Abstract
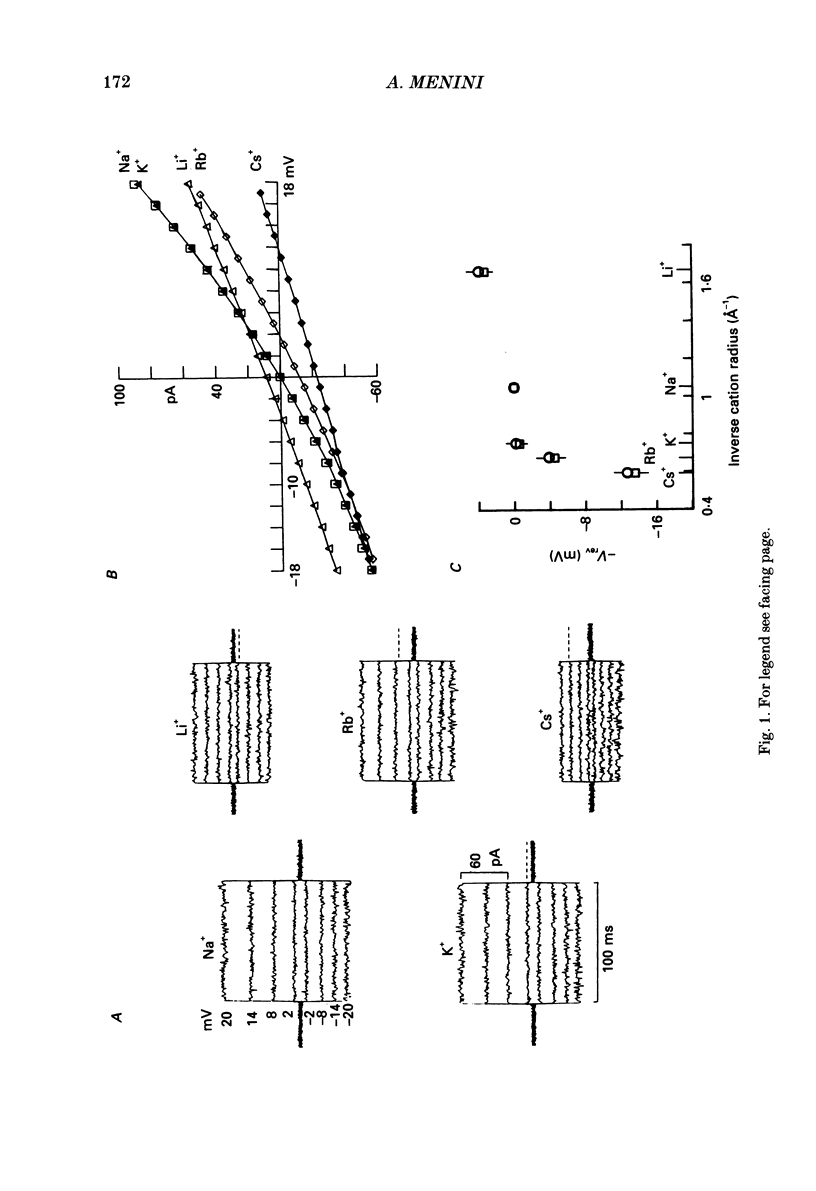
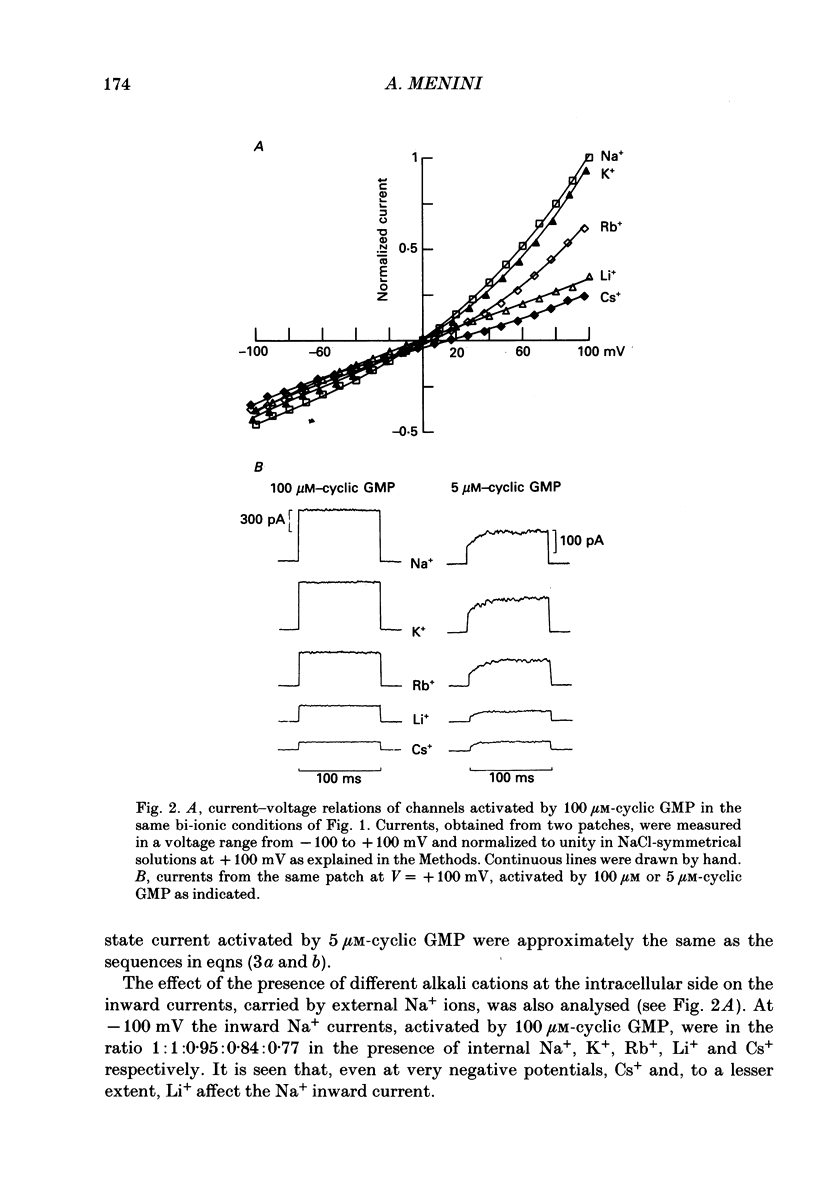
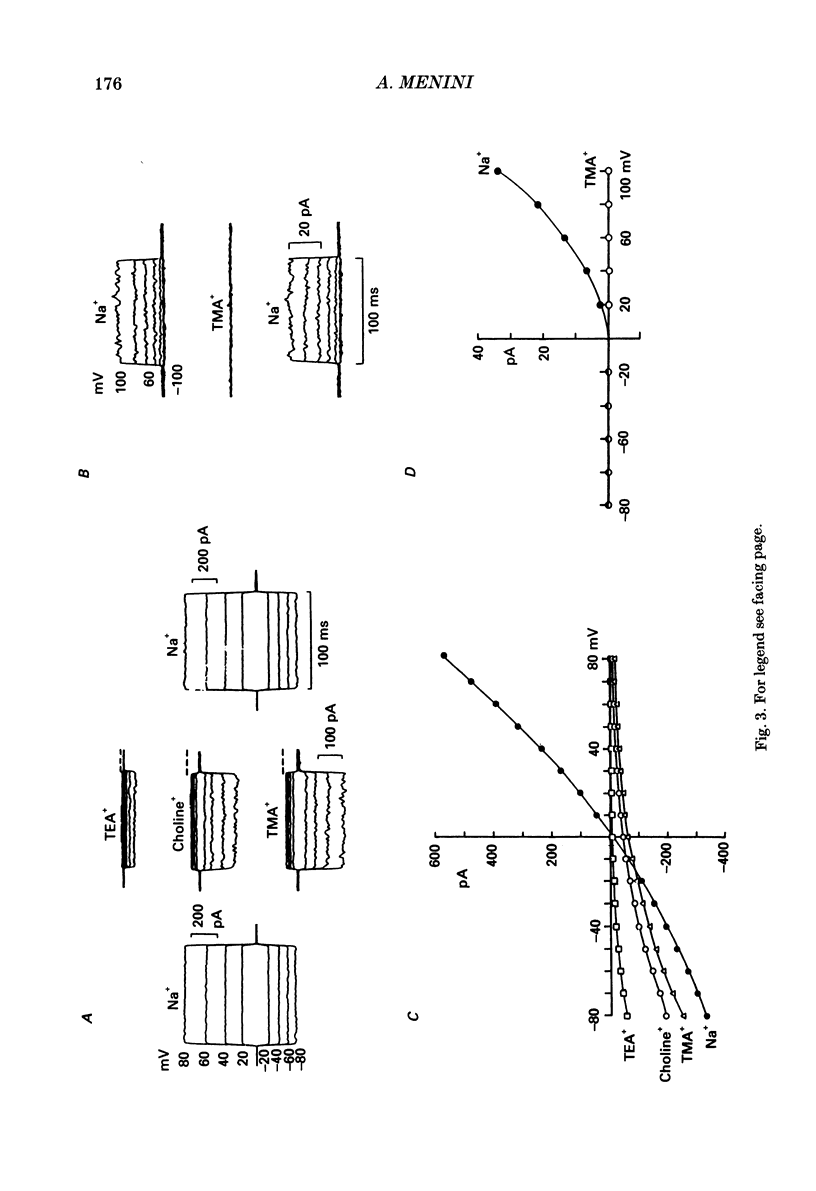
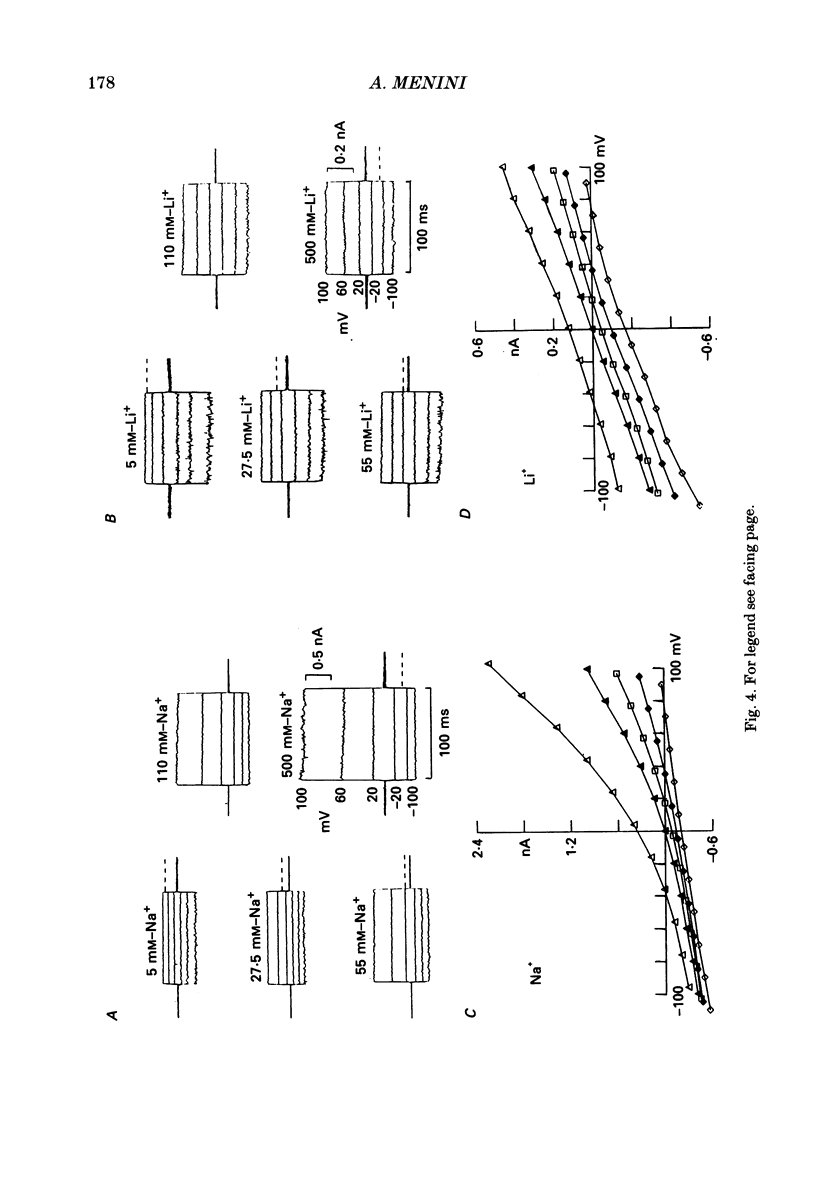
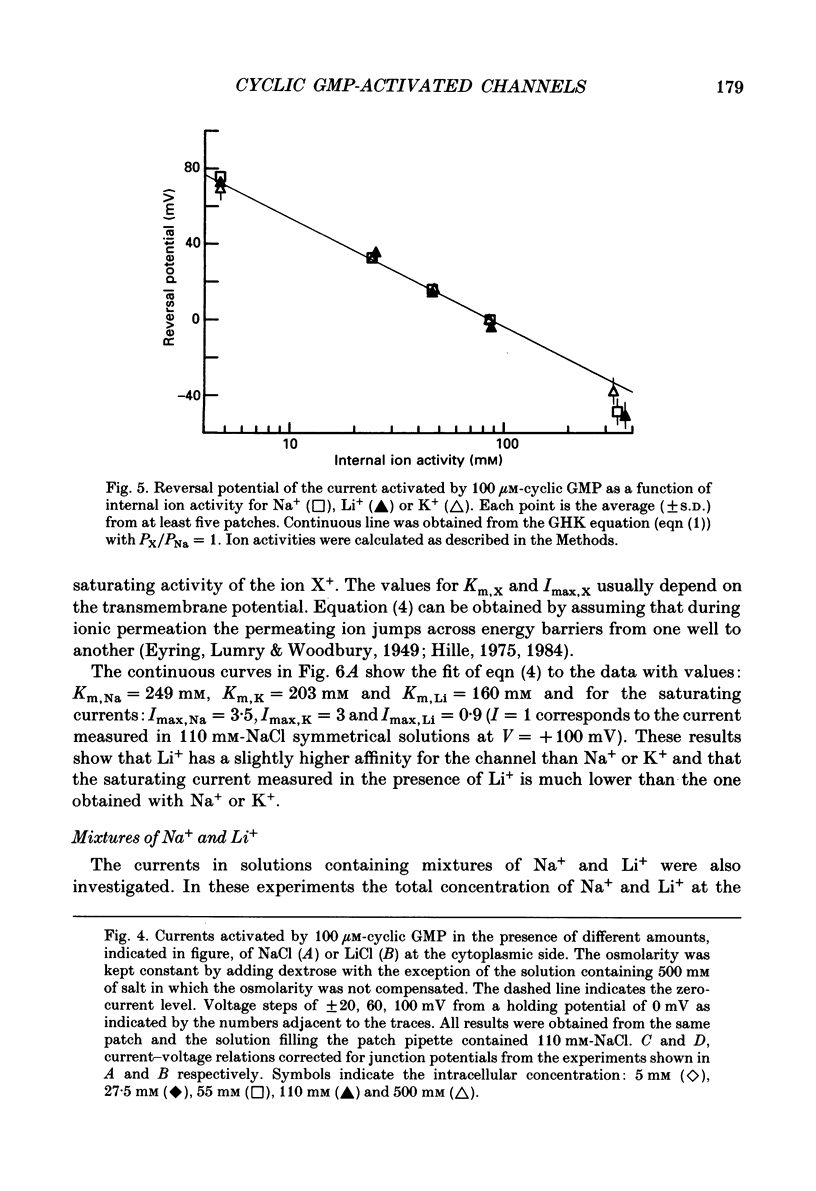
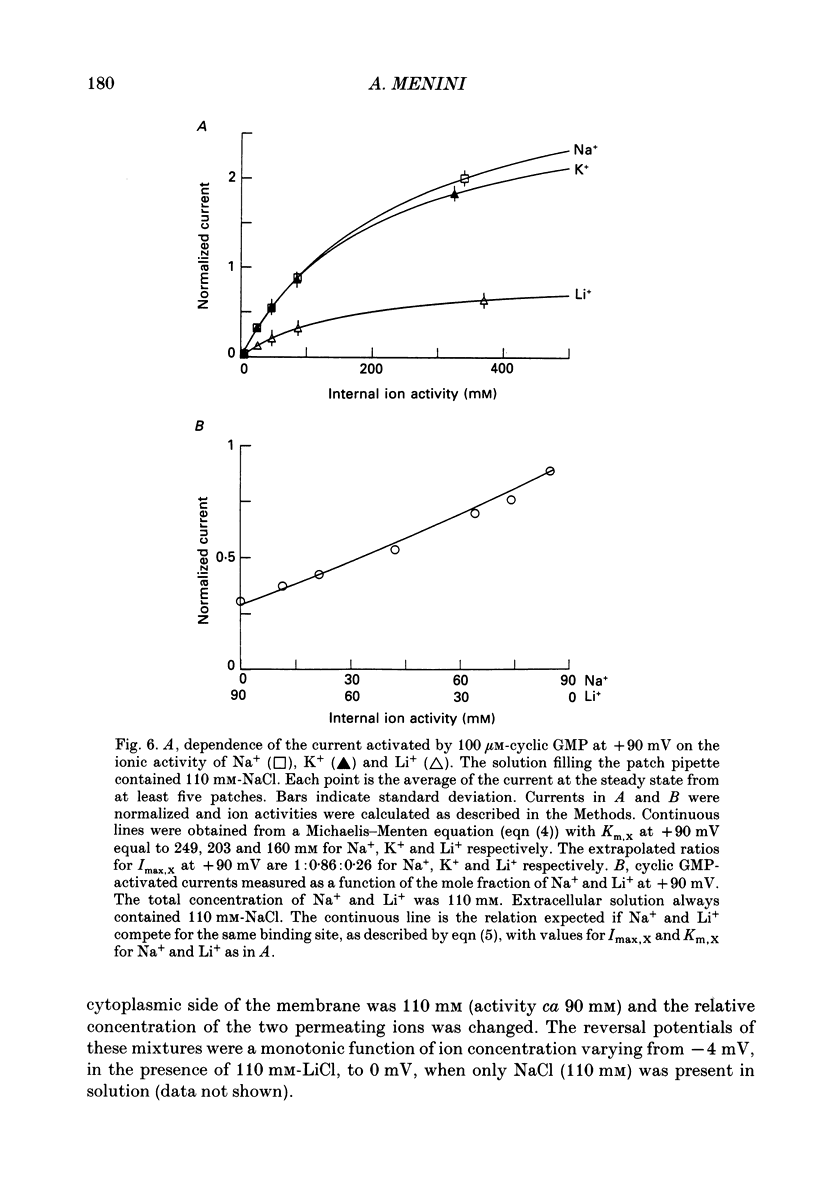
1. Ionic selectivity and affinity for monovalent cations of channels activated by guanosine 3',5'-cyclic monophosphate (cyclic GMP) were studied in excised inside-out patches of plasma membrane from retinal rods of the tiger salamander. Channels were activated by addition of cyclic GMP to the medium bathing the cytoplasmic side of the membrane. The ionic solution at the cytoplasmic side was rapidly changed using the method of Nunn (1987 a). 2. Permeability ratios were calculated with the Goldman-Hodgkin-Katz potential equation from reversal potential measurements for alkali monovalent cations in bi-ionic conditions. The permeability sequence was: Li+:Na+:K+:Rb+:Cs+ = 1.14:1:0.98:0.84:0.58. 3. The selectivity sequence obtained from macroscopic current measurements in bi-ionic conditions at +100 mV was: Na+:K+:Rb+:Li+:Cs+ = 1:1:0.67:0.36:0.25. 4. The organic cations tetramethylammonium (TMA+), choline and tetraethylammonium (TEA+) were not permeant through the cyclic GMP-activated channels and caused a reduction of the Na+ inward current. At -100 mV the current ratio for inward current was 1:0.75:0.58:0.2 in the presence, at the cytoplasmic side, of 110 mM-Na+, TMA+, choline or TEA+ respectively. 5. The concentration dependence of the macroscopic current and the reversal potential was studied by changing the internal concentration of Na+ or K+ or Li+ from 5 mM to 500 mM. The permeability ratios were nearly constant regardless of the permeant ion concentration. 6. The current as a function of internal ion activity could be described by a Michaelis-Menten relation with a half-saturating activity, Km, at +90 mV equal to 249, 203 and 160 mM for Na+, K+ and Li+ respectively. The ratio of the extrapolated saturating current Imax at +90 mV was 1:0.86:0.26 for Na+, K+ and Li+ respectively. 7. The outward currents and the reversal potentials measured in different mixtures of Na+ and Li+ were monotonic function of the mole fraction. 8. These results can be explained by assuming that, at least in a narrow region, the cyclic GMP-activated channel is a one-ion channel, possibly with other poorly voltage-dependent binding sites in a large inner vestibule.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J. Electrical properties of the light-sensitive conductance of rods of the salamander Ambystoma tigrinum. J Physiol. 1986 Feb;371:115–145. doi: 10.1113/jphysiol.1986.sp015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G., Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76(3):197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Haynes L., Yau K. W. Cyclic GMP-sensitive conductance in outer segment membrane of catfish cones. Nature. 1985 Sep 5;317(6032):61–64. doi: 10.1038/317061a0. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen J. W., Zimmerman A. L., Stryer L., Baylor D. A. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1287–1291. doi: 10.1073/pnas.85.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D. An inexpensive digital tape recorder suitable for neurophysiological signals. J Neurosci Methods. 1985 Oct;15(1):1–13. doi: 10.1016/0165-0270(85)90057-3. [DOI] [PubMed] [Google Scholar]
- Matthews G., Watanabe S. Properties of ion channels closed by light and opened by guanosine 3',5'-cyclic monophosphate in toad retinal rods. J Physiol. 1987 Aug;389:691–715. doi: 10.1113/jphysiol.1987.sp016678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menini A., Rispoli G., Torre V. The ionic selectivity of the light-sensitive current in isolated rods of the tiger salamander. J Physiol. 1988 Aug;402:279–300. doi: 10.1113/jphysiol.1988.sp017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. 1987 Jan 29-Feb 4Nature. 325(6103):442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol. 1988 Jan;395:695–729. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Guanosine 3',5'-cyclic monophosphate-activated conductance studied in a truncated rod outer segment of the toad. J Physiol. 1988 Jan;395:731–753. doi: 10.1113/jphysiol.1988.sp016943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Cation selectivity of light-sensitive conductance in retinal rods. Nature. 1984 May 24;309(5966):352–354. doi: 10.1038/309352a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Karpen J. W., Baylor D. A. Hindered diffusion in excised membrane patches from retinal rod outer segments. Biophys J. 1988 Aug;54(2):351–355. doi: 10.1016/S0006-3495(88)82966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


