Abstract

This Perspective explores the use of biomacromolecules in natural materials synthesized by living organisms, such as spider silk, in the development of sustainable synthetic materials. Currently employed synthetic polymers lack the hierarchical complexity and unique properties of natural materials composed of biomacromolecules. By understanding the composition of these natural materials, it may be able to reproduce their properties synthetically. Additionally, research directions involving the use of renewable resources such as nitrogen and carbon dioxide from the air and seawater to develop biomacromolecules such as spider silk and biopolyester via photosynthetic organisms are reviewed. Next-generation biomacromolecule research will aid in the creation of a sustainable global society, advancing fields such as biomanufacturing, agriculture, aquaculture, and other industries.
I. Introduction
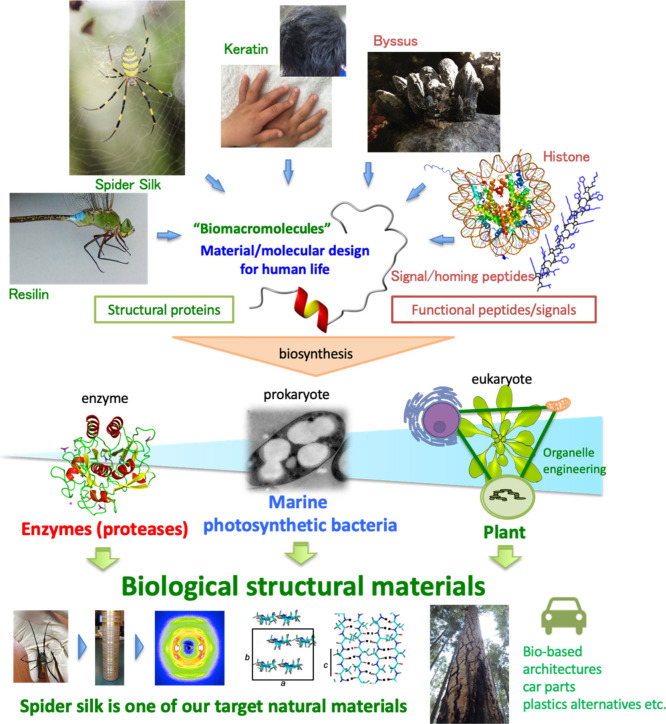
The various physical and biological properties and functions of naturally occurring polymers and composite materials have not yet been able to be replicated in synthetic polymers and artificial materials.1−3 Many of the unique properties of these natural materials depend on the hierarchical structure, including the primary structure (chemical structure), of the biopolymers produced by a particular species. If we can elucidate these hierarchical structures and clarify the mechanisms that contribute to the physical properties of natural materials, it will be possible for humans to artificially mimic the unique physical properties of natural polymers and biopolymers. While we have learned from silkworm silk and developed nylon, no polymer that combines the toughness, lightness, and interfacial affinity of spider silk has been developed. Thus, it is necessary to move away from the current biomimetic science research and establish an academic field where biological materials can be artificially created and used in a practical manner and to establish a process where materials used by humans do not put the global environment and the natural environment at risk and do not compete with food and energy production.
In nature, there are many attractive biomacromolecules, such as protein,4 polyhydroxyalkanoate,5,6 natural rubber,7,8 cellulose,9−12 lignin,13,14 chitin,15−17 and nucleic acid.18−21 Among them, I have been continuously studying biomacromolecules synthesized by living organisms, such as proteins, biopolyesters, and natural rubber.1,3,22−25 In recent years, I have attempted to develop new processes for the artificial production of biomacromolecules such as cellulose, polyhydroxyalkanoate (PHA), and those found in spider silk and natural rubber by using inexhaustible resources (nitrogen and carbon dioxide) from air and seawater and photosynthetic organisms (Figure 1).26,27 If this series of studies reveals promising results, then it will be possible to prevent resource depletion and reduce greenhouse gas emissions while simultaneously establishing a material cycle that is symbiotic with nature. While building a production process based on the fixation of nitrogen and carbon dioxide via photosynthetic organisms, we aim to design molecules and materials that are stable during use and return to the natural environment after use. This process of recycling materials is important for low-resource countries and regions. Moreover, this process does not place a burden on the global environment by utilizing carbon and nitrogen fixation via photosynthetic organisms. I suggest that the creation of next-generation biomanufacturing industries, as well as chemical industries, will lead to the realization of a harmonious global society through organic linkages with next-generation agriculture, animal husbandry, fisheries, and forestry industries. In this Perspective, structural proteins are introduced as representative examples of biopolymers, and the decomposition of carbon dioxide and biomacromolecule biosynthesis from carbon dioxide are outlined.
Figure 1.
Overview of the design and sustainable biosynthesis of spider silk-based materials. Using carbon dioxide and nitrogen, artificial silk was synthesized from marine red photosynthetic bacteria (left figure).28−30 The spinning mechanism of spiders has been elucidated at the molecular level, and a hierarchical structure similar to spider silk was successfully reproduced for the first time in the world (lower right figure).31−35 A database of spider silk proteins was constructed, and the use of material informatics enabled the development and commercialization of artificial silk with excellent water resistance (upper right figure).36,37 Copyright 2020 AAAS.
II-i. Structural Proteins
For the past 10 years, I have focused on structural proteins, which are biological materials, as indispensable materials for a sustainable global society. A structural protein is a protein that possesses a characteristic amino acid sequence or motif with tandem repeats, according to previous literature.2 Structural proteins can be fibrillar proteins but partially similar to intrinsically disordered proteins.38−40 In most cases, such motifs form higher-order structures via intermolecular or intramolecular interactions, resulting in biological materials with specific physical characteristics. However, considering amorphous protein materials, the requirement of a hierarchical structure is not included in this definition. Structural proteins in nature include spider silk, silkworm silk and other silks, collagen, elastin, resilin, reflectin, and keratin (Figure 2).41−57 Moreover, biomineralization, glycosylation, and post-translational modifications can be combined to achieve even more diverse physical and biological properties.58−61 Thus, it is important to understand materials in nature in terms of their molecular units and use this information to design innovative biopolymers and develop artificial materials. I expect to contribute to the construction of a sustainable society by creating biomacromolecular materials with both mechanical properties and high environmental degradability that fundamentally solve the problems caused by the use of polymeric materials, such as microplastics in the ocean and carbon dioxide emissions from petroleum raw materials and processes.
Figure 2.
Pictures of silk producers and silk threads. (a) Trichonephila clavata spider and threads; (b) Nephila pilipes spider on the author’s hand; other silk producers, (c) silkworms and (d) bagworms; (e) spider egg case; and (f) silkworm cocoons and silk threads. (g) Examples of structural proteins are found in nature. Their roles/functions in nature and typical repeat sequences are listed.2 Copyright 2020 Springer-Nature.
II-ii. Spider Silk
Spider silk, which is composed of the structural protein silk, has attracted attention from various research fields and industries as a material that exhibits a variety of physical properties that cannot be achieved with existing structural materials.37,62,63 Using spidroin, the main component of spider silks, as a model polymer, we have clarified its hierarchical structure (especially crystal formation) and the mechanisms of its physical properties (Figure 3).23,64,65 The spider draglines are composed of multiple proteins called major ampullate spidroins (MaSps), but how many types of MaSps exist is still under debate worldwide.37,62,63 Currently, many types have been reported, including MaSp1, MaSp2, and MaSp3, whereas spider-silk constituting element (SpiCE), which is present in trace amounts but has been found to significantly affect the mechanical properties, can be an essential component to design artificial spider silk.37 I studied the self-assembly behavior of MaSp1 and MaSp2 via various techniques, and the interaction between MaSp leads to dimerization of the C-terminal domain,32,66 followed by liquid–liquid phase separation (LLPS) to form a fibrillar network with N-terminal domain dimerization;31−33,66 then, dehydration and shear stress occur to form a bundle-like structure of microfibrils.31,32,67 The ionic conditions affect the self-assembly behavior of the terminal domains of MaSp; for example, chaotropic ions cause MaSp to have more random structures, but kosmotropic ions, including phosphate ions, induce more hydrogen bonding and self-assembly such as LLPS.31,34,35 The effect of water molecules on the formation process of the beta-sheet structure has also been studied widely with various silks.68,69 Since I have published other reviews on the spinning process of these spider silk threads,23,64 I will not got into depth here. However, the molecular mechanism for the construction of traction threads involves a complex system with multiple proteins and is not fully understood. In the future, it will be necessary to address the self-assembly behavior of multiple proteins and the formation mechanism of hierarchical structures and to elucidate the mechanism underlying the unique mechanical properties resulting from the presence of heterogeneous proteins.
Figure 3.
Overview of studies on the self-assembly behaviors and structures of spider silk MaSp proteins.65 Copyright 2024 Wiley-VCH GmbH.
As mentioned above, spider silk is a natural fiber that has high strength and toughness and is attracting attention as a biomaterial that can replace existing petroleum-derived polymer materials. Since spider silk has a wide range of physical properties depending on its type and protein composition, artificial spider silk materials are expected to be used for a variety of applications, including next-generation high-strength structural materials.37,62,63 However, the types of natural spider silk proteins and the amino acid sequences that affect their physical properties have not been fully elucidated. There have been several reports on the relationship between the amino acid sequence and the physical properties of the proteins of traction threads, which show particularly high toughness among the multiple types of spider silk, but these reports are limited. An international research group including the author has been collecting various types of spiders distributed worldwide and constructing a database on the structure and physical properties of diverse spider silk proteins.36 After the collection of more than 1000 different spider species from various regions of the world, including Asia, America, and Europe, RNA was extracted from the cells of each spider, and genetic information such as the type and amino acid sequence of the spider silk proteins in the spider silk was analyzed by Arakawa and his colleagues. They also measured 12 properties of traction threads collected from each spider, including mechanical properties (tensile strength, elongation rate, and toughness), thermal stability, and thread diameter. The collected spiders were phylogenetically classified, and a database was created by linking the genetic information (RNA base sequence and amino acid sequence) to the physical properties of the biomacromolecules using RNA. The spider silk proteins for which amino acid sequences have been identified thus far have been derived from 52 species of spiders, and the number of protein types is limited; however, spider silk protein data from many spider species was obtained from the database in this study. This is the first comprehensive database combining spider silk protein structures and spider silk properties and has been published as the Spider Silkome Database (Silkome, https://spider-silkome.org/).36 Currently, various molecules are designed using Silkome, which uses the sequences associated with specific properties of interest. For example, the correlation between the amino acid sequence of the spider silk protein and hypercontraction, which indicates sensitivity to water, revealed that the glutamine-glutamine (diglutamine, QQ) motif and proline in MaSp2 were significantly positively correlated, indicating that these motifs are the main factors responsible for sensitivity to water.65,70 By replacing these amino acids/motifs with hydrophobic amino acids, it was possible to develop artificial silk with excellent water resistance, which is a rare success story from the viewpoint of material informatics in polymers.65 In the future, artificial spider silk materials can be created at will by rational molecular design on the basis of the prediction of physical properties from the amino acid sequence.
III-i. Precise Polymer Degradation
One of the attractive properties of structural proteins is the combination of biodegradability and mechanical properties. In this section, I discuss the biodegradability and degradation products of structural proteins. Polymer science textbooks generally include the synthesis, properties, structures, and functions of numerous polymers. This might be because many polymer textbooks originated from Principles of Polymer Chemistry by P. J. Flory.71 Even in the latest textbooks, there are few descriptions of polymer degradation, with only a few references to thermal degradation, photodegradation (photooxidation), oxidative degradation, and biodegradation. However, there is a strong trade-off relationship between physical properties and degradability, and it is difficult to reconcile functionality and mechanical properties with degradability. In fact, there is a demand for polymeric materials that can be quickly degraded after use by inserting some molecular switches (in response to external and/or multiple stimuli); many researchers are working on such materials, but they have not yet been realized. In this context, basic molecular theory is needed to precisely induce or inhibit the degradation of polymers without interfering with their physical properties and functions. In fact, the precise degradation of polymers is necessary and attractive in many fields. For example, in the design and synthesis of new polymers, prediction of the degradation behavior is needed, and in the study of deformation and disintegration, it is necessary to chemically understand the molecular cleavage that occurs at the fracture interface. In the natural environment, additionally, the design of monomers and polymers must consider physical disintegration, chemical hydrolysis and photodegradation, and enzymatic degradation and biological metabolism involving living organisms (Figure 4). Thus, a systematic understanding of polymer degradation is important for a wide variety of disciplines.
Figure 4.
Various possibilities for the precise degradation of polymers.
As described above, it is necessary to understand precise polymer degradation mechanisms, such as physical degradation, chemical cleavage, and biological metabolism, which will lead to the establishment of new polymer material design guidelines. To the best of our knowledge, biopolymers and biomacromolecules are indispensable in these material design guidelines worldwide. Considering the wide range of environments in which polymeric/biomacromolecular materials are used, it is scientifically important and an interesting research topic to clarify the molecular mechanisms that dynamically influence the physical properties and functions of biopolymers under the conditions of physical breakdown, chemical degradation, biodegradation, and biological metabolism in vivo and in the natural environment.72,73 When this series of studies is accomplished, it is expected to be applicable in a wide range of academic fields for creating biomacromolecules with precisely designed and controlled degradability, such as polymers that are safe even when released into the environment, polymers that can be safely used in the body for a long time, and polymers that can be recycled in a closed-loop manner. One of the candidate biomacromolecules with such controllable biodegradability is structural proteins, including spider silk.
III-ii. Biodegradation into CO2 and/or Natural Molecules at Their Natural Concentrations
Do you think that structural proteins such as silk are sustainable plastics or polymers? In the white paper from Chemical Sciences and Society Summit (CS3), London, 2019 (“Science to enable sustainable plastics”, https://www.rsc.org/news-events/articles/2020/jun/science-to-enable-sustainable-plastics/), structural proteins such as spider silk were also introduced as an environmentally degradable polymer. Examples of the use of plastics that are biodegradable, especially examples of the use of on-demand biodegradation that induces degradation after use, are very limited. Precision control of polymer degradation is very important not only for ensuring the properties of degradable polymers but also for extending the long-term stability of polymer materials. Currently, it is challenging to suppress the deterioration and decomposition of polymer materials and improve their stability for several decades, preventing their use as major structural materials like metals. However, it is possible to address these problems related to the long-term stability and controlled degradability of polymer materials by understanding the decomposition of polymers from a multilevel perspective.
Silk, as a structural protein and a natural polymer, is biodegradable, which makes it a promising alternative to synthetic petroleum-based polymers. I have studied the biodegradability of silk resins through biochemical oxygen demand (BOD) tests and confirmed their breakdown in natural environments (Figure 5). Compared with synthetic polymers or biodegradable polyesters, natural structural proteins and artificial silk materials (resins, films, gels, coatings, composites, and fibers) can be degraded in the natural environment,74−77 and the degradation products are nontoxic to model organisms, e.g., Daphnia magna.78−80 These degradation productions are unique compared with biodegradable polyesters and other materials.80 Further tests were performed to assess the impact of coating silk films with fluorinated polypeptides, which increased water repellency without significantly affecting biodegradability.76 Scanning electron microscopy (SEM) revealed that the coated films developed surface roughness and cracks when exposed to seawater over time, indicating successful degradation. These results suggest that fluorinated polypeptides can alter the material properties of silk without compromising environmental friendliness, paving the way for more sustainable polymer materials.
Figure 5.
Biodegradation and environmental effects of model silk fragments, namely, polymers and oligomers of Ala and different nylon units. (a) BOD tests of poly(AlaNylXAla) and polyAla in seawater at 25 °C.78 (b) Daphnia magna used for the environmental toxicity test. (c) Kaplan–Meier survival curves for evaluating the effects of poly(AlaNylXAla) and polyAla on the aquatic model animal Daphnia magna at 20 °C. Control denotes the background values without any test samples.78 Copyright 2020 Royal Society of Chemistry.
IV-i. Biomacromolecule Synthesis from CO2
To realize a sustainable society and a circular economy, polymer scientists need to create biomacromolecular materials that are attractive from an environmental, social, and governance (ESG) perspective. To solve the social problems caused by polymeric materials such as plastics, it is important for both industry and academia to create environmentally degradable and recyclable polymer materials that are friendly to the earth by making full use of biotechnology as well as CO2 and nitrogen as raw materials. In particular, I aim to achieve biopolymer production using photosynthetic organisms through a process with a low environmental impact that does not require organic solvents, high-temperature/pressure, or harsh conditions.22,81,82 For example, the spinning process of artificial silk fibers using only aqueous solvents is progressing with an emphasis on zero-carbon processes.32,65,67,83−85 Not only are the final biopolymer products environmentally degradable, but the synthesis and process also do not use fossil resources as raw materials, which coincides with zero-carbon initiatives.
Among photosynthetic organisms, purple photosynthetic bacteria are unique. In addition to CO2 and nitrogen fixation, purple photosynthetic bacteria, as exemplified by PHA and spider silk protein production, have been reported to be beneficial for biopolymer production.26,28,82,86−89 Industrial fermentation and production technologies generally utilize heterotrophic microorganisms, which require sugar or vegetable oil as a carbon source and fresh water as a culture medium for the growth of heterotrophic microorganisms, and there are concerns that as the scale of production increases, there will be competition for human food and water resources. Marine purple photosynthetic bacteria do not need a carbon source and do not compete with freshwater resources when they carry out photosynthesis under anaerobic conditions to promote CO2 fixation. Furthermore, biopolymer/biomacromolecular production with marine photosynthetic bacteria has advantages in terms of raw material costs and large-scale production. Recently, genetic modification technology for marine photosynthetic purple bacteria has made it possible to produce exogenous proteins and metabolites.29,30,90 The author studied and developed a system to synthesize biopolymeric materials from purple photosynthetic bacteria via the use of inexhaustible seawater, CO2, and nitrogen as starting materials (Figure 6).26,27,88,91 This bioproduction using autotrophic microorganisms has some drawbacks, and it is necessary to meet the need for an electron donor since electrons cannot be extracted from water splitting.
Figure 6.

Photosynthetic bacterial biopolymer synthesis.
I constructed a basic technology for the artificial synthesis of structural protein materials and biopolymers, such as spider silk, using Rhodovulum sulfidophilum, a marine purple photosynthetic bacterium isolated from seawater, as the main strain.26,87,88R. sulfidophilum, which can be cultured under seawater conditions and has also been isolated from the seawaters in Japan, fixes carbon dioxide via photosynthesis and nitrogen via nitrogenase activity.91 So far, the common genetic recombination is by gene transfer via Escherichia coli, but the “peptide method” I developed has made it possible to prepare recombinants in a high-throughput manner. The peptide method is a gene delivery system that uses fusion peptides to introduce exogenous DNA/RNA into various organisms as well as specific organelles (Figure 7).30,90,92−100 The promoter, terminator, and selection marker genes used to express the desired structural protein by genetic recombination were also optimized, and an artificial silk, i.e., a biopolymer with a polyamide backbone, was successfully synthesized from R. sulfidophilum.28 The process by which photosynthetic bicarbonate ions are metabolized into amino acids and the process by which nitrogen gas is incorporated into amino acids via nitrogenase activity have also been confirmed from 13C and 15N labeling experiments, providing scientific evidence of carbon dioxide and nitrogen fixation.91 Thus, the use of photosynthetic organisms with carbon dioxide and nitrogen fixation abilities resolves the issue of limited carbon and nitrogen sources and reveals the possibility of circular polymer production.
Figure 7.
Schematic of the peptide method.22,98,101 A carrier peptide consisting of two domains is designed and synthesized and complexed with the molecule to be delivered, such as DNA or protein (upper left figure). Moreover, macropinocytosis-inducing peptides are processed into target organisms (upper right figure), enabling efficient transformation and functional modification of cells (lower figure).
IV-ii. Artificial Silk Production via Photosynthesis (Air Silk)
As described in the previous section, the biosynthesis of spider silk protein by marine purple photosynthetic bacteria was performed via genetic modification.28 The key technology for the future is how to utilize carbon dioxide and nitrogen for material production. Recently, a collaborating team and I succeeded in creating a zero-carbon protein fiber (Air Silk) using marine purple photosynthetic bacteria (Figure 8).65 These protein fibers were prepared in a similar manner to natural spider silk;67 hence, the coagulations were performed with weak acidic buffers. Air Silk can be prepared without an organic solvent and strong acid/alkaline conditions and therefore is expected to contribute to a sustainable next-generation textile industry as a new alternative to synthetic fibers.
Figure 8.
Development of Air Silk using photosynthetic bacteria. A scheme for introducing a gene encoding MaSp2 from spider silk into marine red photosynthetic bacteria and biosynthesizing silk protein through fermentation and culture.28 The aim is to create a new value-added fiber material by converting the obtained silk protein into fiber.65 Copyright 2024 Wiley-VCH GmbH.
IV-iii. Potential of Biological Synthesis of Biomacromolecules
In the last 20 years, I have synthesized various biopolymers through various biological processes (Figure 9). Recently, using plant cells, intact plants, cyanobacteria, and marine purple photosynthetic bacteria, we established a platform for the artificial synthesis of PHA, a biomass plastic, and structural proteins such as spider silk.27,28,81,82,102,103
Figure 9.
How can we design, synthesize, and process biomacromolecules? Schematic illustration of the author’s research strategy. Sustainable biomacromolecular design, biosynthesis, and material processing are needed to achieve optimized physical, chemical, and biological properties/functions.3
The synthesis method that I ultimately aimed to generate various biomacromolecules via photosynthetic organisms was performed using abundant raw materials such as air. As introduced above, the purple photosynthetic bacterial production is one of the successful examples. In addition to marine purple photosynthetic bacteria, my target photosynthetic organisms include plants, trees, cultured plant cells, algae, cyanobacteria, and photosynthetic bacteria.104−112 Using the inexhaustible resources of air (CO2 and nitrogen) and seawater and photosynthetic organisms, my research group has attempted to achieve new production processes for biopolymers and proteins such as spider silk, cellulose, natural rubber, and other types of biopolymers. If this kind of research is accomplished successfully, a material cycle that is symbiotic with nature can be established while reducing resource depletion and rising greenhouse gas emissions. By building a production process based on the fixation of nitrogen and carbon dioxide via photosynthetic organisms, the next step can be designing and developing molecules and materials that are stable during use and return to the natural environment after use. This ultimate resource recycling of materials can be important for low-resource countries and regions, especially in Japan, by reducing the burden on the environment through the use of carbon dioxide and nitrogen as raw materials. Instead of conventional petroleum-derived plastics and synthetic fibers, I would like to promote the spread of environmentally friendly zero-carbon materials and products in terms of raw materials, manufacturing methods, and life cycles.
V. Conclusions and Future Perspectives
While silkworm silk has been extensively studied in the context of nylon development, no polymer that combines the toughness, lightness, and interfacial affinity of spider silk has been developed. In addition, although natural rubber is widely used and indispensable to modern society, elucidation of the mechanisms underlying its physical properties, such as strain-induced crystallization and artificial reproduction of its mechanical properties, has not been achieved. To the best of the author’s knowledge, these scientific limitations may be because only the chemical structure has been elucidated and not the hierarchical or network structure. In the future, I would like to continue to investigate biomacromolecules, including their hierarchical structure, and clarify the relationship between structure and function to provide novel insights for the development of artificial biological materials.
Using spider silk as an example in this Perspective, it is important to understand biological materials in nature in terms of their biomacromolecules and other constituent units and to elucidate the relationships between their properties and molecular composition, which leads to sustainable material innovation. The creation of polymeric materials with both optimized physical properties and environmental degradability will fundamentally solve the problems caused by polymeric materials, such as microplastics in the ocean and carbon dioxide emissions from petroleum raw materials and processes, and contribute to the construction of a sustainable and circular society. On a slightly larger scale, it is necessary to move away from the current biomimetic research and establish an academic domain where biological materials can be created and used in a practical manner and establish a process where materials used by humans do not put the global environment and the natural environment at risk and do not compete with food and energy production. I am convinced that such an academic field is essential for a sustainable global society and can be important for low-resource countries and regions, including Japan. Although not mentioned in this Perspective, efforts are underway in the fish industry to develop zero-carbon fishery feed by generating optimized proteins via photosynthetic bacteria and in the agricultural sector to develop zero-carbon fertilizers such as amino acid-based nitrogen fertilizer.113 I hope to contribute to the development of a sustainable global society not only through chemical research but also through biology studies and through tight linkages with diverse fields such as next-generation aquaculture and agriculture.
Acknowledgments
I would like to thank all the students, postdocs, lab members, and collaborators who contributed to this biomacromolecule research.
Author Contributions
K.N. designed, wrote, and edited the manuscript.
This work was supported by the Japan Science and Technology Agency (JST) Exploratory Research for Advanced Technology (ERATO, JPMJER1602), JST COI-NEXT (Grant Number JPMJPF2114), and the MEXT Program: Data Creation and Utilization-Type Material Research and Development Project Grant Number JPMXP1122714694.
The author declares no competing financial interest.
Dedication
This Perspective is dedicated to the 25th Anniversary of Biomacromolecules.
References
- Numata K. Poly(amino acid)s/polypeptides as potential functional and structural materials. Polym. J. 2015, 47 (8), 537–545. 10.1038/pj.2015.35. [DOI] [Google Scholar]
- Numata K. How to define and study structural proteins as biopolymer materials. Polym. J. 2020, 52 (9), 1043–1056. 10.1038/s41428-020-0362-5. [DOI] [Google Scholar]
- Numata K. In Biopolymer science for proteins and peptides, 1st ed.; Elsevier, 2021. [Google Scholar]
- Omenetto F. G.; Kaplan D. L. New opportunities for an ancient material. Science 2010, 329 (5991), 528–31. 10.1126/science.1188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudesh K.; Abe H.; Doi Y. Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog. Polym. Sci. 2000, 25 (10), 1503–1555. 10.1016/S0079-6700(00)00035-6. [DOI] [Google Scholar]
- Vert M.; Doi Y.; Hellwich K. H.; Hess M.; Hodge P.; Kubisa P.; Rinaudo M.; Schué F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84 (2), 377–408. 10.1351/PAC-REC-10-12-04. [DOI] [Google Scholar]
- Payungwong N.; Wu J. R.; Sakdapipanich J. Unlocking the potential of natural rubber: A review of rubber particle sizes and their impact on properties. Polymer 2024, 308, 127419. 10.1016/j.polymer.2024.127419. [DOI] [Google Scholar]
- Yamashita S.; Takahashi S. Molecular Mechanisms of Natural Rubber Biosynthesis. Annual Review of Biochemistry, Vol 89 2020, 89, 821–851. 10.1146/annurev-biochem-013118-111107. [DOI] [PubMed] [Google Scholar]
- Isogai A. Emerging Nanocellulose Technologies: Recent Developments. Adv. Mater. 2021, 33 (28), 2000630 10.1002/adma.202000630. [DOI] [PubMed] [Google Scholar]
- Isogai A.; Saito T.; Fukuzumi H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3 (1), 71–85. 10.1039/C0NR00583E. [DOI] [PubMed] [Google Scholar]
- Li T.; Chen C.; Brozena A. H.; Zhu J. Y.; Xu L.; Driemeier C.; Dai J.; Rojas O. J.; Isogai A.; Wagberg L.; Hu L. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590 (7844), 47–56. 10.1038/s41586-020-03167-7. [DOI] [PubMed] [Google Scholar]
- Okita Y.; Fujisawa S.; Saito T.; Isogai A. TEMPO-oxidized cellulose nanofibrils dispersed in organic solvents. Biomacromolecules 2011, 12 (2), 518–22. 10.1021/bm101255x. [DOI] [PubMed] [Google Scholar]
- Grossman A.; Vermerris W. Lignin-based polymers and nanomaterials. Curr. Opin Biotechnol 2019, 56, 112–120. 10.1016/j.copbio.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Ralph J.; Lapierre C.; Boerjan W. Lignin structure and its engineering. Curr. Opin Biotechnol 2019, 56, 240–249. 10.1016/j.copbio.2019.02.019. [DOI] [PubMed] [Google Scholar]
- Pacheco N.; Garnica-Gonzalez M.; Gimeno M.; Barzana E.; Trombotto S.; David L.; Shirai K. Structural characterization of chitin and chitosan obtained by biological and chemical methods. Biomacromolecules 2011, 12 (9), 3285–90. 10.1021/bm200750t. [DOI] [PubMed] [Google Scholar]
- Trombotto S.; Ladaviere C.; Delolme F.; Domard A. Chemical preparation and structural characterization of a homogeneous series of chitin/chitosan oligomers. Biomacromolecules 2008, 9 (7), 1731–8. 10.1021/bm800157x. [DOI] [PubMed] [Google Scholar]
- Younes I.; Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 2015, 13 (3), 1133–74. 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L.; Bai C.; Platnich C. M.; Rizzuto F. J. Divergent Polymer Superstructures from Protonated Poly(adenine) DNA and RNA. Biomacromolecules 2024, 25 (5), 3163–3168. 10.1021/acs.biomac.4c00271. [DOI] [PubMed] [Google Scholar]
- Knappe G. A.; Wamhoff E. C.; Bathe M. Functionalizing DNA origami to investigate and interact with biological systems. Nat. Rev. Mater. 2023, 8 (2), 123–138. 10.1038/s41578-022-00517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler T.; Herrmann A. DNA block copolymers: functional materials for nanoscience and biomedicine. Acc. Chem. Res. 2012, 45 (9), 1419–30. 10.1021/ar200211a. [DOI] [PubMed] [Google Scholar]
- Wang D.; Hu Y.; Liu P.; Luo D. Bioresponsive DNA Hydrogels: Beyond the Conventional Stimuli Responsiveness. Acc. Chem. Res. 2017, 50 (4), 733–739. 10.1021/acs.accounts.6b00581. [DOI] [PubMed] [Google Scholar]
- Law S. S. Y.; Miyamoto T.; Numata K. Organelle-targeted gene delivery in plants by nanomaterials. Chem. Commun. (Camb) 2023, 59 (47), 7166–7181. 10.1039/D3CC00962A. [DOI] [PubMed] [Google Scholar]
- Malay A. D.; Craig H. C.; Chen J.; Oktaviani N. A.; Numata K. Complexity of Spider Dragline Silk. Biomacromolecules 2022, 23 (5), 1827–1840. 10.1021/acs.biomac.1c01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata K.; Abe H.; Doi Y. Enzymatic processes for biodegradation of poly(hydroxyalkanoate)s crystals. Can. J. Chem. 2008, 86 (6), 471–483. 10.1139/v08-004. [DOI] [Google Scholar]
- Numata K.; Abe H.; Iwata T. Biodegradability of Poly(hydroxyalkanoate) Materials. Materials 2009, 2 (3), 1104–1126. 10.3390/ma2031104. [DOI] [Google Scholar]
- Higuchi-Takeuchi M.; Numata K. Marine Purple Photosynthetic Bacteria as Sustainable Microbial Production Hosts. Front Bioeng Biotech 2019, 7, 258. 10.3389/fbioe.2019.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisawat P.; Higuchi-Takeuchi M.; Numata K. Microbial autotrophic biorefineries: Perspectives for biopolymer production. Polym. J. 2022, 54 (10), 1139–1151. 10.1038/s41428-022-00675-3. [DOI] [Google Scholar]
- Foong C. P.; Higuchi-Takeuchi M.; Malay A. D.; Oktaviani N. A.; Thagun C.; Numata K. A marine photosynthetic microbial cell factory as a platform for spider silk production. Commun. Biol. 2020, 3 (1), 357. 10.1038/s42003-020-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi-Takeuchi M.; Morisaki K.; Numata K. Method for the facile transformation of marine purple photosynthetic bacteria using chemically competent cells. MicrobiologyOpen 2020, 9 (1), e00953 10.1002/mbo3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T.; Toyooka K.; Chuah J. A.; Odahara M.; Higchi-Takeuchi M.; Goto Y.; Motoda Y.; Kigawa T.; Kodama Y.; Numata K. A Synthetic Multidomain Peptide That Drives a Macropinocytosis-Like Mechanism for Cytosolic Transport of Exogenous Proteins into Plants. JACS Au 2022, 2 (1), 223–233. 10.1021/jacsau.1c00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malay A. D.; Oktaviani N. A.; Chen J.; Numata K. Spider Silk: Rapid, Bottom-Up Self-Assembly of MaSp1 into Hierarchically Structured Fibers Through Biomimetic Processing. Adv. Funct. Mater. 2024, 2408175. 10.1002/adfm.202408175. [DOI] [Google Scholar]
- Malay A. D.; Suzuki T.; Katashima T.; Kono N.; Arakawa K.; Numata K. Spider silk self-assembly via modular liquid-liquid phase separation and nanofibrillation. Sci. Adv. 2020, 6 (45), eabb6030. 10.1126/sciadv.abb6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktaviani N. A.; Malay A. D.; Matsugami A.; Hayashi F.; Numata K. Unusual pK(a) Values Mediate the Self-Assembly of Spider Dragline Silk Proteins. Biomacromolecules 2023, 24 (4), 1604–1616. 10.1021/acs.biomac.2c01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktaviani N. A.; Matsugami A.; Hayashi F.; Numata K. Ion effects on the conformation and dynamics of repetitive domains of a spider silk protein: implications for solubility and beta-sheet formation. Chem. Commun. (Camb) 2019, 55 (66), 9761–9764. 10.1039/C9CC03538A. [DOI] [PubMed] [Google Scholar]
- Oktaviani N. A.; Matsugami A.; Malay A. D.; Hayashi F.; Kaplan D. L.; Numata K. Conformation and dynamics of soluble repetitive domain elucidates the initial beta-sheet formation of spider silk. Nat. Commun. 2018, 9 (1), 2121. 10.1038/s41467-018-04570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa K.; Kono N.; Malay A. D.; Tateishi A.; Ifuku N.; Masunaga H.; Sato R.; Tsuchiya K.; Ohtoshi R.; Pedrazzoli D.; Shinohara A.; Ito Y.; Nakamura H.; Tanikawa A.; Suzuki Y.; Ichikawa T.; Fujita S.; Fujiwara M.; Tomita M.; Blamires S. J.; Chuah J. A.; Craig H.; Foong C. P.; Greco G.; Guan J.; Holland C.; Kaplan D. L.; Sudesh K.; Mandal B. B.; Norma-Rashid Y.; Oktaviani N. A.; Preda R. C.; Pugno N. M.; Rajkhowa R.; Wang X.; Yazawa K.; Zheng Z.; Numata K. 1000 spider silkomes: Linking sequences to silk physical properties. Sci. Adv. 2022, 8 (41), eabo6043 10.1126/sciadv.abo6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono N.; Nakamura H.; Mori M.; Yoshida Y.; Ohtoshi R.; Malay A. D.; Pedrazzoli Moran D. A.; Tomita M.; Numata K.; Arakawa K. Multicomponent nature underlies the extraordinary mechanical properties of spider dragline silk. Proc. Natl. Acad. Sci. U.S.A. 2021, 118 (31), e2107065118. 10.1073/pnas.2107065118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield C. J.; Dunker A. K. Intrinsically disordered proteins and intrinsically disordered protein regions. Annu. Rev. Biochem. 2014, 83, 553–84. 10.1146/annurev-biochem-072711-164947. [DOI] [PubMed] [Google Scholar]
- Ruff K. M.; Pappu R. V. AlphaFold and Implications for Intrinsically Disordered Proteins. J. Mol. Biol. 2021, 433 (20), 167208. 10.1016/j.jmb.2021.167208. [DOI] [PubMed] [Google Scholar]
- Wright P. E.; Dyson H. J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16 (1), 18–29. 10.1038/nrm3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman G. H.; Diaz F.; Jakuba C.; Calabro T.; Horan R. L.; Chen J.; Lu H.; Richmond J.; Kaplan D. L. Silk-based biomaterials. Biomaterials 2003, 24 (3), 401–16. 10.1016/S0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- Blackledge T. A.; Perez-Rigueiro J.; Plaza G. R.; Perea B.; Navarro A.; Guinea G. V.; Elices M. Sequential origin in the high performance properties of orb spider dragline silk. Sci. Rep. 2012, 2, 782. 10.1038/srep00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crookes W. J.; Ding L. L.; Huang Q. L.; Kimbell J. R.; Horwitz J.; McFall-Ngai M. J. Reflectins: The unusual proteins of squid reflective tissues. Science 2004, 303 (5655), 235–238. 10.1126/science.1091288. [DOI] [PubMed] [Google Scholar]
- DeMartini D. G.; Izumi M.; Weaver A. T.; Pandolfi E.; Morse D. E. Structures, Organization, and Function of Reflectin Proteins in Dynamically Tunable Reflective Cells. J. Biol. Chem. 2015, 290 (24), 15238–15249. 10.1074/jbc.M115.638254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling L. M.; Crewther W. G.; Inglis A. S. The primary structure of component 8c-1, a subunit protein of intermediate filaments in wool keratin. Relationships with proteins from other intermediate filaments. Biochem. J. 1986, 236 (3), 695–703. 10.1042/bj2360695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvin C. M.; Carr A. G.; Huson M. G.; Maxwell J. M.; Pearson R. D.; Vuocolo T.; Liyou N. E.; Wong D. C.; Merritt D. J.; Dixon N. E. Synthesis and properties of crosslinked recombinant pro-resilin. Nature 2005, 437 (7061), 999–1002. 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- Kramer R. M.; Crookes-Goodson W. J.; Naik R. R. The self-organizing properties of squid reflectin protein. Nat. Mater. 2007, 6 (7), 533–538. 10.1038/nmat1930. [DOI] [PubMed] [Google Scholar]
- Lee C. H.; Singla A.; Lee Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221 (1–2), 1–22. 10.1016/S0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- Leisk G. G.; Lo T. J.; Yucel T.; Lu Q.; Kaplan D. L. Electrogelation for protein adhesives. Adv. Mater. 2010, 22 (6), 711–5. 10.1002/adma.200902643. [DOI] [PubMed] [Google Scholar]
- Mamat-Noorhidayah; Yazawa K.; Numata K.; Norma-Rashid Y. Morphological and mechanical properties of flexible resilin joints on damselfly wings (Rhinocypha spp.). PLoS One 2018, 13 (3), e0193147 10.1371/journal.pone.0193147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton K. L.; Phan L.; Leung E. M.; Kautz R.; Lin Q. Y.; Van Dyke Y.; Marmiroli B.; Sartori B.; Arvai A.; Li S.; Pique M. E.; Naeim M.; Kerr J. P.; Aquino M. J.; Roberts V. A.; Getzoff E. D.; Zhu C. H.; Bernstorff S.; Gorodetsky A. A. Self-Assembly of the Cephalopod Protein Reflectin. Adv. Mater. 2016, 28 (38), 8405–8412. 10.1002/adma.201601666. [DOI] [PubMed] [Google Scholar]
- Pauling L.; Corey R. B. Compound helical configurations of polypeptide chains: structure of proteins of the alpha-keratin type. Nature 1953, 171 (4341), 59–61. 10.1038/171059a0. [DOI] [PubMed] [Google Scholar]
- Qin G. K.; Hu X.; Cebe P.; Kaplan D. L. Mechanism of resilin elasticity. Nat. Commun. 2012, 3, 1003. 10.1038/ncomms2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G. K.; Lapidot S.; Numata K.; Hu X.; Meirovitch S.; Dekel M.; Podoler I.; Shoseyov O.; Kaplan D. L. Expression, Cross-Linking, and Characterization of Recombinant Chitin Binding Resilin. Biomacromolecules 2009, 10 (12), 3227–3234. 10.1021/bm900735g. [DOI] [PubMed] [Google Scholar]
- Qin G. K.; Rivkin A.; Lapidot S.; Hu X.; Preis I.; Arinus S. B.; Dgany O.; Shoseyov O.; Kaplan D. L. Recombinant exon-encoded resilins for elastomeric biomaterials. Biomaterials 2011, 32 (35), 9231–9243. 10.1016/j.biomaterials.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termonia Y. Molecular Modeling of Spider Silk Elasticity. Macromolecules 1994, 27 (25), 7378–7381. 10.1021/ma00103a018. [DOI] [Google Scholar]
- Zhang Y. Q. Applications of natural silk protein sericin in biomaterials. Biotechnol Adv. 2002, 20 (2), 91–100. 10.1016/S0734-9750(02)00003-4. [DOI] [PubMed] [Google Scholar]
- Wong Po Foo C.; Patwardhan S. V.; Belton D. J.; Kitchel B.; Anastasiades D.; Huang J.; Naik R. R.; Perry C. C.; Kaplan D. L. Novel nanocomposites from spider silk-silica fusion (chimeric) proteins. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (25), 9428–33. 10.1073/pnas.0601096103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q.; Yadavalli S. S.; Zhang S.; Sherman S. E.; Fiorin E.; da Silva L.; Wilson D. A.; Hammer D. A.; Andre S.; Gabius H. J.; Klein M. L.; Goulian M.; Percec V. Bioactive cell-like hybrids coassembled from (glyco)dendrimersomes with bacterial membranes. Proc. Natl. Acad. Sci. U.S.A. 2016, 113 (9), E1134 10.1073/pnas.1525589113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig H. C.; Malay A. D.; Hayashi F.; Mori M.; Arakawa K.; Numata K. Posttranslational modifications in spider silk influence conformation and dimerization dynamics. MRS Bull. 2024, 49, 1192. 10.1557/s43577-024-00771-0. [DOI] [Google Scholar]
- Numata K.; Baker P. J. Synthesis of adhesive peptides similar to those found in blue mussel (Mytilus edulis) using papain and tyrosinase. Biomacromolecules 2014, 15 (8), 3206–12. 10.1021/bm5009052. [DOI] [PubMed] [Google Scholar]
- Babb P. L.; Lahens N. F.; Correa-Garhwal S. M.; Nicholson D. N.; Kim E. J.; Hogenesch J. B.; Kuntner M.; Higgins L.; Hayashi C. Y.; Agnarsson I.; Voight B. F. The Nephila clavipes genome highlights the diversity of spider silk genes and their complex expression. Nat. Genet. 2017, 49 (6), 895–903. 10.1038/ng.3852. [DOI] [PubMed] [Google Scholar]
- Sonavane S.; Hassan S.; Chatterjee U.; Soler L.; Holm L.; Mollbrink A.; Greco G.; Fereydouni N.; Vinnere Pettersson O.; Bunikis I.; Churcher A.; Lantz H.; Johansson J.; Reimegard J.; Rising A. Origin, structure, and composition of the spider major ampullate silk fiber revealed by genomics, proteomics, and single-cell and spatial transcriptomics. Sci. Adv. 2024, 10 (33), eadn0597 10.1126/sciadv.adn0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C.; Numata K.; Rnjak-Kovacina J.; Seib F. P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc Mater. 2019, 8 (1), 1800465 10.1002/adhm.201800465. [DOI] [PubMed] [Google Scholar]
- Numata K.; Kaplan D. L. Silk Proteins: Designs from Nature with Multipurpose Utility and Infinite Future Possibilities. Adv. Mater. 2024, 2411256. 10.1002/adma.202411256. [DOI] [PubMed] [Google Scholar]
- Hagn F.; Eisoldt L.; Hardy J. G.; Vendrely C.; Coles M.; Scheibel T.; Kessler H. A conserved spider silk domain acts as a molecular switch that controls fibre assembly. Nature 2010, 465 (7295), 239–42. 10.1038/nature08936. [DOI] [PubMed] [Google Scholar]
- Chen J.; Tsuchida A.; Malay A. D.; Tsuchiya K.; Masunaga H.; Tsuji Y.; Kuzumoto M.; Urayama K.; Shintaku H.; Numata K. Replicating shear-mediated self-assembly of spider silk through microfluidics. Nat. Commun. 2024, 15 (1), 527. 10.1038/s41467-024-44733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa K.; Ishida K.; Masunaga H.; Hikima T.; Numata K. Influence of Water Content on the beta-Sheet Formation, Thermal Stability, Water Removal, and Mechanical Properties of Silk Materials. Biomacromolecules 2016, 17 (3), 1057–1066. 10.1021/acs.biomac.5b01685. [DOI] [PubMed] [Google Scholar]
- Yazawa K.; Malay A.; Masunaga H.; Norma-Rashid Y.; Numata K. Simultaneous effect of strain rate and humidity on the structure and mechanical behavior of spider silk. Commun. Mater. 2020, 1 (1), 10. 10.1038/s43246-020-0011-8. [DOI] [Google Scholar]
- Malay A. D.; Arakawa K.; Numata K. Analysis of repetitive amino acid motifs reveals the essential features of spider dragline silk proteins. PLoS One 2017, 12 (8), e0183397 10.1371/journal.pone.0183397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory P. J. In Principles of Polymer Chemistry; Cornell University Press, 1983; Vol. 186. [Google Scholar]
- Hayashi K.; Okamoto F.; Hoshi S.; Katashima T.; Zujur D. C.; Li X.; Shibayama M.; Gilbert E. P.; Chung U.; Ohba S.; Oshika T.; Sakai T. Fast-forming hydrogel with ultralow polymeric content as an artificial vitreous body. Nat. Biomed Eng. 2017, 1, 0044. 10.1038/s41551-017-0044. [DOI] [Google Scholar]
- Iwata T. Biodegradable and bio-based polymers: future prospects of eco-friendly plastics. Angew. Chem., Int. Ed. Engl. 2015, 54 (11), 3210–5. 10.1002/anie.201410770. [DOI] [PubMed] [Google Scholar]
- Numata K.; Cebe P.; Kaplan D. L. Mechanism of enzymatic degradation of beta-sheet crystals. Biomaterials 2010, 31 (10), 2926–33. 10.1016/j.biomaterials.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata K.; Ifuku N.; Masunaga H.; Hikima T.; Sakai T. Silk Resin with Hydrated Dual Chemical-Physical Cross-Links Achieves High Strength and Toughness. Biomacromolecules 2017, 18 (6), 1937–1946. 10.1021/acs.biomac.7b00376. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K.; Ifuku N.; Koyama Y.; Numata K. Development of regenerated silk films coated with fluorinated polypeptides to achieve high water repellency and biodegradability in seawater. Polym. Degrad. Stab. 2019, 160, 96–101. 10.1016/j.polymdegradstab.2018.12.016. [DOI] [Google Scholar]
- Arai T.; Freddi G.; Innocenti R.; Tsukada M. Biodegradation of Bombyx mori silk fibroin fibers and films. J. Appl. Polym. Sci. 2004, 91 (4), 2383–2390. 10.1002/app.13393. [DOI] [Google Scholar]
- Gudeangadi P. G.; Uchida K.; Tateishi A.; Terada K.; Masunaga H.; Tsuchiya K.; Miyakawa H.; Numata K. Poly(alanine-nylon-alanine) as a Bioplastic: chemoenzymatic synthesis, thermal properties and biological degradation effects. Polym. Chem-Uk 2020, 11 (30), 4920–4927. 10.1039/D0PY00137F. [DOI] [Google Scholar]
- Matsumoto M.; Ito H.; Tateishi A.; Kobayashi Y.; Satoh K.; Numata K.; Miyakawa H. Effects of polycaprolactone degradation products on the water flea, Daphnia magna: Carbodiimide additives have acute and chronic toxicity. J. Appl. Toxicol 2023, 43 (12), 1840–1848. 10.1002/jat.4516. [DOI] [PubMed] [Google Scholar]
- Yoshinaga N.; Tateishi A.; Kobayashi Y.; Kubo T.; Miyakawa H.; Satoh K.; Numata K. Effect of Oligomers Derived from Biodegradable Polyesters on Eco- and Neurotoxicity. Biomacromolecules 2023, 24 (6), 2721–2729. 10.1021/acs.biomac.3c00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizumi T.; Yamada M.; Higuchi-Takeuchi M.; Matsumoto K.; Taguchi S.; Matsui M.; Numata K. Sucrose supplementation suppressed the growth inhibition in polyhydroxyalkanoate-producing plants. Plant Biotechnol (Tokyo) 2017, 34 (1), 39–43. 10.5511/plantbiotechnology.16.1121a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thagun C.; Suzuki T.; Kodama Y.; Numata K. C-Terminal Domain Controls Protein Quality and Secretion of Spider Silk in Tobacco Cells. Adv. Biol. (Weinh) 2023, 7 (12), 2300011 10.1002/adbi.202300011. [DOI] [PubMed] [Google Scholar]
- Andersson M.; Jia Q.; Abella A.; Lee X. Y.; Landreh M.; Purhonen P.; Hebert H.; Tenje M.; Robinson C. V.; Meng Q.; Plaza G. R.; Johansson J.; Rising A. Biomimetic spinning of artificial spider silk from a chimeric minispidroin. Nat. Chem. Biol. 2017, 13 (3), 262–264. 10.1038/nchembio.2269. [DOI] [PubMed] [Google Scholar]
- Koeppel A.; Holland C. Progress and Trends in Artificial Silk Spinning: A Systematic Review. Acs Biomater Sci. Eng. 2017, 3 (3), 226–237. 10.1021/acsbiomaterials.6b00669. [DOI] [PubMed] [Google Scholar]
- Rising A.; Johansson J. Toward spinning artificial spider silk. Nat. Chem. Biol. 2015, 11 (5), 309–15. 10.1038/nchembio.1789. [DOI] [PubMed] [Google Scholar]
- Foong C. P.; Higuchi-Takeuchi M.; Numata K. Optimal iron concentrations for growth-associated polyhydroxyalkanoate biosynthesis in the marine photosynthetic purple bacterium Rhodovulum sulfidophilum under photoheterotrophic condition. PLoS One 2019, 14 (4), e0212654 10.1371/journal.pone.0212654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi-Takeuchi M.; Morisaki K.; Numata K. A Screening Method for the Isolation of Polyhydroxyalkanoate-Producing Purple Non-sulfur Photosynthetic Bacteria from Natural Seawater. Front Microbiol 2016, 7, 1509. 10.3389/fmicb.2016.01509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi-Takeuchi M.; Morisaki K.; Toyooka K.; Numata K. Synthesis of High-Molecular-Weight Polyhydroxyalkanoates by Marine Photosynthetic Purple Bacteria. PLoS One 2016, 11 (8), e0160981 10.1371/journal.pone.0160981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi-Takeuchi M.; Numata K. Acetate-Inducing Metabolic States Enhance Polyhydroxyalkanoate Production in Marine Purple Non-sulfur Bacteria Under Aerobic Conditions. Front Bioeng Biotech 2019, 7, 118. 10.3389/fbioe.2019.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi-Takeuchi M.; Miyamoto T.; Foong C. P.; Goto M.; Morisaki K.; Numata K. Peptide-Mediated Gene Transfer into Marine Purple Photosynthetic Bacteria. Int. J. Mol. Sci. 2020, 21 (22), 8625. 10.3390/ijms21228625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisawat P.; Higuchi-Takeuchi M.; Honda R.; Shirai T.; Kondo A.; Hoshino Y.; Numata K. Engineered Nanogel Particles Enhance the PhotoautotrophicBiosynthesis of Polyhydroxyalkanoate in Marine PhotosyntheticBacteria. Acs Sustain Chem. Eng. 2022, 10 (13), 4133–4142. 10.1021/acssuschemeng.1c07252. [DOI] [Google Scholar]
- Abe N.; Fujita S.; Miyamoto T.; Tsuchiya K.; Numata K. Plant Mitochondrial-Targeted Gene Delivery by Peptide/DNA Micelles Quantitatively Surface-Modified with Mitochondrial Targeting and Membrane-Penetrating Peptides. Biomacromolecules 2023, 24 (8), 3657–3665. 10.1021/acs.biomac.3c00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan M.; Kodama Y.; Yoshizumi T.; Sudesh K.; Numata K. Rapid and efficient gene delivery into plant cells using designed peptide carriers. Biomacromolecules 2013, 14 (1), 10–6. 10.1021/bm301275g. [DOI] [PubMed] [Google Scholar]
- Law S. S. Y.; Liou G.; Nagai Y.; Gimenez-Dejoz J.; Tateishi A.; Tsuchiya K.; Kodama Y.; Fujigaya T.; Numata K. Polymer-coated carbon nanotube hybrids with functional peptides for gene delivery into plant mitochondria. Nat. Commun. 2022, 13 (1), 2417. 10.1038/s41467-022-30185-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T.; Tsuchiya K.; Numata K. Block Copolymer/Plasmid DNA Micelles Postmodified with Functional Peptides via Thiol-Maleimide Conjugation for Efficient Gene Delivery into Plants. Biomacromolecules 2019, 20 (2), 653–661. 10.1021/acs.biomac.8b01304. [DOI] [PubMed] [Google Scholar]
- Miyamoto T.; Tsuchiya K.; Numata K. Dual Peptide-Based Gene Delivery System for the Efficient Transfection of Plant Callus Cells. Biomacromolecules 2020, 21 (7), 2735–2744. 10.1021/acs.biomac.0c00481. [DOI] [PubMed] [Google Scholar]
- Miyamoto T.; Tsuchiya K.; Toyooka K.; Goto Y.; Tateishi A.; Numata K. Relaxation of the Plant Cell Wall Barrier via Zwitterionic Liquid Pretreatment for Micelle-Complex-Mediated DNA Delivery to Specific Plant Organelles. Angew. Chem., Int. Ed. Engl. 2022, 61 (32), e202204234 10.1002/anie.202204234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K.; Odahara M.; Miyamoto T.; Numata K. Fusion Peptide-Based Biomacromolecule Delivery System for Plant Cells. Acs Biomater Sci. Eng. 2021, 7 (6), 2246–2254. 10.1021/acsbiomaterials.1c00227. [DOI] [PubMed] [Google Scholar]
- Yoshinaga N.; Miyamoto T.; Goto M.; Tanaka A.; Numata K. Phenylboronic Acid-Functionalized Micelles Dual-Targeting Boronic Acid Transporter and Polysaccharides for siRNA Delivery into Brown Algae. JACS Au 2024, 4 (4), 1385–1395. 10.1021/jacsau.3c00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga N.; Miyamoto T.; Odahara M.; Takeda-Kamiya N.; Toyooka K.; Nara S.; Nishimura H.; Ling F.; Su’etsugu M.; Yoshida M.; Numata K. Design of an Artificial Peptide Inspired by Transmembrane Mitochondrial Protein for Escorting Exogenous DNA into the Mitochondria to Restore their Functions by Simultaneous Multiple Gene Expression. Adv. Funct. Mater. 2024, 34 (8), 2306070. 10.1002/adfm.202306070. [DOI] [Google Scholar]
- Miyamoto T.; Numata K. Advancing Biomolecule Delivery in Plants: Harnessing Synthetic Nanocarriers to Overcome Multiscale Barriers for Cutting-Edge Plant Bioengineering. B Chem. Soc. Jpn. 2023, 96 (9), 1026–1044. 10.1246/bcsj.20230147. [DOI] [Google Scholar]
- Osanai T.; Numata K.; Oikawa A.; Kuwahara A.; Iijima H.; Doi Y.; Tanaka K.; Saito K.; Hirai M. Y. Increased Bioplastic production with an RNA polymerase sigma factor SigE during nitrogen starvation in Synechocystis sp. PCC 6803. DNA Res. 2013, 20 (6), 525–35. 10.1093/dnares/dst028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K.; Numata K. Chemoenzymatic Synthesis of Polypeptides for Use as Functional and Structural Materials. Macromol. Biosci 2017, 17 (11), 1700177. 10.1002/mabi.201700177. [DOI] [PubMed] [Google Scholar]
- Balabanova L.; Slepchenko L.; Son O.; Tekutyeva L. Biotechnology Potential of Marine Fungi Degrading Plant and Algae Polymeric Substrates. Front Microbiol 2018, 9, 1527. 10.3389/fmicb.2018.01527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabris M.; Abbriano R. M.; Pernice M.; Sutherland D. L.; Commault A. S.; Hall C. C.; Labeeuw L.; McCauley J. I.; Kuzhiuparambil U.; Ray P.; Kahlke T.; Ralph P. J. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front Plant Sci. 2020, 11, 279. 10.3389/fpls.2020.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastropetros S. G.; Pispas K.; Zagklis D.; Ali S. S.; Kornaros M. Biopolymers production from microalgae and cyanobacteria cultivated in wastewater: Recent advances. Biotechnol Adv. 2022, 60, 107999. 10.1016/j.biotechadv.2022.107999. [DOI] [PubMed] [Google Scholar]
- Sheridan C. A second chance for plant biotechnology in Europe. Nat. Biotechnol. 2024, 42 (5), 687–689. 10.1038/s41587-024-02246-8. [DOI] [PubMed] [Google Scholar]
- Snell K. D.; Singh V.; Brumbley S. M. Production of novel biopolymers in plants: recent technological advances and future prospects. Curr. Opin Biotechnol 2015, 32, 68–75. 10.1016/j.copbio.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Sudesh K.; Taguchi K.; Doi Y. Effect of increased PHA synthase activity on polyhydroxyalkanoates biosynthesis in Synechocystis sp. PCC6803. Int. J. Biol. Macromol. 2002, 30 (2), 97–104. 10.1016/S0141-8130(02)00010-7. [DOI] [PubMed] [Google Scholar]
- van Beilen J. B.; Poirier Y. Production of renewable polymers from crop plants. Plant J. 2008, 54 (4), 684–701. 10.1111/j.1365-313X.2008.03431.x. [DOI] [PubMed] [Google Scholar]
- Varshney P.; Mikulic P.; Vonshak A.; Beardall J.; Wangikar P. P. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour. Technol. 2015, 184, 363–372. 10.1016/j.biortech.2014.11.040. [DOI] [PubMed] [Google Scholar]
- Visch W.; Rad-Menendez C.; Nylund G. M.; Pavia H.; Ryan M. J.; Day J. Underpinning the Development of Seaweed Biotechnology: Cryopreservation of Brown Algae (Saccharina latissima) Gametophytes. Biopreserv Biobank 2019, 17 (5), 378–386. 10.1089/bio.2018.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey-Yagi S. R.; Kinoshita Y.; Motoki K.; Iwahashi Y.; Hanh D. D.; Kato S.; Nakano R.; Ochiai K.; Kobayashi M.; Nakazaki T.; Numata K. Utilization of lysed and dried bacterial biomass from the marine purple photosynthetic bacterium Rhodovulum sulfidophilum as a sustainable nitrogen fertilizer for plant production. npj Sustainable Agriculture 2024, 2 (1), 10. 10.1038/s44264-024-00018-0. [DOI] [Google Scholar]