Abstract
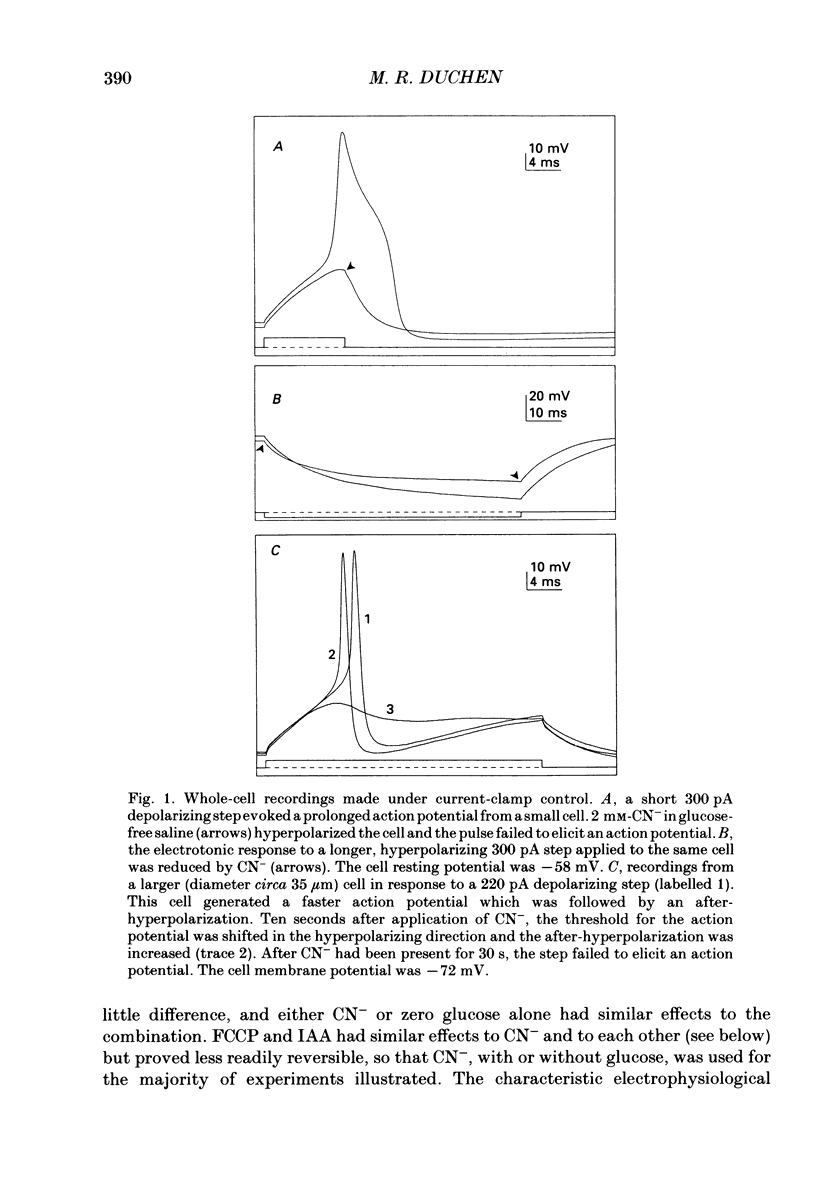
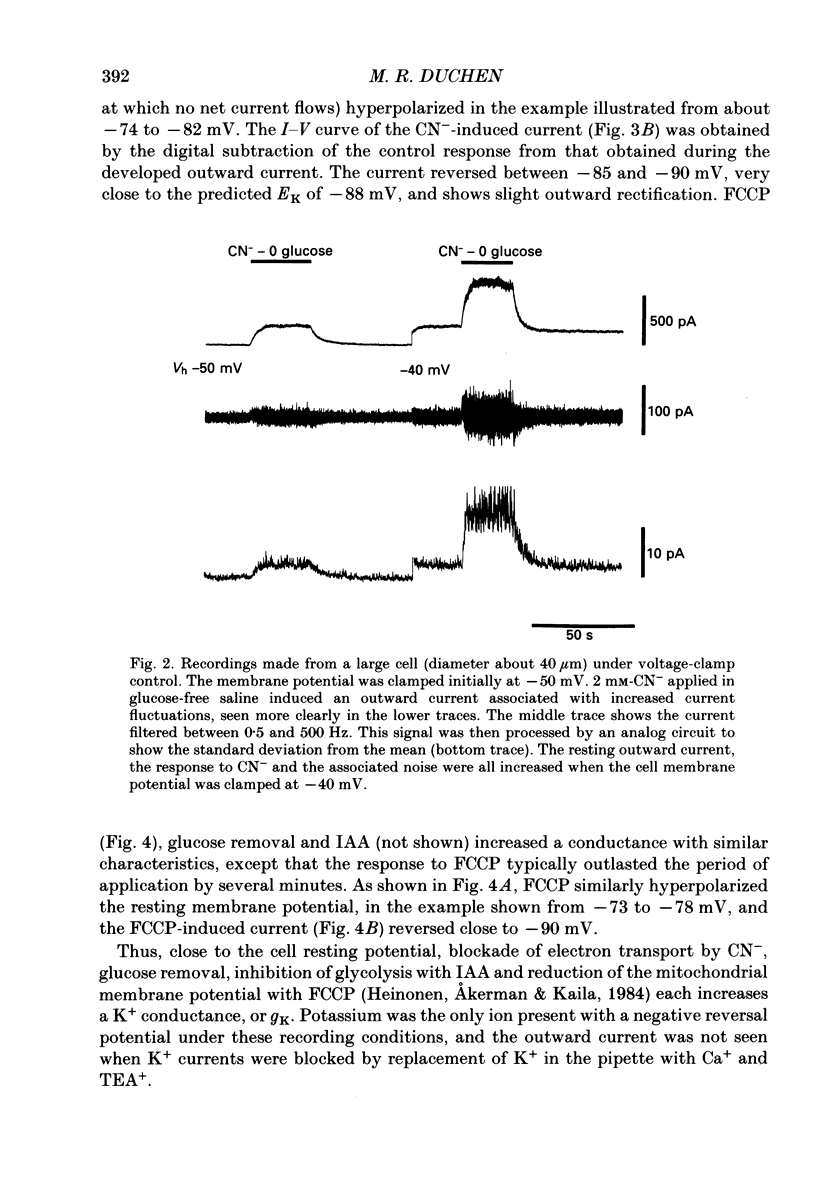
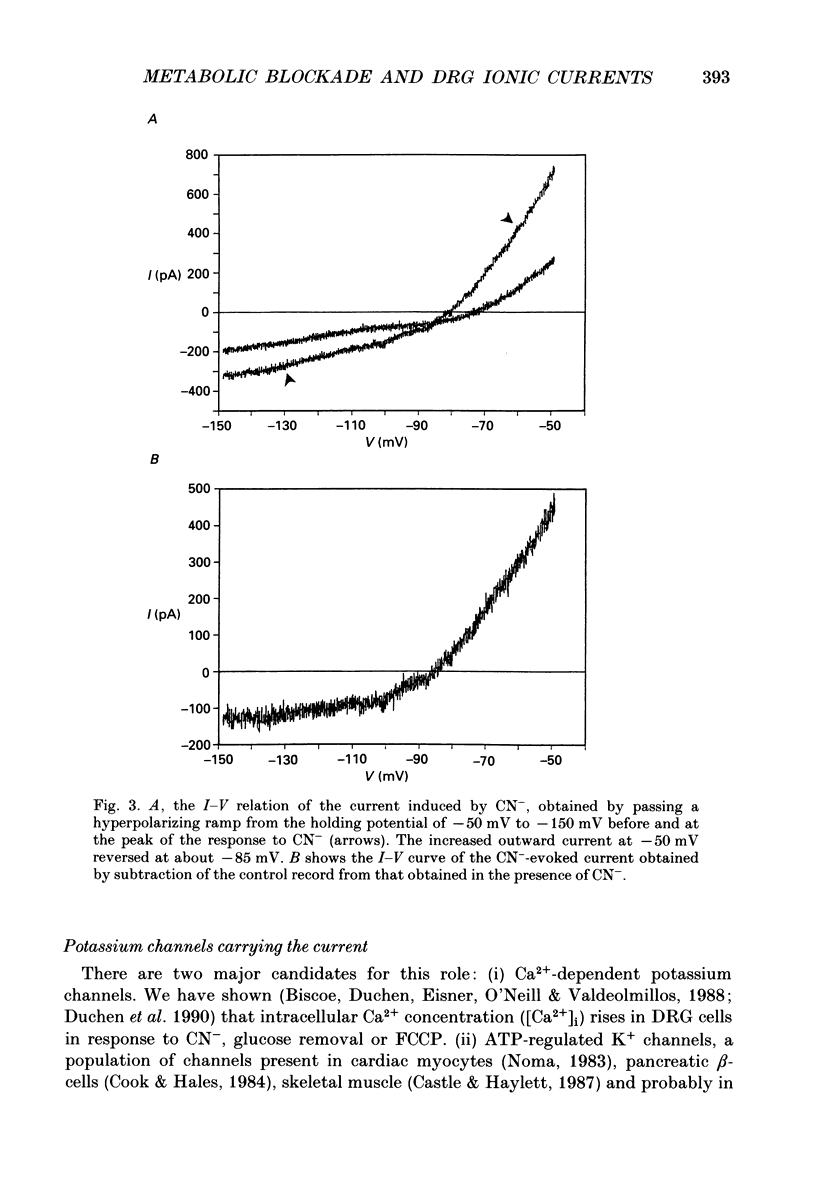
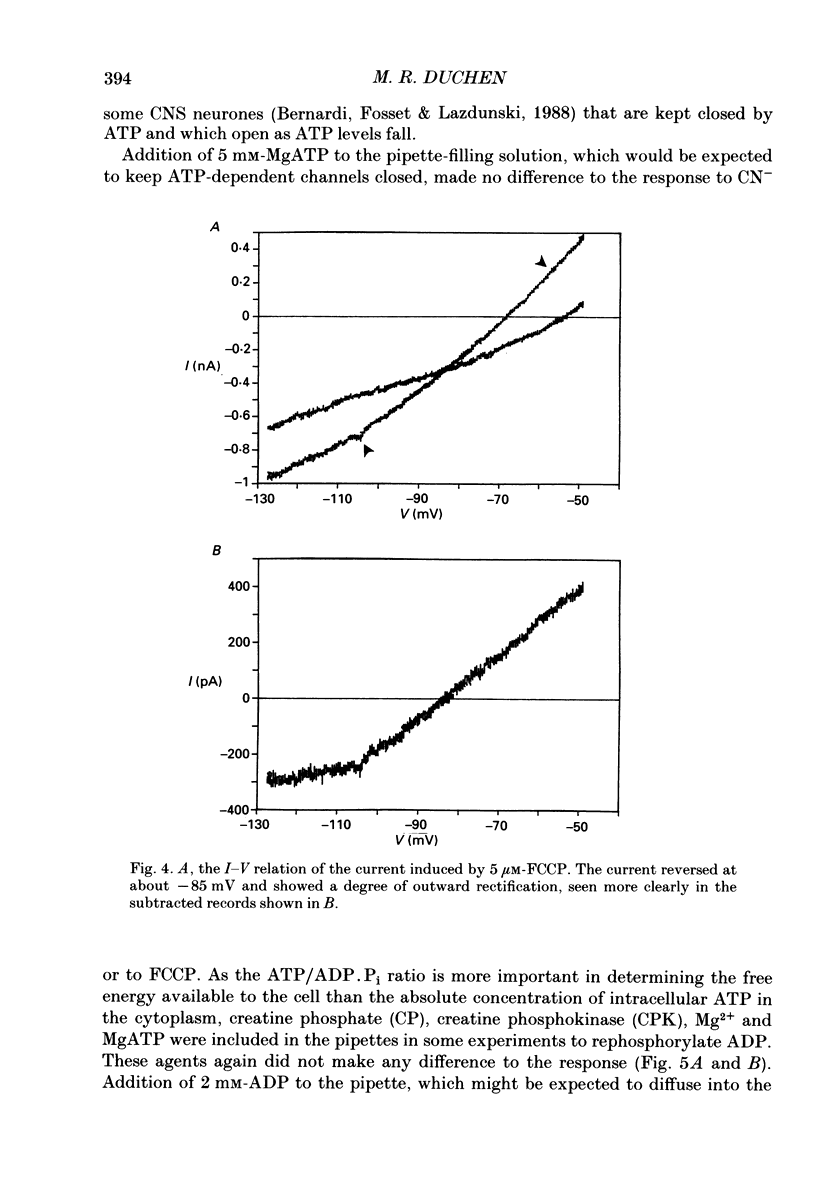
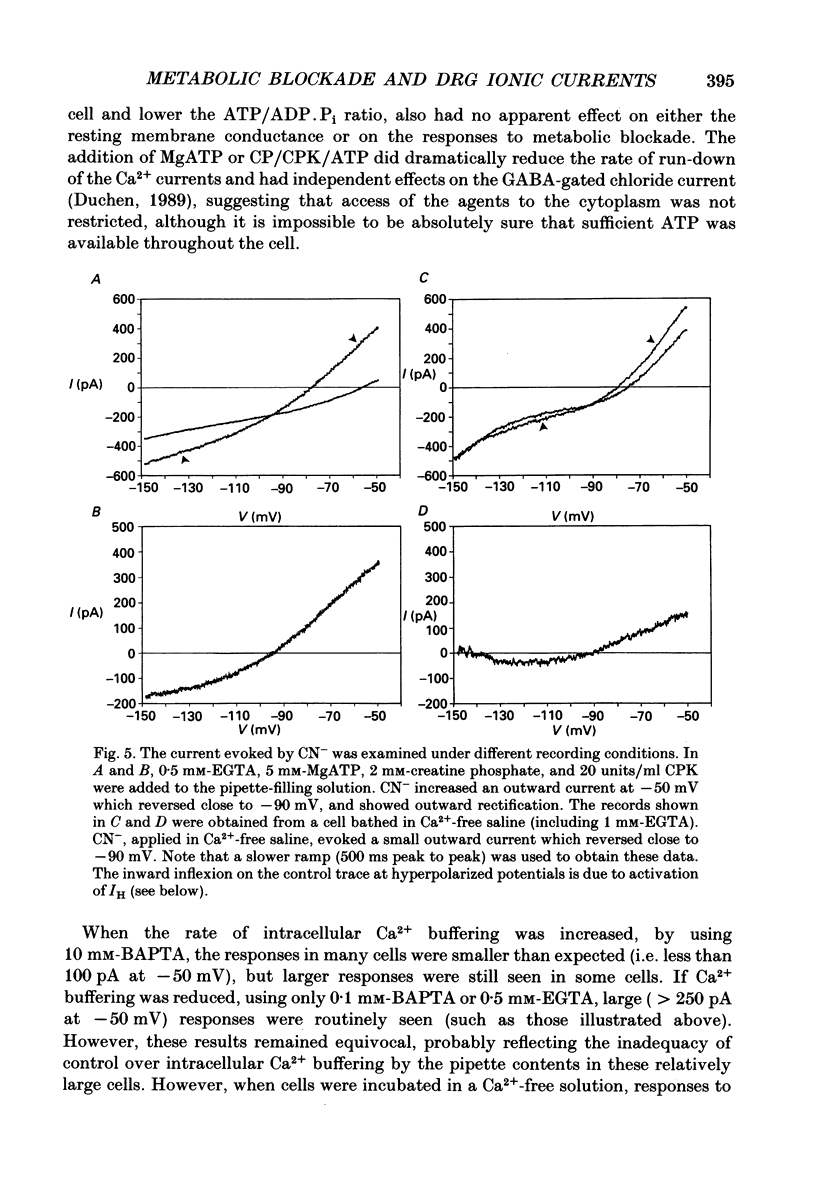
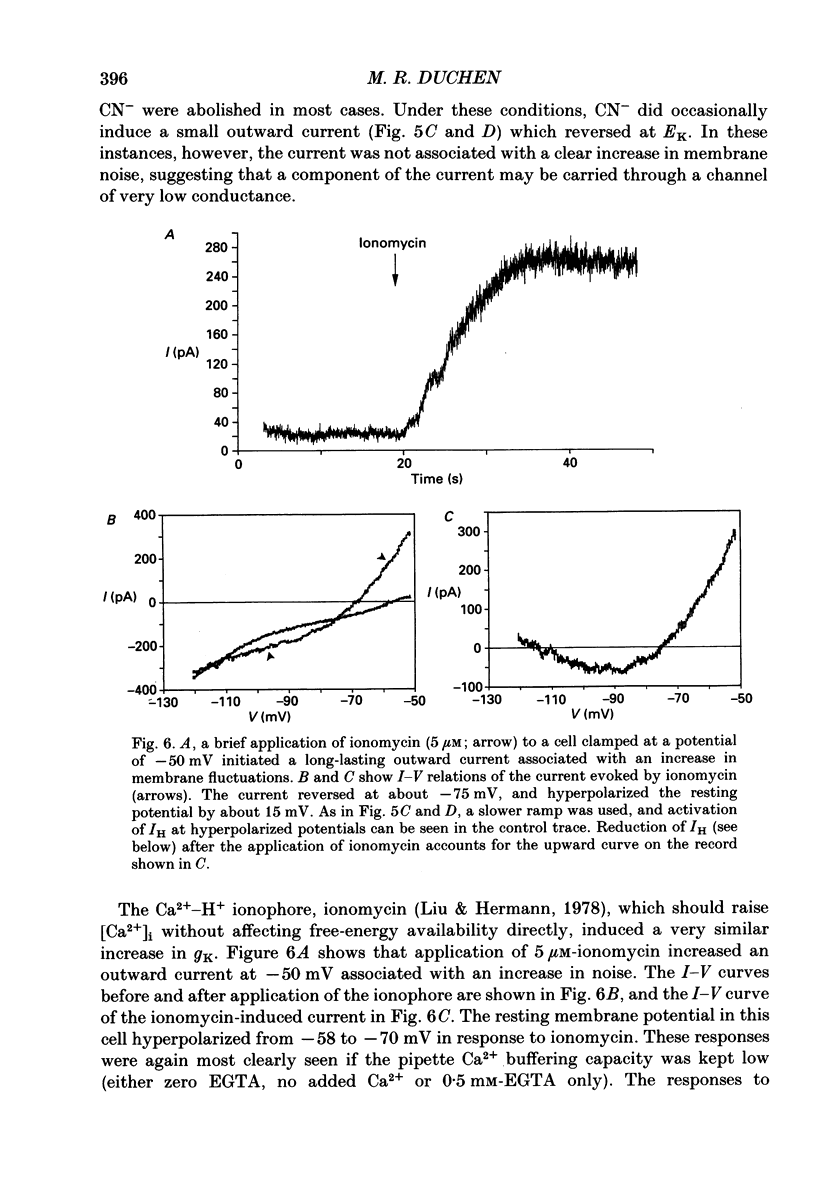
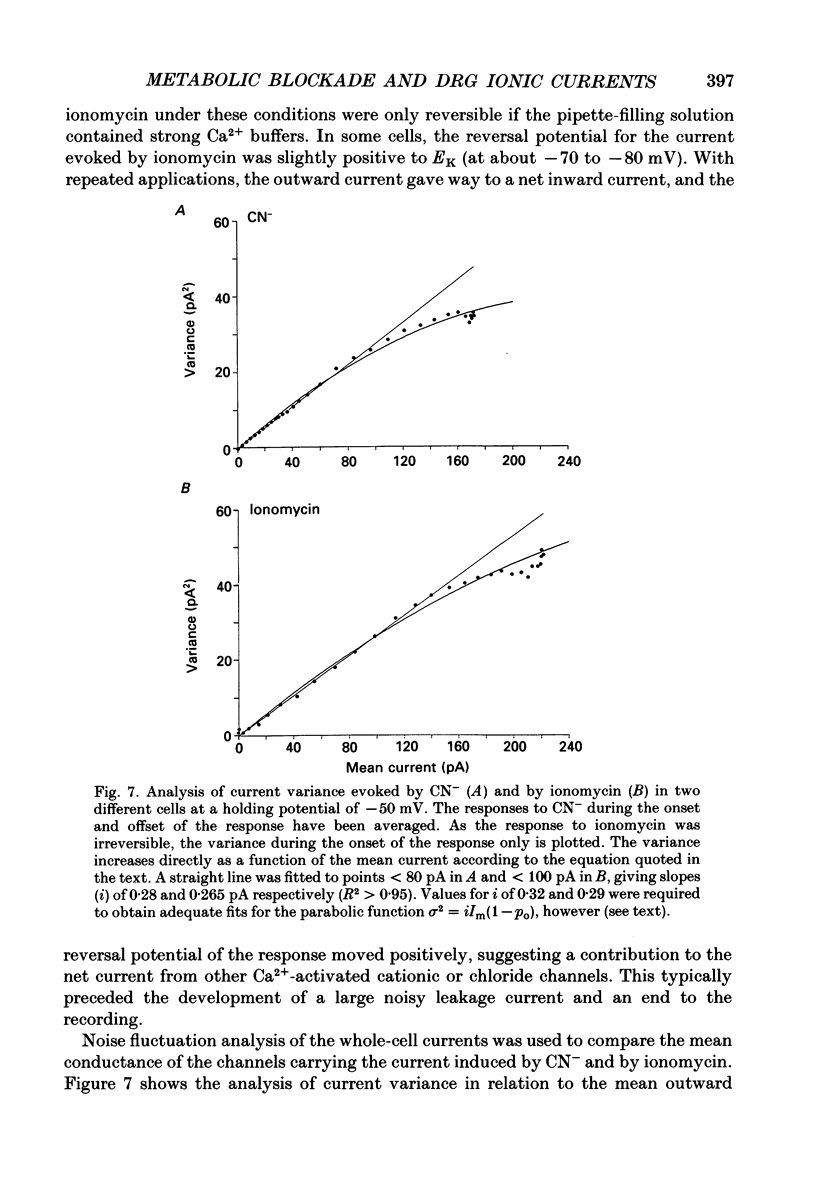
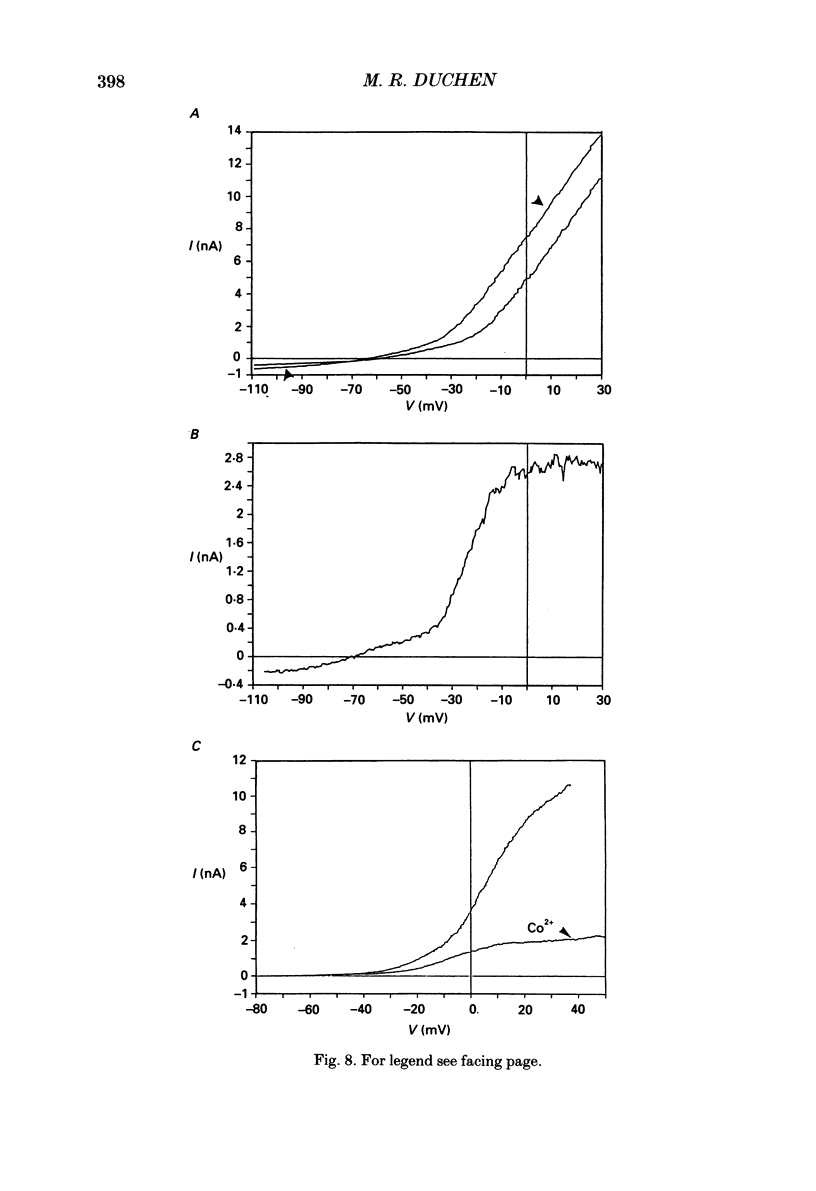
1. The patch-clamp technique has been used to investigate the mechanisms that couple membrane excitability to metabolism in neurones isolated from mouse dorsal root ganglia. 2. Blockade of electron transport by cyanide (CN-), reduction of the mitochondrial membrane potential with carbonyl cyanide p-trifluoromethoxyphenyl hydrazone (FCCP), removal of glucose or inhibition of glycolysis with idoacetic acid (IAA), all increased a K+ conductance (gK), which could be sufficient to shunt action potentials. 3. The K+ conductance was reduced by incubation of cells in Ca2(+)-free solutions or by increasing the Ca2+ buffering power of pipette-filling solutions. The Ca2+ ionophore, ionomycin, also increased a K+ conductance, and current fluctuation analysis showed that the channels carrying the current induced by both ionomycin and by CN- had a similar mean conductance of circa 9 pS. Thus, increased gK was a Ca2(+)-dependent K+ conductance, gK(Ca), reflecting a rise in resting [Ca2+]i. 4. The conductance was not affected by inclusion of ATP or an ATP-regenerating system in the pipette, suggesting that the underlying rise in [Ca2+] is not due directly to loss of ATP, and confirming that the increased gK is not carried through ATP-dependent K+ channels. 5. Voltage-gated K+ currents evoked by membrane depolarization were increased by CN- or glucose removal. The current-voltage relation of the increased gK mirrored the voltage dependence of Ca2+ entry, and thus reflects impaired cellular handling of the Ca2+ load imposed by depolarization. 6. The rise in [Ca2+]i and altered Ca2+ buffering capacity induced by metabolic blockade affected several other conductances: (i) a Ca2(+)-dependent chloride current was increased. (ii) Both the low-threshold transient and high-threshold sustained voltage-gated Ca2+ currents were attenuated and their thresholds were shifted in the hyperpolarizing direction. (iii) The inward current activated by hyperpolarization. IH, seen in large cells, was attenuated by either metabolic blockade or ionomycin. 7. The responses of these neurones to impaired metabolism thus depend largely on the effects of raised [Ca2+]i on the populations of channels expressed by the cells. These changes in membrane properties could account for some of the changes in neuronal behaviour seen during the clinical states of hypoxia or hypoglycaemia, underlying changes in central nervous system function.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Takeda K., Umbach J. A. Inhibitors of calcium buffering depress evoked transmitter release at the squid giant synapse. J Physiol. 1985 Dec;369:145–159. doi: 10.1113/jphysiol.1985.sp015893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnaes E., Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol. 1975 Jun;248(2):285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelard H. S., Cox D. W., Feeney J., Morris P. G. 31P nuclear-magnetic-resonance studies on superfused cerebral tissues. Biochem Soc Trans. 1985 Oct;13(5):835–839. doi: 10.1042/bst0130835. [DOI] [PubMed] [Google Scholar]
- Benveniste H., Drejer J., Schousboe A., Diemer N. H. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984 Nov;43(5):1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Bernardi H., Fosset M., Lazdunski M. Characterization, purification, and affinity labeling of the brain [3H]glibenclamide-binding protein, a putative neuronal ATP-regulated K+ channel. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9816–9820. doi: 10.1073/pnas.85.24.9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R., Eisner D. A., O'Neill S. C., Valdeolmillos M. Measurements of intracellular Ca2+ in dissociated type I cells of the rabbit carotid body. J Physiol. 1989 Sep;416:421–434. doi: 10.1113/jphysiol.1989.sp017769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Electrophysiological responses of dissociated type I cells of the rabbit carotid body to cyanide. J Physiol. 1989 Jun;413:447–468. doi: 10.1113/jphysiol.1989.sp017663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Synaptic physiology of spinal motoneurones of normal and spastic mice: an in vitro study. J Physiol. 1986 Oct;379:275–292. doi: 10.1113/jphysiol.1986.sp016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosley T. M., Woodhams P. L., Gordon R. D., Balázs R. Effects of anoxia on the stimulated release of amino acid neurotransmitters in the cerebellum in vitro. J Neurochem. 1983 Jan;40(1):189–201. doi: 10.1111/j.1471-4159.1983.tb12670.x. [DOI] [PubMed] [Google Scholar]
- Branston N. M., Strong A. J., Symon L. Extracellular potassium activity, evoked potential and tissue blood flow. Relationships during progressive ischaemia in baboon cerebral cortex. J Neurol Sci. 1977 Jul;32(3):305–321. doi: 10.1016/0022-510x(77)90014-4. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Carafoli E. The calcium cycle of mitochondria. FEBS Lett. 1979 Aug 1;104(1):1–5. doi: 10.1016/0014-5793(79)81073-x. [DOI] [PubMed] [Google Scholar]
- Castle N. A., Haylett D. G. Effect of channel blockers on potassium efflux from metabolically exhausted frog skeletal muscle. J Physiol. 1987 Feb;383:31–43. doi: 10.1113/jphysiol.1987.sp016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G. D., Rothman S. M. Blockade of excitatory amino acid receptors protects anoxic hippocampal slices. Neuroscience. 1987 Jun;21(3):665–671. doi: 10.1016/0306-4522(87)90027-3. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Bachelard H. S. Attenuation of evoked field potentials from dentate granule cells by low glucose, pyruvate + malate, and sodium fluoride. Brain Res. 1982 May 13;239(2):527–534. doi: 10.1016/0006-8993(82)90527-3. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Morris P. G., Feeney J., Bachelard H. S. 31P-n.m.r. studies on cerebral energy metabolism under conditions of hypoglycaemia and hypoxia in vitro. Biochem J. 1983 May 15;212(2):365–370. doi: 10.1042/bj2120365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande J. K., Siesjö B. K., Wieloch T. Calcium accumulation and neuronal damage in the rat hippocampus following cerebral ischemia. J Cereb Blood Flow Metab. 1987 Feb;7(1):89–95. doi: 10.1038/jcbfm.1987.13. [DOI] [PubMed] [Google Scholar]
- Duchen M. R., Caddy K. W., Kirby G. C., Patterson D. L., Ponte J., Biscoe T. J. Biophysical studies of the cellular elements of the rabbit carotid body. Neuroscience. 1988 Jul;26(1):291–311. doi: 10.1016/0306-4522(88)90146-7. [DOI] [PubMed] [Google Scholar]
- Duchen M. R., Valdeolmillos M., O'Neill S. C., Eisner D. A. Effects of metabolic blockade on the regulation of intracellular calcium in dissociated mouse sensory neurones. J Physiol. 1990 May;424:411–426. doi: 10.1113/jphysiol.1990.sp018074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. M., Loyning Y., Oshima T. Effects of hypoxia on the monosynaptic reflex pathway in the cat spinal cord. J Neurophysiol. 1966 Mar;29(2):315–331. doi: 10.1152/jn.1966.29.2.315. [DOI] [PubMed] [Google Scholar]
- Fujiwara N., Higashi H., Shimoji K., Yoshimura M. Effects of hypoxia on rat hippocampal neurones in vitro. J Physiol. 1987 Mar;384:131–151. doi: 10.1113/jphysiol.1987.sp016447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego R., Ivorra I., Morales A. Effects of central or peripheral axotomy on membrane properties of sensory neurones in the petrosal ganglion of the cat. J Physiol. 1987 Oct;391:39–56. doi: 10.1113/jphysiol.1987.sp016724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind J. M., Kawamura H., Krnjević K., Pumain R. Actions of dinitrophenol and some other metabolic inhibitors on cortical neurones. J Physiol. 1971 May;215(1):199–222. doi: 10.1113/jphysiol.1971.sp009465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hansen A. J. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985 Jan;65(1):101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Hansen A. J., Hounsgaard J., Jahnsen H. Anoxia increases potassium conductance in hippocampal nerve cells. Acta Physiol Scand. 1982 Jul;115(3):301–310. doi: 10.1111/j.1748-1716.1982.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Lawson S. N. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol. 1985 Feb;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen E., Akerman K. E., Kaila K. Depolarization of the mitochondrial membrane potential increases free cytosolic calcium in synaptosomes. Neurosci Lett. 1984 Aug 24;49(1-2):33–37. doi: 10.1016/0304-3940(84)90132-0. [DOI] [PubMed] [Google Scholar]
- Kauppinen R. A., Nicholls D. G. Synaptosomal bioenergetics. The role of glycolysis, pyruvate oxidation and responses to hypoglycaemia. Eur J Biochem. 1986 Jul 1;158(1):159–165. doi: 10.1111/j.1432-1033.1986.tb09733.x. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G. Metabolic control of ionic channels in the neuronal membrane. Neuroscience. 1984 Dec;13(4):983–989. doi: 10.1016/0306-4522(84)90282-3. [DOI] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P., Whittingham T. S. Reduced ATP concentration as a basis for synaptic transmission failure during hypoxia in the in vitro guinea-pig hippocampus. J Physiol. 1982 Apr;325:51–65. doi: 10.1113/jphysiol.1982.sp014135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P., Whittingham T. S. The effect of hypoxia on evoked potentials in the in vitro hippocampus. J Physiol. 1979 Feb;287:427–438. doi: 10.1113/jphysiol.1979.sp012668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Hermann T. E. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978 Sep 10;253(17):5892–5894. [PubMed] [Google Scholar]
- Mayer M. L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985 Jul;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L. Selective block of inward but not outward rectification in rat sensory neurones infected with herpes simplex virus. J Physiol. 1986 Jun;375:327–338. doi: 10.1113/jphysiol.1986.sp016119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Davies N. W., Kaplan J. H., Lux H. D. Inactivation and block of calcium channels by photo-released Ca2+ in dorsal root ganglion neurons. Science. 1988 Aug 12;241(4867):842–844. doi: 10.1126/science.2457253. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A. Regulation of cytosolic calcium concentration in presynaptic nerve endings isolated from rat brain. J Physiol. 1985 Jun;363:87–101. doi: 10.1113/jphysiol.1985.sp015697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G., Akerman K. E. Biochemical approaches to the study of cytosolic calcium regulation in nerve endings. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):115–122. doi: 10.1098/rstb.1981.0176. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Owen D. G., Segal M., Barker J. L. A Ca-dependent Cl- conductance in cultured mouse spinal neurones. Nature. 1984 Oct 11;311(5986):567–570. doi: 10.1038/311567a0. [DOI] [PubMed] [Google Scholar]
- Rasgado-Flores H., Blaustein M. P. ATP-dependent regulation of cytoplasmic free calcium in nerve terminals. Am J Physiol. 1987 Jun;252(6 Pt 1):C588–C594. doi: 10.1152/ajpcell.1987.252.6.C588. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Scarpa A. Calcium uptake and membrane potential in mitochondria. Biochemistry. 1974 Nov 5;13(23):4811–4817. doi: 10.1021/bi00720a020. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab. 1981;1(2):155–185. doi: 10.1038/jcbfm.1981.18. [DOI] [PubMed] [Google Scholar]
- VAN HARREVELD A. Compounds in brain extracts causing spreading depression of cerebral cortical activity and contraction of crustacean muscle. J Neurochem. 1959 Feb;3(4):300–315. doi: 10.1111/j.1471-4159.1959.tb12636.x. [DOI] [PubMed] [Google Scholar]


