Top reasons for crovalimab preference were that the treatment required fewer hospital visits, was easier to administer, took less time to administer, and provided a better quality of life. In the COMMODORE 1 non-randomized arm, 9 of 15 patients (60%) who switched from ravulizumab to crovalimab preferred crovalimab, with similar reasons for preference.
Conclusion: Patients across COMMODORE 1 and 2 preferred crovalimab to eculizumab and ravulizumab and rated it as more convenient than eculizumab. With Q4W SC injection and the option for self-administration outside of a healthcare setting, crovalimab potentially offers a new treatment option for patients with PNH that is less burdensome than existing therapies for this chronic lifelong disease.
References
Röth A. et al. Am J Hematol. 2024 Sep;99(9):1768-1777
Scheinberg P. et al. Am J Hematol. 2024 Sep;99(9):1757-1767.
Liu ASH 2022; #293
Evaluating the Neuroprotective Effects of Caplacizumab in Immune-mediated Thrombotic Thrombocytopenic Purpura
Fahad Hannan BSc, Daniel Mendes BSc, Lee Ting-Yim PhD, Christopher J. Patriquin MD, Katerina Pavenski MD, Jonathan D. Thiessen PhD, Shih-Han Susan. Huang MD
Lawson Health Research Institute, London, ON, Canada
Disclosures
This study makes use of Dr. Lee Ting-Yim’s CT perfusion protocol which is licensed to GE Healthcare.
Despite modern methods to treat immune-mediated thrombotic thrombocytopenic purpura (iTTP), patients often suffer from increased risks of cerebrovascular disease, cognitive decline, and depression. Caplacizumab has shown to be an effective treatment for iTTP by targeting bindings zones on ultra-large von Willebrand factors, preventing platelets from aggregating and forming clots. This study investigates whether the use of Caplacizumab can reduce/prevent brain tissue damage compared to standard of care treatment (SOC).
We conducted a multi-center study involving 13 iTTP patients, six of whom were refractory to SOC treatment and received Caplacizumab. Participants underwent MRI and a contrast-enhanced dynamic CT perfusion scan to assess gross pathology, blood-brain barrier (BBB) permeability, cerebral blood flow, and volume. Additionally, a comprehensive cognitive and neuropsychiatric assessment were conducted to evaluate reasoning, short-term memory, verbal memory, concentration, and depression scores. Data collection was conducted at two timepoints; baseline, 30-days post-remission, and follow-up within one year.
BBB disruption was evident in all patients regardless of treatment method. Baseline whole brain mean permeability surface (PS) product for patients receiving Caplacizumab was 0.35 +/- 0.09 mL/min/100g, compared to SOC at 0.27 +/- 0.10mL/min/100g. A significant reduction (p = 0.047) in BBB integrity to 0.24 +/- 0.08 mL/min/100g was found in the Caplacizumab group in the follow-up visit (See Figure 1). Cognitive scores remained low across all cognitive domains for both groups with persistent white matter hyperintensities observed on MRI scans. Depression assessments revealed consistent concentration difficulties and depressive symptoms across all participants.
Figure 1.

Blood brain barrier permeability values for Patients on SOC and Caplacizumab at baseline and follow-up.
** indicates a significant p-value.
This study highlights the persistent challenges of managing iTTP post-remission, particularly regarding neurocognitive health. The compromised BBB integrity observed in patients, regardless of remission, underscores the chronic nature of iTTP's impact on the brain. Despite patients on Caplacizumab having a higher whole brain mean PS product at baseline, likely due to being refractory, follow-up scans show a significant reduction, highlighting Caplacizumab's potential neuroprotective effects. However, no improvement was seen in patient cognitive scores and depression scores. However, to demonstrate impact in the cognitive and depression function, we will need to have more patients. While Caplacizumab shows promise in reducing BBB permeability and potentially offering a neuroprotective effect, further longitudinal studies are needed to see if this translated into neurocognitive changes.
Figure 2.
Representative blood brain barrier permeability surface product maps for each patient at baseline and follow-up.
Mapping White Matter Degeneration in iTTP Survivors: A Diffusion Tensor Imaging Study
Fahad Hannan BSc, Stefan E. Poirier MSc, Christopher J. Patriquin MD, Katerina Pavenski MD, Jonathan D. Thiessen PhD, Shih-Han S. Huang MD
Lawson Health Research Institute, London, ON, Canada
Disclosures
None
The purpose of this study is to explore whether white matter damage in immune-mediated thrombotic thrombocytopenic purpura (iTTP) survivors contributes to the cognitive decline observed in this patient population. iTTP, despite advances in treatment such as immunosuppressants and plasma exchange, leaves over 50% of survivors at increased risk of developing cognitive impairments and cerebrovascular disease. The mechanisms underlying these neurological complications remain poorly understood. We hypothesize that damage to specific white matter tracts, which play a crucial role in cognitive processing, may be a key factor contributing to the cognitive decline seen in iTTP survivors.
We utilized diffusion tensor imaging (DTI), a sensitive technique for detecting microstructural changes in white matter, to evaluate 23 iTTP patients (mean age = 48 +/- 14, female = 19) at 30 days post-remission compared to 22 healthy controls (mean age = 47 +/- 15, female = 16). DTI metrics such as fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) were measured across eight white matter tracts strongly correlated with cognitive function for a total of 25 regions of interest (ROIs) (Figure 1). Tracts were analyzed as ROIs and along 100 equidistant points along tracts. Alongside MRI scans, cognitive performance was assessed using Cambridge Brain Sciences across four cognitive domains: reasoning, short-term memory, verbal memory, and concentration.
Figure 1.

Representative diffusion tracts of eight white matter bundles: 1) arcuate fasciculus 2) anterior thalamic radiation 3) corpus callosum 4) cingulum 5) inferior longitudinal fasciculus 6) superior longitudinal fasciculus 7) uncinate fasciculus 8) thalamo-parietal. Colors indicate the direction of diffusion.
Our analysis revealed that iTTP survivors exhibited significant breakdown of brain white matter microstructure when compared to healthy controls. Specifically, FA was significantly decreased in 15 of the 25 ROIs, reflecting more isotropic water diffusion, which is indicative of axonal degeneration or demyelination. In addition, increased MD was observed in 2 of 25 ROIs, while RD was significantly elevated in 11 of 25 ROIs, suggesting possible edema, neuroinflammation, or myelin damage. These microstructural alterations were particularly pronounced in white matter tracts known to be involved in memory, such as the superior longitudinal fasciculus (Figure 2). Cognitive assessments further demonstrated that iTTP survivors had significantly reduced scores in the domains of short-term memory and concentration, which directly corresponded with the observed DTI changes (Figure 3).
Figure 2.
Along tract analysis of the superior longitudinal fasciculus II between iTTP patients and controls. Measurements are taken at 100 equidistant points along the tract. Red-dotted lines are p < 0.05 (FWE-corrected). A) fractional anisotropy B) axial diffusivity C) radial diffusivity D) mean diffusivity.
Figure 3.

Cambridge Brain Sciences cognitive test findings for iTTP patients and controls across four cognitive domains: reasoning, short-term memory, verbal memory, concentration. Scores are standardized to normative data. Categories with significant differences are indicated with a p-value.
The findings of this study suggest that iTTP survivors experience significant white matter damage, particularly in tracts that are critical for cognitive function. The observed decreases in FA and increases in RD in these regions indicate that iTTP may lead to axonal degeneration, demyelination, and possible inflammatory processes, contributing to cognitive decline. The fact that these structural changes are mirrored by deficits in short-term memory and concentration provides compelling evidence of a link between white matter damage and cognitive impairment in iTTP survivors. This study highlights the importance of long-term neurological follow-up for iTTP survivors. Further exploration into the role of inflammation and microvascular injury as potential contributors to white matter damage will be critical for developing effective therapeutic strategies.
Role of the C5b-9 complex in kidney disease
Srishti Sahu, Msc1,2, Amandine Badie, PhD1,2, Natacha Patey, MD, PhD2,3, Stéphan Troyanov, MD4,5, Arnaud Bonnefoy, PhD6, Anne-Laure Lapeyraque, MD1,7, Alexandra Cambier, MD, PhD1,2,7
1Department of Microbiology, Infectiology and Immunology, Faculty of Medicine, University of Montreal, Montreal, Quebec, Canada
2Immunology and Cancer axis, CHU Sainte Justine Research Centre, Montreal, Quebec, Canada
3Pathology Department, CHU Sainte Justine, Montreal, Quebec, Canada
4Division of Nephrology, Hôpital du Sacré-Coeur-de-Montréal, Montreal, Quebec, Canada
5Division of Nephrology, Hôtel-Dieu de St-Jérôme, St-Jerome, Montreal, Quebec, Canada
6Division of Hematology, CHU Sainte Justine, Montreal, Quebec, Canada
7Division of Nephrology, CHU Sainte Justine, Montreal, Quebec, Canada
Disclosures
None
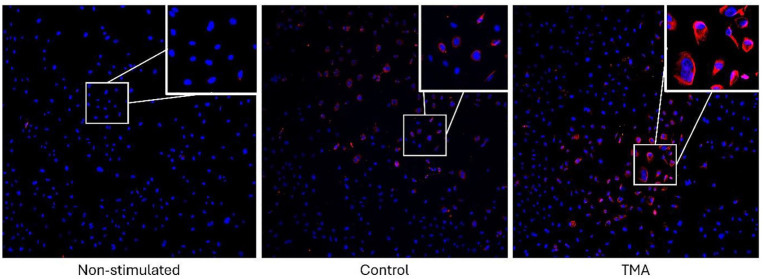
Aim: Thrombotic microangiopathy (TMA) with renal involvement is diagnosed by histological analysis of renal biopsies. Currently, there is a lack of specific and sensitive biomarkers to demonstrate complement activation in all TMA patients including aHUS patients, thus we are seeking to identify new biomarkers. These biomarkers would also be of interest in other complement-mediated glomerulonephritis. Our hypothesis posits that complement activation primarily occurs on the endothelium rather than in the fluid phase. We aim to establish correlations between clinical phenotypes, levels of plasma and urinary C5b-9, concentrations of C3 and C4, genetic mutations, and levels of Factor H and Factor I in TMA including aHUS with C5b-9 formation on endothelial cells.
Methods: Glomerular cell types were cultured: human umbilical vein endothelial cells (HUVECs) and an endothelial-like cell that was isolated from the endothelium of the foreskin of a male patient (HMEC-1, ATCC). These cells were activated using adenosine diphosphate (ADP) and stimulated with serum from patients with renal TMA with varying levels of glomerular inflammation, or from healthy patients. The C5b-9 complex was detected using an antibody against the C9 neoantigen, and its expression was analyzed by immunofluorescence and observed by microscopy.
Results: We have showed that C5b-9 can form on the surface of HUVECs when stimulated by patient serum through complement activation by immunofluorescence. These deposits predominate when HUVECs are stimulated with serum from TMA patients compared to control patients (Figure). The same results are expected for HMEC-1 cells.
Figure.
Expression of C5b-9 on the surface of HUVECs after stimulation or not with serum from TMA patients or controls. Dapi in blue, C5b-9 in red. Objective x20.
Conclusions: We were able to find a correlation between TMA patient and C5b-9 deposition in endothelial cells. The differential expression of C5b-9 in HUVECs provide insight into the mechanisms involved in renal TMA but also in other glomerulonephritis such as IgAN. In addition, these results will provide a better understanding of complement-mediated immunity and its potential role as a diagnostic marker and/or therapeutic target in glomerulonephritis.
Efficacy and safety of iptacopan in patients with C3G glomerulopathy: 12 months results from the phase III APPEAR C3G
Richard J Smith1 David Kavanagh2-3, Marina Vivarelli 4, Carla M Nester5, Giuseppe Remuzzi6, Ming-Hui Zhao7, Edwin K. Wong7, Yaqin Wang8, Angelo J Trapani8, Induja Krishnan8, Nicholas J Webb9, Matthias Meier9, Natacha Bastien10, Andrew Bomback11
1The University of Iowa Roy and Lucille Carver College of Medicine Iowa City,IA, US
2Newcastle University, Newcastle upon Tyne, United Kingdom
3National Renal Complement Therapeutics Center Royal Victoria Infirmary Newcastle upon Tyne, UK
4Ospedate Pediatrico Bambino Gesu, Roma, Italy
5The University of Iowa stead family Children's Hospital, Iowa City, IA, US
6IRCCS Istituto Di Ricerche Farmacologiche Mario Negri Centro Anna Maria Astori, Bergamo, Lombardia, Italy
7Peking Union Medical College Hospital, Beijing, China
8Novartis Pharmaceutical Corporation, East Hanover, NJ, US
9Novartis Pharma AG, Basel, Basel-Stat, Switzerland
10Novartis Pharma Canada inc. Montreal, Canada
11Columbia University Vagelos College of Physicians and Surgeons, New York, NY, US
Disclosures
Natacha Bastien, Yaqin Wang, Angelo J Trapani, Induja Krishnan Nicholas J Webb and Mathias Meier are Novartis employes
Purpose of the study: The phase III APPEAR C3G study evaluated the efficacy safety and tolerability of iptacopan versus placebo in C3G patients.
Methods: APPEAR C3G was a multicenter randomized double-blind placebo-controlled pivotal phase III study that included adult patients with biopsy confirmed C3G. The study comprised a 6-month randomized double-blind treatment with iptacopan 200 mg bid versus placebo followed by a 6 month open-label iptacopan treatment.
Results: 74 patients were randomized 1:1 to either iptacopan (n= 38) or placebo (n=36). Baseline patient demographics were generally well balanced. The iptacopan arm exhibited a more severe disease phenotype. 43 (58.1%) patients in the iptacopan (n= 22) placebo (n=21) arms completed the 12 months treatment at data cut-off (when all patients completed 6 months of treatment. The study met its primary endpoint demonstrating a statistically significant reduction in 24 hours UPCR with iptacopan treatment at 6 months (35.1%, one sided P = 0.0014, 95% confidence interval: 13.8% 51.1%) versus placebo sustained up to 12 months of treatment. Iptacopan show showed a sustained improvement in patients meeting the composite renal endpoint (≥ 50% reduction in UPCR + ≤15% reduction in eGFR at 12 months), 43.5% (iptacopan versus placebo) and 25.0% (switched to iptacopan). Iptacopan led to an improvement in the trajectory of eGFR compared to patient historical eGFR decline. Iptacopan demonstrated a favorable safety profile with no new safety signals identified. Other 12 months primary and secondary endpoints will be presented.
Conclusion: Iptacopan demonstrated a significant and clinically meaningful proteinuria reduction on top of supportive care at 6 months in the APPEAR C3G study sustained up to 12 months. Iptacopan was well tolerated with its favorable safety profile in C3G patients.
Funding
Novartis Pharma AG
Update to the long-term safety and efficacy of iptacopan in C3G: 33-month extension study data from patients enrolled in a Phase 2 study
Carla M. Nester1, Ute Eisenberger2, Alexandre Karra3, Moglie Le Quintrec4, Liz Lightstone5-6, Manuel Praga7, Giuseppe Remuzzi8, Maria José Soler Romeo9, Junhao Liu10, Matthias Meier11, Nicholas Webb11, Tathyana M. A. Franco12, Edwin Wong13-14
1Stead Family Children’s Hospital, University of Iowa, US
2Department of Nephrology, University Hospital Essen, University of Duisburg-Essen, Germany
3Hôpital Européen Georges Pompidou, France
4Service de Néphrologie et Transplantation Rénale, Centre Hospitalier Universitaire de Montpellier Montpellier, France
5Centre for Inflammatory Disease, Department of Immunology and Inflammation, Imperial College London, UK
6Imperial College Renal and Transplant Centre, Imperial College Healthcare NHS Trust, Hammersmith Hospital, UK
7Department of Medicine, Complutense, University. Spain
8Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Italy
9Nephrology Department, Hospital Universitari, Spain
10Novartis Pharmaceuticals Corporation, US
11Novartis Pharma AG, Basel, Switzerland
12Novartis Inc. Canada
13National Renal Complement Therapeutics Centre, UK
14Newcastle University, UK
Disclosures
Junhao Liu, Matthias Meier are Novartis employes
Purpose of the study: Complement 3 glomerulopathy (C3G), due to dysregulation of the alternative complement pathway (AP), is an ultra-rare primary glomerulonephritis. Iptacopan (LNP023) is an oral, proximal complement inhibitor that specifically binds factor B and inhibits the AP. Here we present further long-term efficacy and safety data from patients with C3G and recurrent C3G who completed 33 months of iptacopan treatment.
Method: Adults with C3G (Cohort A) or C3G recurrence post-transplantation (Cohort B) received iptacopan 200 mg bid for at least 12 weeks before entering the open label extension study. Long-term efficacy was assessed by a number of renal endpoints. Long-term safety and tolerability of iptacopan were monitored.
Results: Of 27 patients completing the Ph2 study, 26 (16 Cohort A, 10 Cohort B) entered the extension study treatment with iptacopan; 22 patients (14 Cohort A, 8 Cohort B) completed the 33 months visit. In Cohort A, 42.9% of patients met the 2-component composite renal endpoint criteria at 33 months. Proteinuria (first morning void [FMV] UPCR) was reduced by 41% (p=0.0097) compared to baseline. eGFR stabilized or improved in most patients (13/16) over time and change from baseline at the 33 months time-point was -3.18 mL/min/1.73 m2 (p=0.3512). C3 levels increased by 275% (p<0.0001) from baseline. In Cohort B, eGFR change from baseline at the 33 months time-point was -6.34 mL/min/1.73 m2 (p=0.0571), and C3 levels increased by 102%. Baseline proteinuria values (FMV UPCR) were within the normal range for most participants in Cohort B and remained so during iptacopan treatment. Iptacopan was generally well-tolerated, and most adverse events were of mild severity in both cohorts. Biomarkers demonstrated substantial AP inhibition.
Conclusion: Long-term treatment with iptacopan was associated with sustained reduction in proteinuria in C3G patients and preservation of eGFR. These improvements in kidney function were associated with substantial AP inhibition leading to normalization of serum C3. Iptacopan was well-tolerated with no new safety findings in the long-term including those with recurrence post-transplantation on top of broad triple immunosuppression.
Deciphering Complement Activation Mechanisms in Childhood IgA Nephropathy
Srishti Sahu, MSc1,2, Natacha Patey, MD, PhD1,2,3, Arnaud Bonnefoy, PhD1,4, Stéphan Troyanov, MD1,5, Anne-Laure Lapeyraque, MD1,6, Alexandra Cambier, MD, PhD1,2,6
1University of Montreal, Faculty of Medicine, Montreal, Canada
2CHU Sainte Justine Research Centre, Immunology and Cancer Axis, Montreal, Canada
3CHU Sainte Justine, Pathology Department, Montreal, Canada
4CHU Sainte Justine, Hematology Department, Montreal, Canada
5Sacré-Cœur Hospital of Montreal, Department of Medicine, Montreal, Canada
6CHU Sainte Justine, Nephrology Department, Montreal, Canada
Disclosures
None
Objectives: The complement pathway is crucial in the development of IgA nephropathy (IgAN), as evidenced by the association of complement C3 with IgA deposits. However, the specific mechanisms that trigger complement pathway activation remain poorly understood in IgAN. CX1 (protein name hidden), a newly identified initiator of the lectin complement pathway, has been recently highlighted in research. Additionally, soluble CD89 (sCD89) has been implicated in kidney inflammation among childhood IgAN (cIgAN) patients. Therefore, this study aims to understand how sCD89 activates CX1, leading to the formation of C5b-9 and subsequent kidney inflammation.
Methods: A prospective cohort of cIgAN patients was enrolled in the study. The levels of soluble C5b-9 (sC5b-9) and CX1 were determined in these patients' urine and plasma using ELISA. These levels were correlated with biological, histological, and clinical data. Kidney biopsies were assessed for inflammation and C5b-9 deposition. Next, we evaluated the expression and secretion of CX1 in human mesangial cells (HMCs) using western blot and ELISA respectively. HMCs were stimulated with cIgAN plasma or recombinant sCD89 (rsCD89). Additionally, to detect the presence of CX1 within circulating immune complexes (CICs) we used sCD89 immunoprecipitation.
Results: Our research findings demonstrate several significant associations in cIgAN patients. sC5b-9 correlates with increased proteinuria. Elevated levels of sC5b-9 were also linked to cellular inflammation, glomerulosclerosis, and crescents. Notably, we found that plasma sC5b-9 is linked to endocapillary deposition of C5b-9 in kidney glomeruli. Furthermore, CX1 levels are elevated in cIgAN patients compared to healthy controls. A positive correlation exists between plasma C5b-9 and CX1 levels. Our in vitro results indicate that CX1 is expressed and secreted by the HMCs, with its upregulation upon stimulation by cIgAN plasma and rsCD89. Interestingly, we also observe the presence of CX1 within the CICs.
Conclusions: According to our research, sC5b-9 and CX1 are linked to the severity of cIgAN. More specifically, proliferative cIgAN is indicated by plasma sC5b-9 levels above 250 ng/ml. They may eliminate the necessity for invasive kidney biopsy procedures by acting as promising prognostic indicators for cIgAN. Most significantly, the interplay between CX1 and sCD89 may provide insights into the underlying mechanisms of complement pathway activation in cIgAN.
Complement Activation Distinguishes Focal Segmental Glomerulosclerosis from Minimal Change Disease
Alexandra Cambier1, Natacha Patey2, Virginie Royal3, François Gougeon4, Dominique S. Genest5, Soumeya Brachemi6, Guillaume Bollée6, Clémence Merlen7, Arnaud Bonnefoy7, Anne-Laure Lapeyraque1 and Stéphan Troyanov5,8
1Division of Nephrology, Centre Hospitalier Universitaire Ste-Justine, University of Montreal, Quebec, Canada
2Pathology Department, Centre Hospitalier Universitaire Ste-Justine, University of Montreal, Quebec, Canada
3Pathology Department, Hôpital Maisonneuve-Rosemont, Quebec, Canada
4Pathology Department, Centre Hospitalier de l’Université de Montréal,Quebec, Canada
5Division of Nephrology, Hôpital du Sacré-Coeur-de-Montréal, Quebec, Canada
6Nephrology Division, Centre Hospitalier de l’Université de Montréal, Quebec, Canada
7Division of Hematology, Centre Hospitalier Universitaire Sainte- Justine, University of Montreal, Quebec, Canada
8Division of Nephrology, Hôtel-Dieu de St-Jérôme, St-Jerome, Quebec, Canada
Disclosures
None
Introduction: Minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) are related podocytopathies with distinct kidney outcomes. Pathology findings distinguish these two diseases, but unsampled FSGS can initially be misdiagnosed as MCD, especially when few glomeruli are sampled. Multiple biomarkers have been studied to differentiate these two entities but were insufficiently sensitive or specific for general clinical use. Surprisingly, elevated urinary activation fragments have been found in FSGS despite little complement deposition on immunofluorescence staining. Whether complement activation distinguishes FSGS from MCD, participating in the development of segmental lesions, remains unknown.
Methods: We performed an observational study between 2006 and 2023 in 4 hospitals affiliated with the University of Montreal in subjects with adults, pediatrics MCD and FSGS, and proteinuria ≥ 1 g/g of creatinine. We included both primary and secondary or unknown causes. We compared urinary fragments of terminal pathway activation, sC5b9 and C5a expressed as creatinine ratios, between MCD and FSGS.
Results: Patients with FSGS (n=41) had a serum albumin of 31±10 g/L and proteinuria of 5.1 (2.6-9.1) g/g at sampling, while those with MCD (n=15) had a lower serum albumin (22±9 g/L, p=0.002), and a proteinuria of 3.8 (1.9-7.7) g/g (p=0.40). Urinary sC5b9 and C5a were 8.7 (1.7- 52.3) and 1.26 (0.45-1.84) μg/mmol of creatinine, respectively in FSGS subjects, compared to 0.8 (0.0-1.5) and 0.06 (0.01-0.15) μg/mmol of creatinine in MCD (p<0.001), respectively. We found no association between urinary complement fragments and age, eGFR, or chronic kidney lesions. When analyzing samples with proteinuria ≥ 3 g/g, the c-statistics for urinary sC5b9 and C5a were 0.96 and 1.00, respectively, in differentiating FSGS from MCD (figure 1).
Figure 1.
Conclusion: Our investigation revealed the absence of urinary complement activation fragments in patients with MCD, contrasting with findings in FSGS, despite comparable levels of proteinuria. This disparity implies a potential involvement of complement activation in the pathogenesis of FSGS and offers a straightforward diagnostic tool for distinguishing between these two conditions, thereby potentially obviating the need for kidney biopsy. Furthermore, our findings prompt consideration of complement inhibition as a therapeutic strategy for FSGS, given its unfavorable prognosis and limited treatment alternatives.