Abstract
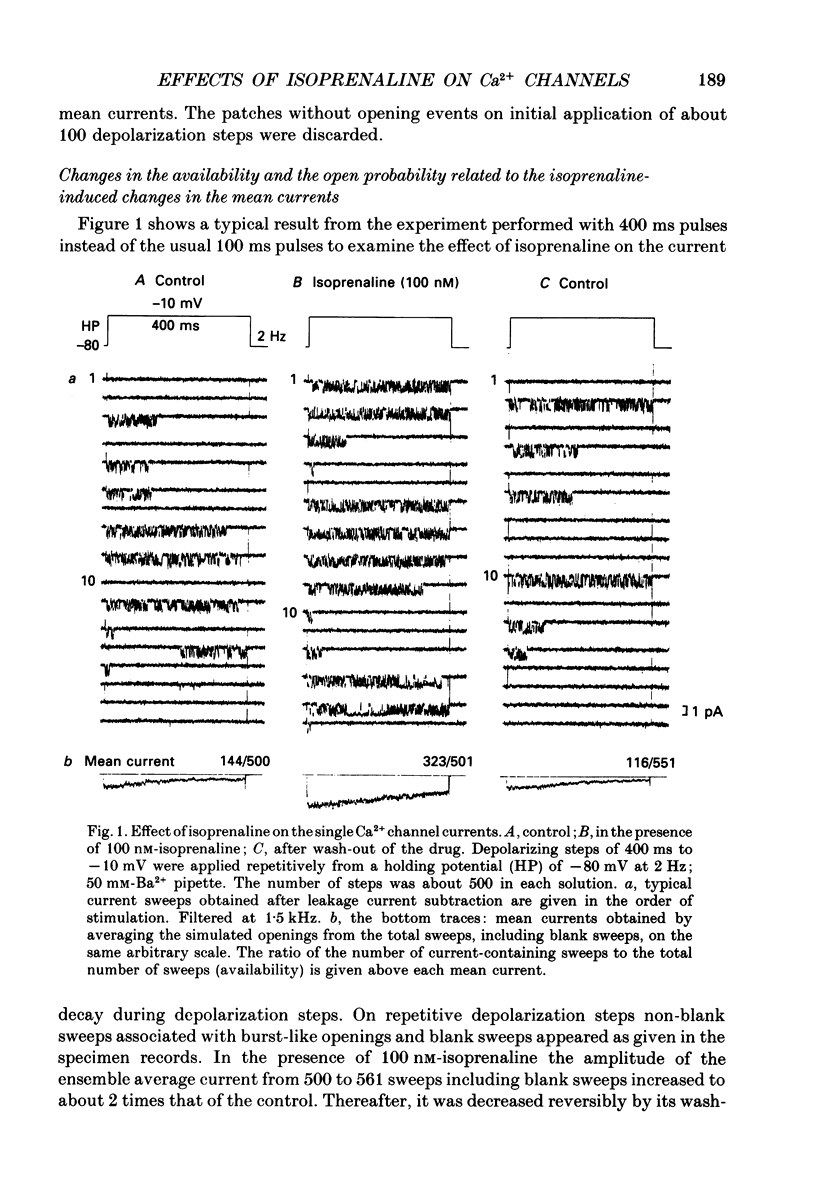
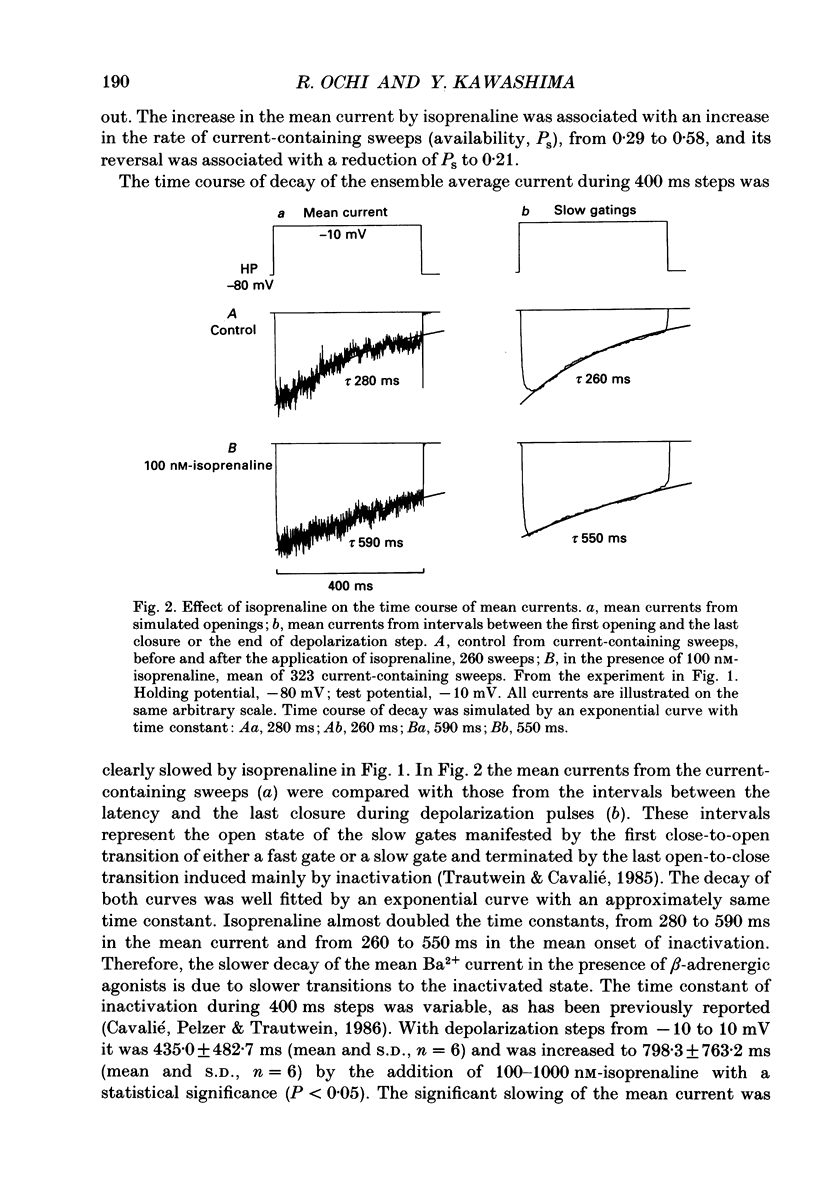
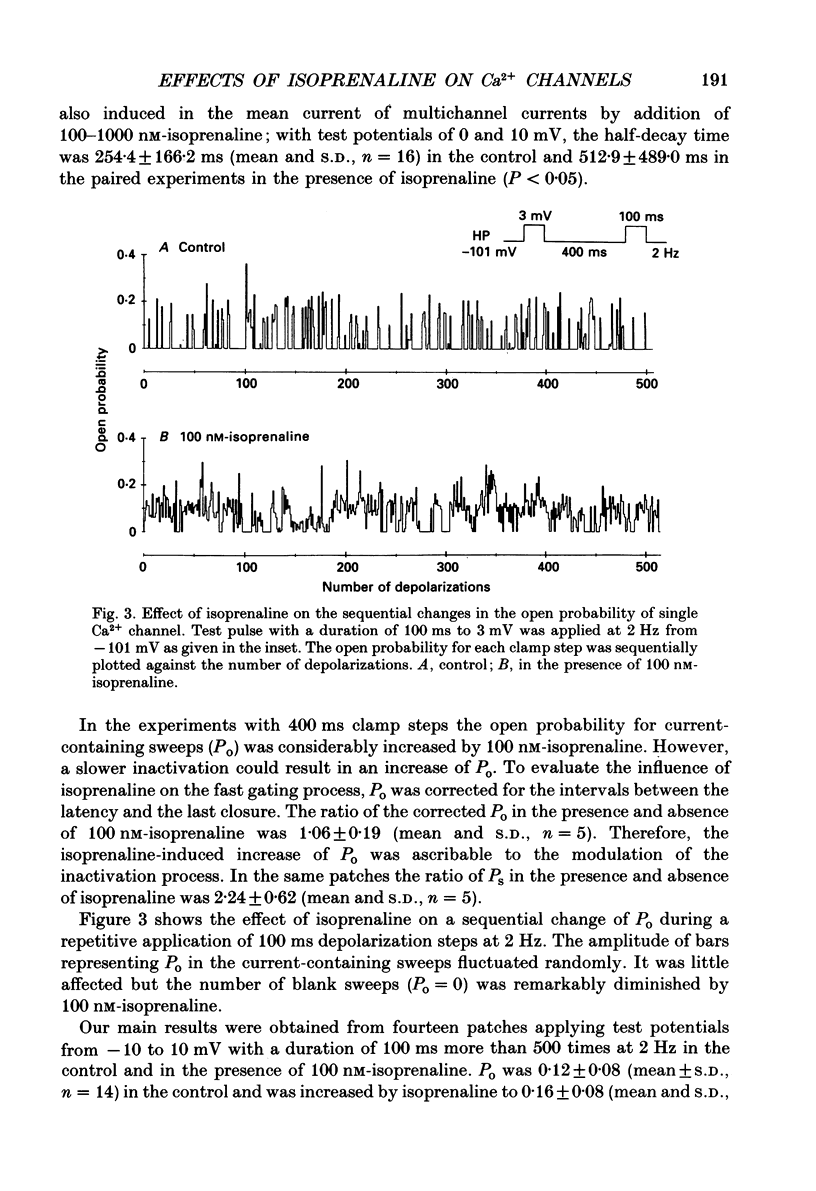
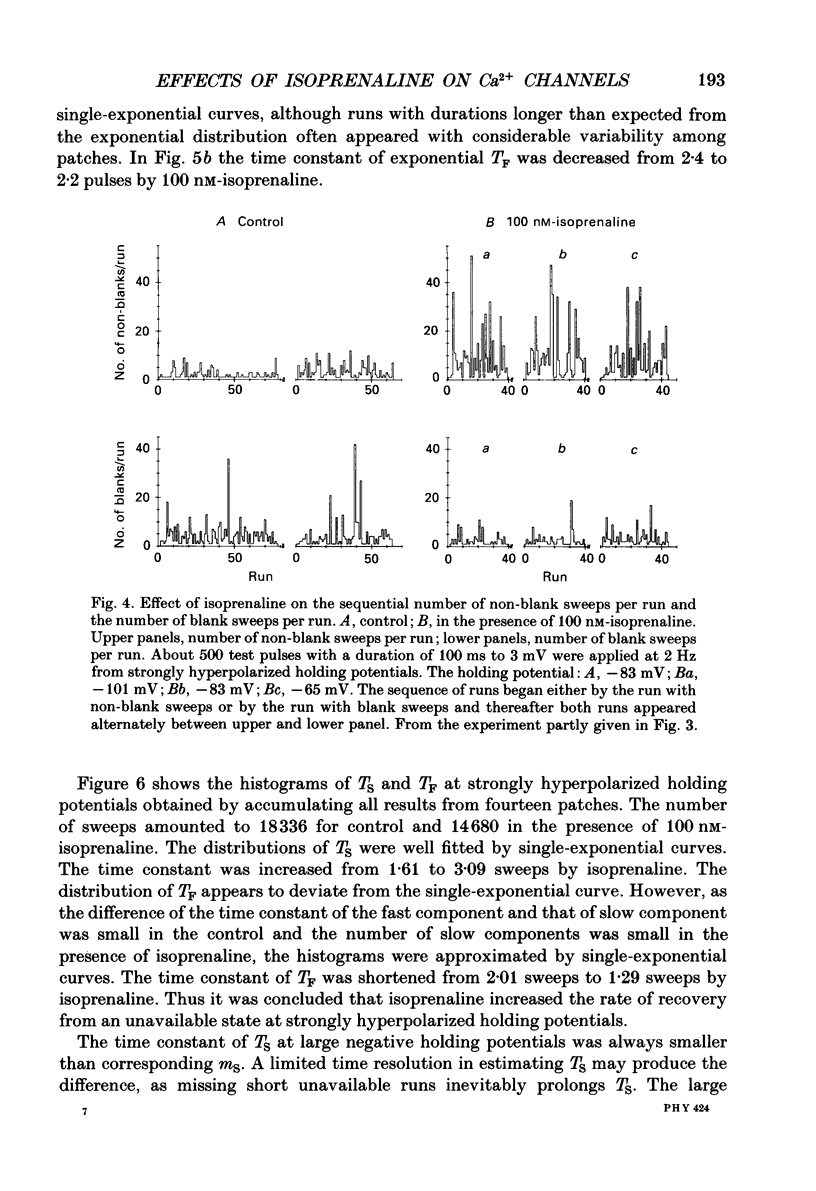
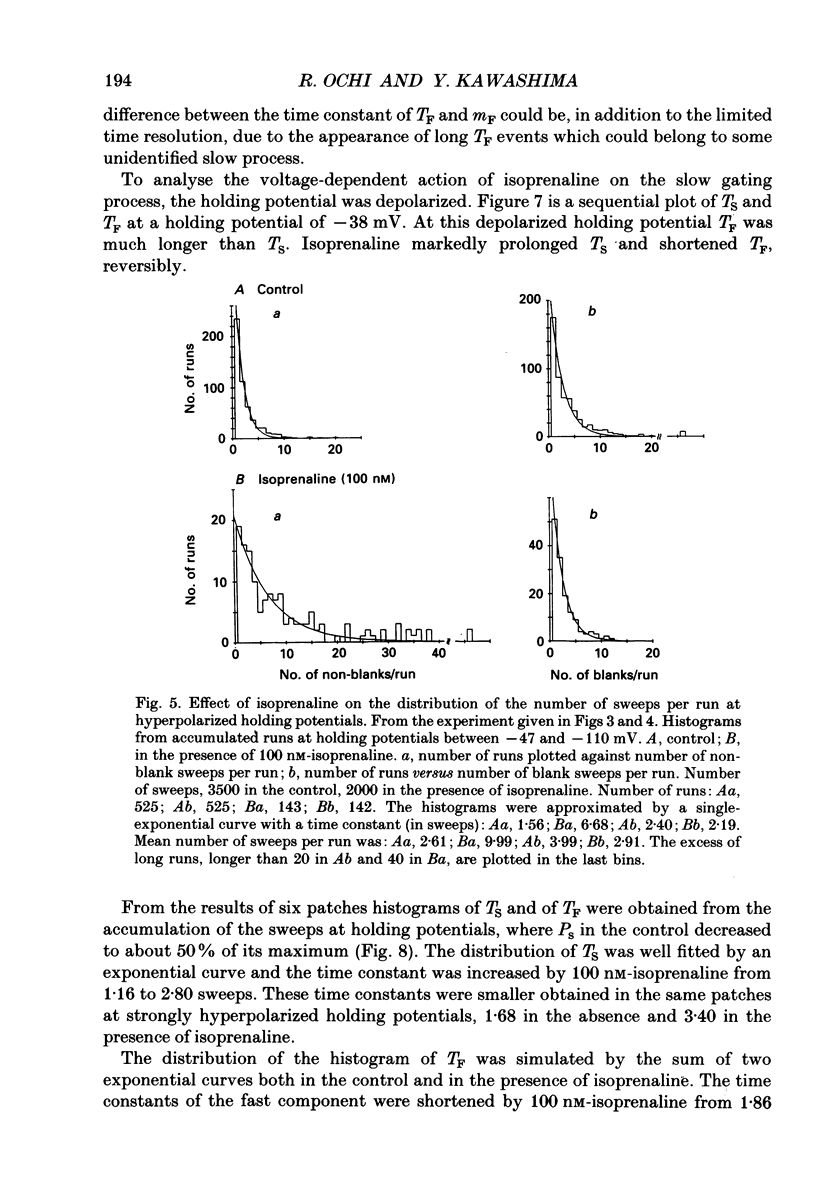
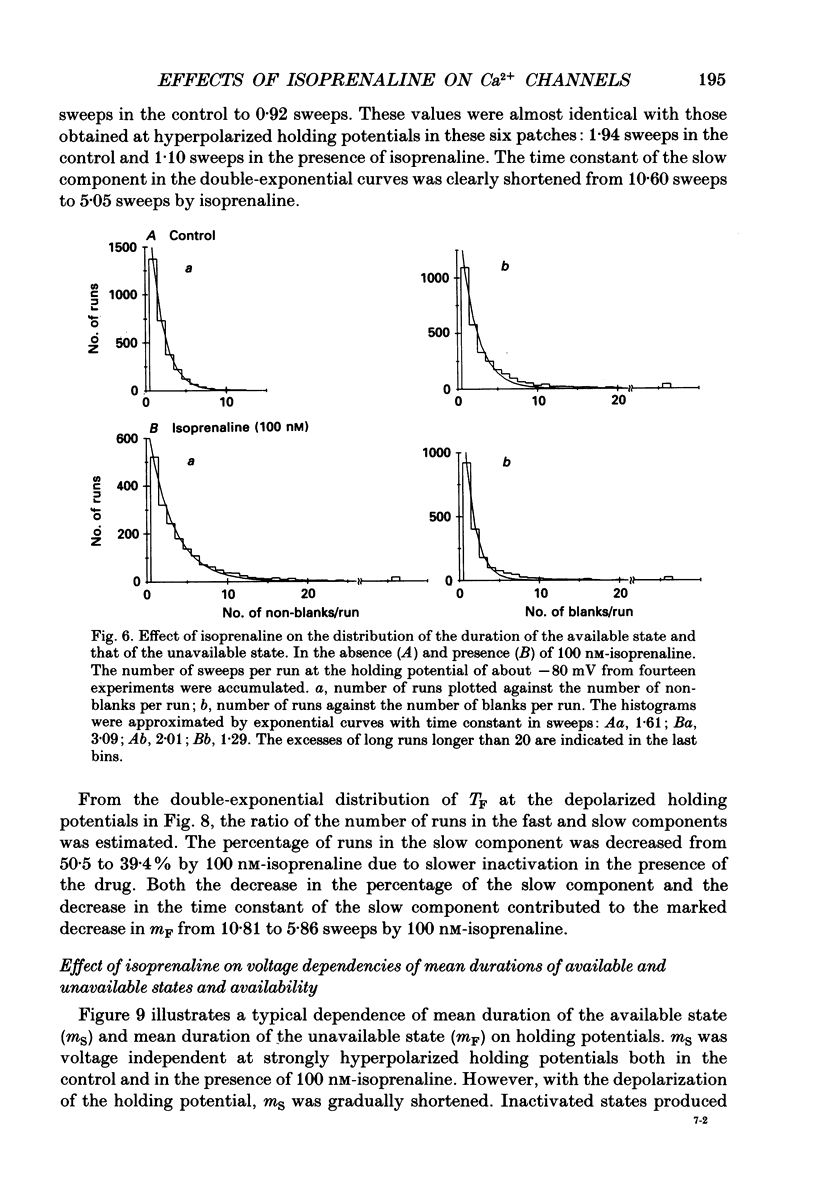
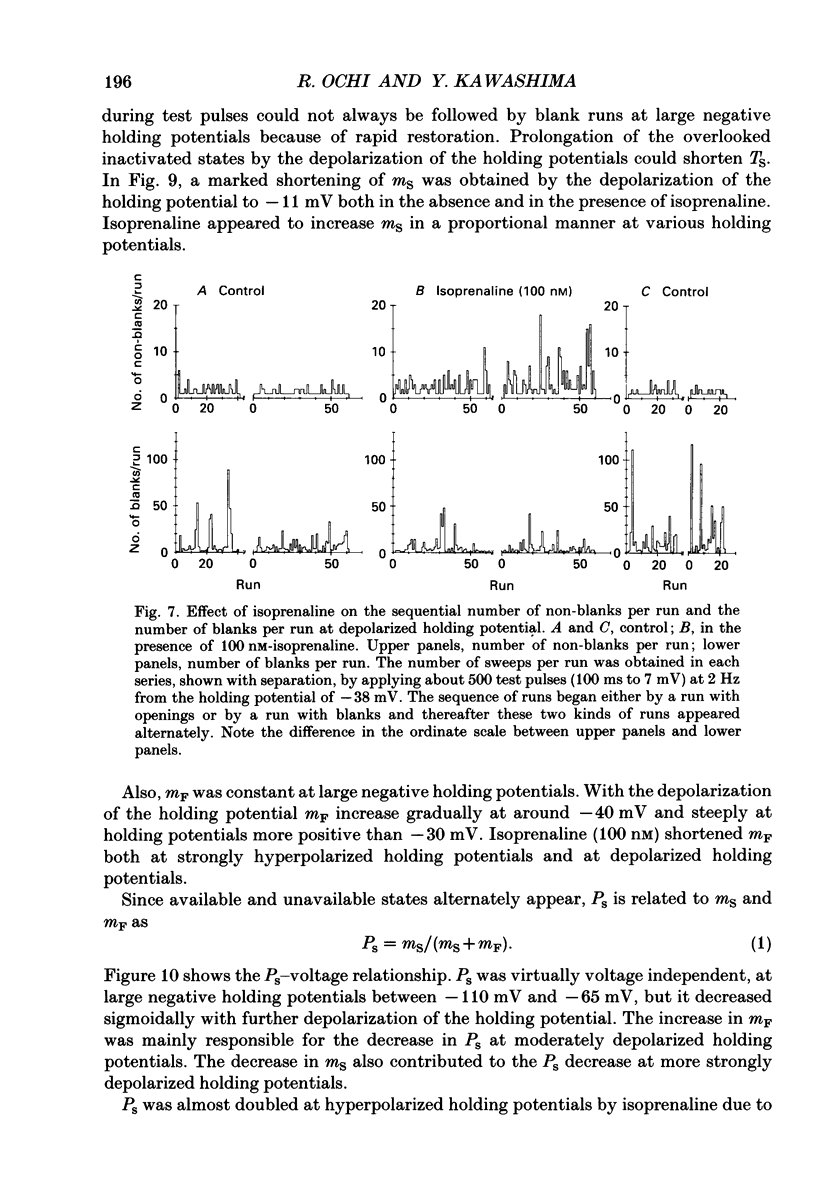
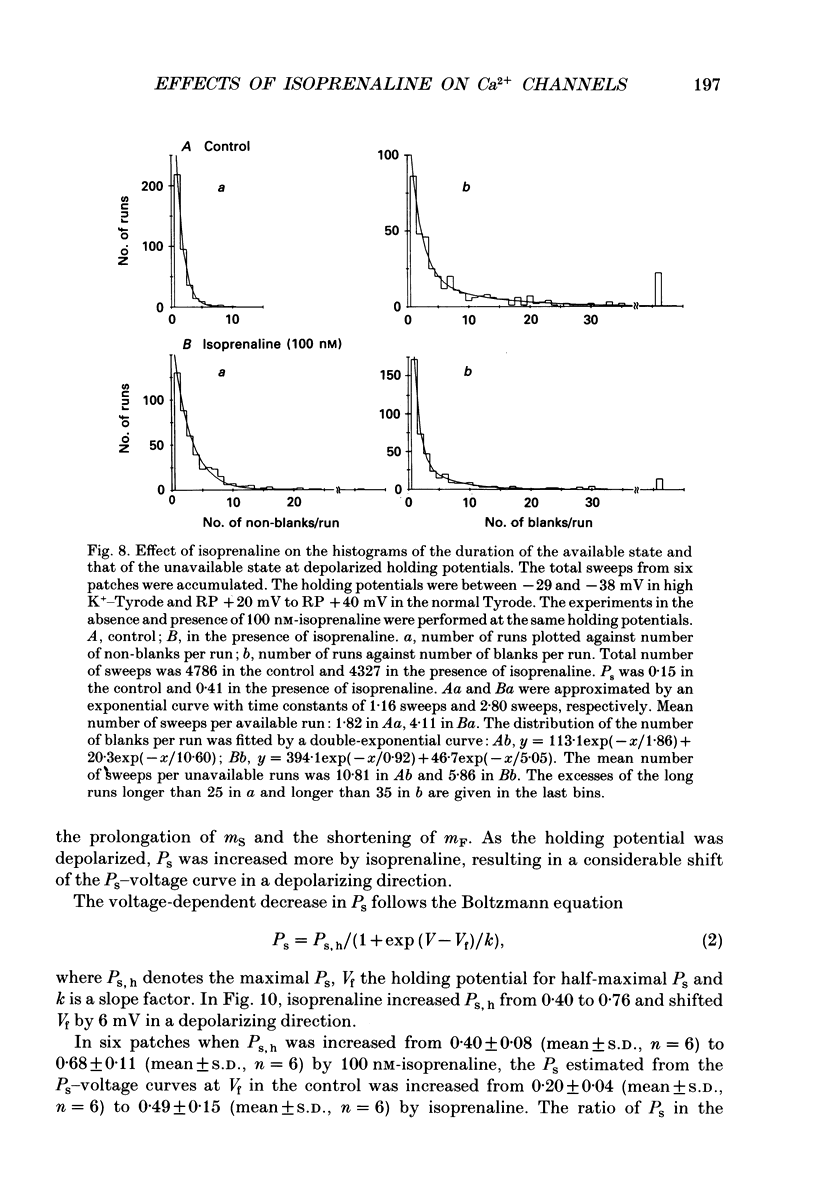
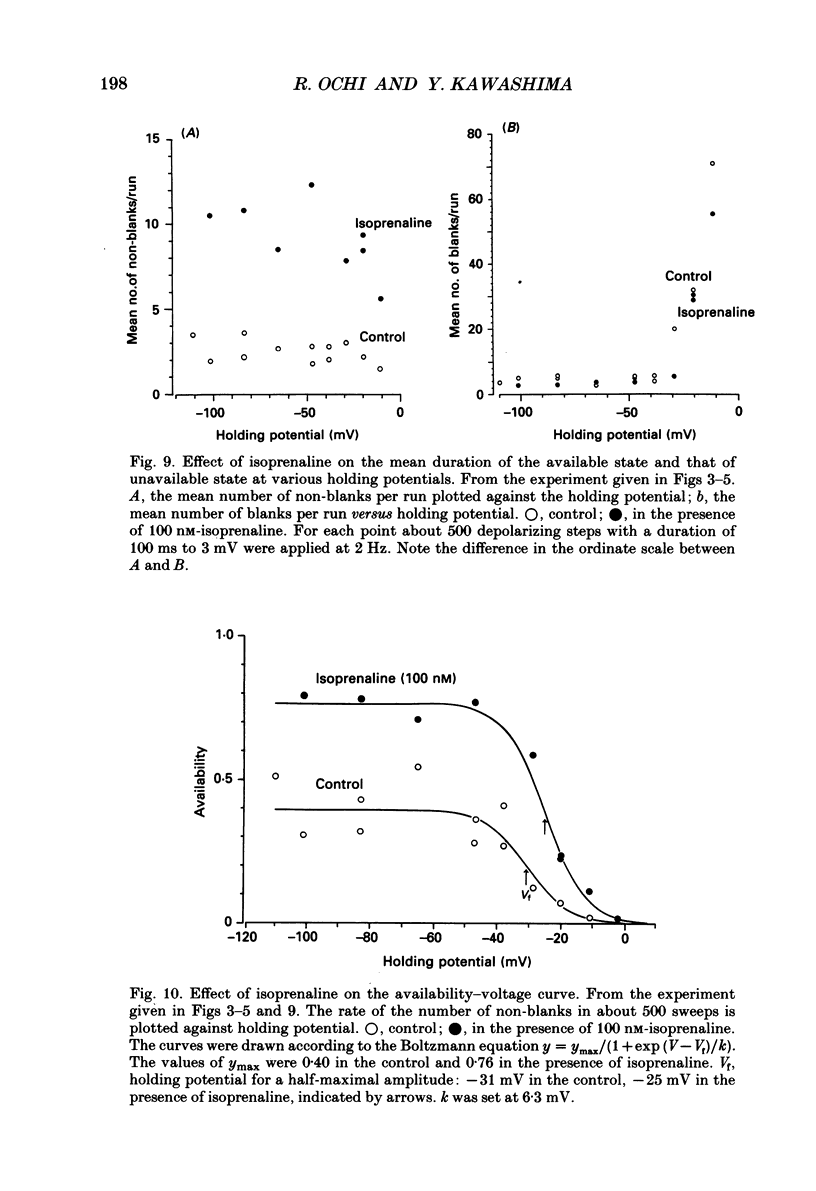
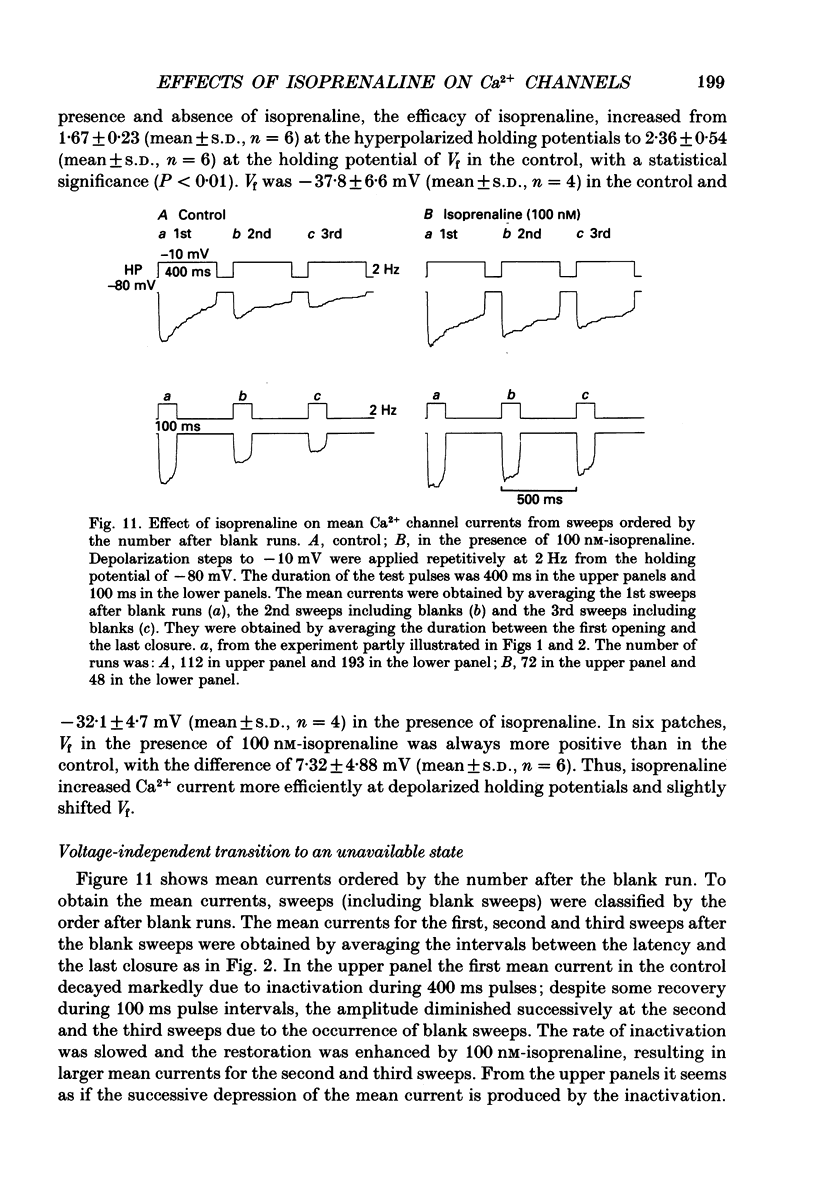
1. The mechanism of enhancement of Ca2+ current by isoprenaline was studied by recording single-channel activity from cell-attached patches on isolated guinea-pig ventricular cells using patch pipettes containing 50 or 100 mM-Ba2+. 2. Isoprenaline (100 nM) increased the amplitude of ensemble average currents by increasing the rate of non-blank sweeps (availability). The current decay during 400 ms steps was significantly slowed by isoprenaline. However, the open probability for the non-blank sweeps elicited by 100 ms steps was only slightly increased by the application of isoprenaline. 3. The durations of the available state (TS) and the unavailable state (TF) were estimated by the number of non-blank and blank sweeps per run, respectively, applying repetitively 100 ms steps at 2 Hz. 4. At large negative holding potentials the distribution of TS was well fitted by an exponential curve, whose time constant was increased from 1.6 to 3.1 sweeps by 100 nM-isoprenaline, while TF distributed approximately single exponentially with a time constant of 2.0 sweeps in control and 1.3 sweeps in the presence of the drug. 5. At depolarized holding potentials a slow voltage-dependent component appeared in the histogram of TF and its time constant was markedly decreased by 100 nM-isoprenaline. 6. The availability-voltage relationship was simulated by the Boltzmann equation with a maximal value of 0.4 in the control. The maximal value was increased to 0.7 and the curve was shifted to a depolarizing direction by 7 mV by 100 nM-isoprenaline. 7. Isoprenaline increased the availability of cardiac Ca2+ channels by increasing the forward rate constant and decreasing the backward rate constant in both voltage-dependent and independent slow state transitions.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brum G., Osterrieder W., Trautwein W. Beta-adrenergic increase in the calcium conductance of cardiac myocytes studied with the patch clamp. Pflugers Arch. 1984 Jun;401(2):111–118. doi: 10.1007/BF00583870. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., de Peyer J. E., Kokubun S., Reuter H. Ca2+ channel modulation by 8-bromocyclic AMP in cultured heart cells. Nature. 1983 Aug 4;304(5925):462–464. doi: 10.1038/304462a0. [DOI] [PubMed] [Google Scholar]
- Cavalié A., Pelzer D., Trautwein W. Fast and slow gating behaviour of single calcium channels in cardiac cells. Relation to activation and inactivation of calcium-channel current. Pflugers Arch. 1986 Mar;406(3):241–258. doi: 10.1007/BF00640910. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A., Lange K. Statistical analysis of single sodium channels. Effects of N-bromoacetamide. Biophys J. 1984 Jan;45(1):323–335. doi: 10.1016/S0006-3495(84)84158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Mieskes G., Trautwein W. The protein-specific phosphatase 1 antagonizes the beta-adrenergic increase of the cardiac Ca current. Pflugers Arch. 1986 Oct;407(4):461–463. doi: 10.1007/BF00652635. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Kawashima Y., Ochi R. Voltage-dependent decrease in the availability of single calcium channels by nitrendipine in guinea-pig ventricular cells. J Physiol. 1988 Aug;402:219–235. doi: 10.1113/jphysiol.1988.sp017201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Localization of beta adrenergic receptors, and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents and tension in mammalian cardiac muscle. J Physiol. 1974 Oct;242(2):429–451. doi: 10.1113/jphysiol.1974.sp010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. L., Hess P., Reeves J. P., Smilowitz H., Tsien R. W. Calcium channels in planar lipid bilayers: insights into mechanisms of ion permeation and gating. Science. 1986 Mar 28;231(4745):1564–1566. doi: 10.1126/science.2420007. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y., Raz S., Gotsman M. S. Two potentially arrhythmogenic mechanisms of adrenaline action in cardiac muscle. J Mol Cell Cardiol. 1984 May;16(5):471–478. doi: 10.1016/s0022-2828(84)80618-5. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R., Ward T. A. Properties of single potassium channels in vesicles formed from the sarcolemma of frog skeletal muscle. J Physiol. 1985 Jul;364:339–358. doi: 10.1113/jphysiol.1985.sp015749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein W., Cavalié A. Cardiac calcium channels and their control by neurotransmitters and drugs. J Am Coll Cardiol. 1985 Dec;6(6):1409–1416. doi: 10.1016/s0735-1097(85)80233-3. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986 Jul;18(7):691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Tsuji Y., Inoue D., Pappano A. J. beta-Adrenoceptor agonist accelerates recovery from inactivation of calcium-dependent action potentials. J Mol Cell Cardiol. 1985 May;17(5):517–521. doi: 10.1016/s0022-2828(85)80057-2. [DOI] [PubMed] [Google Scholar]



