Abstract
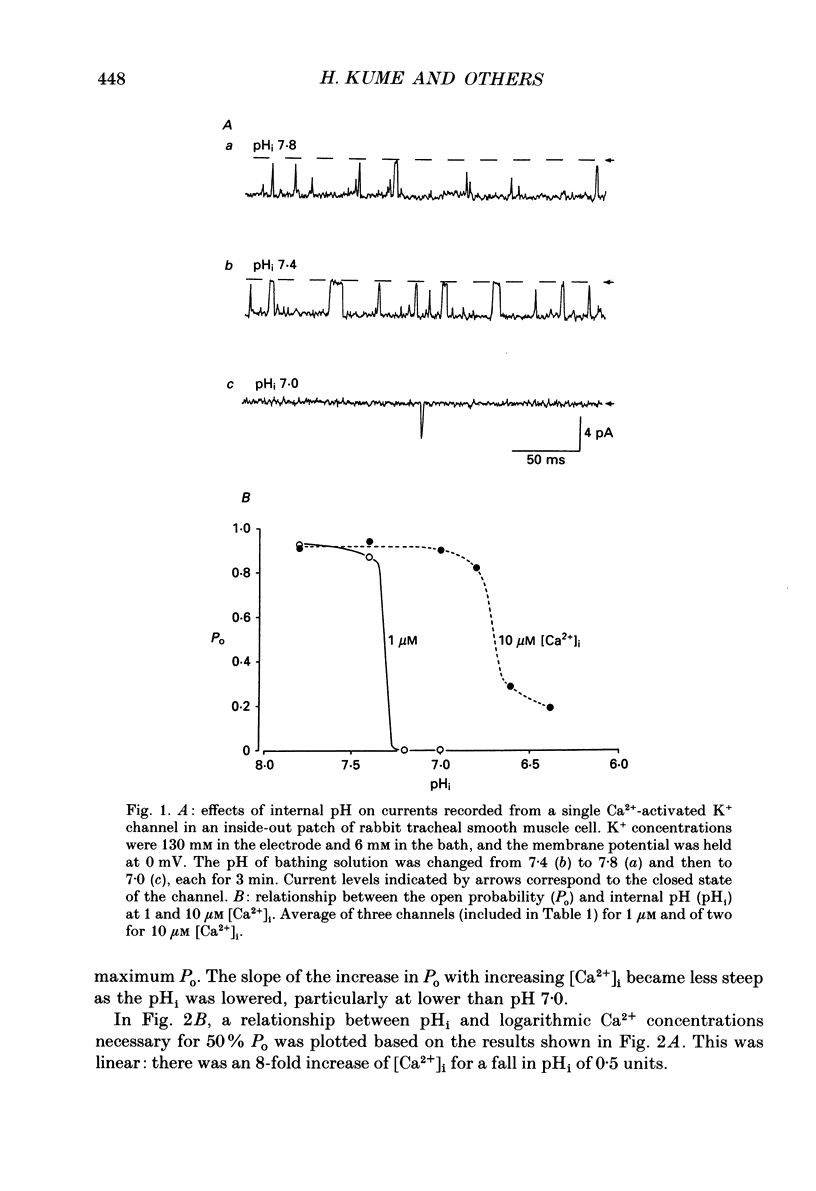
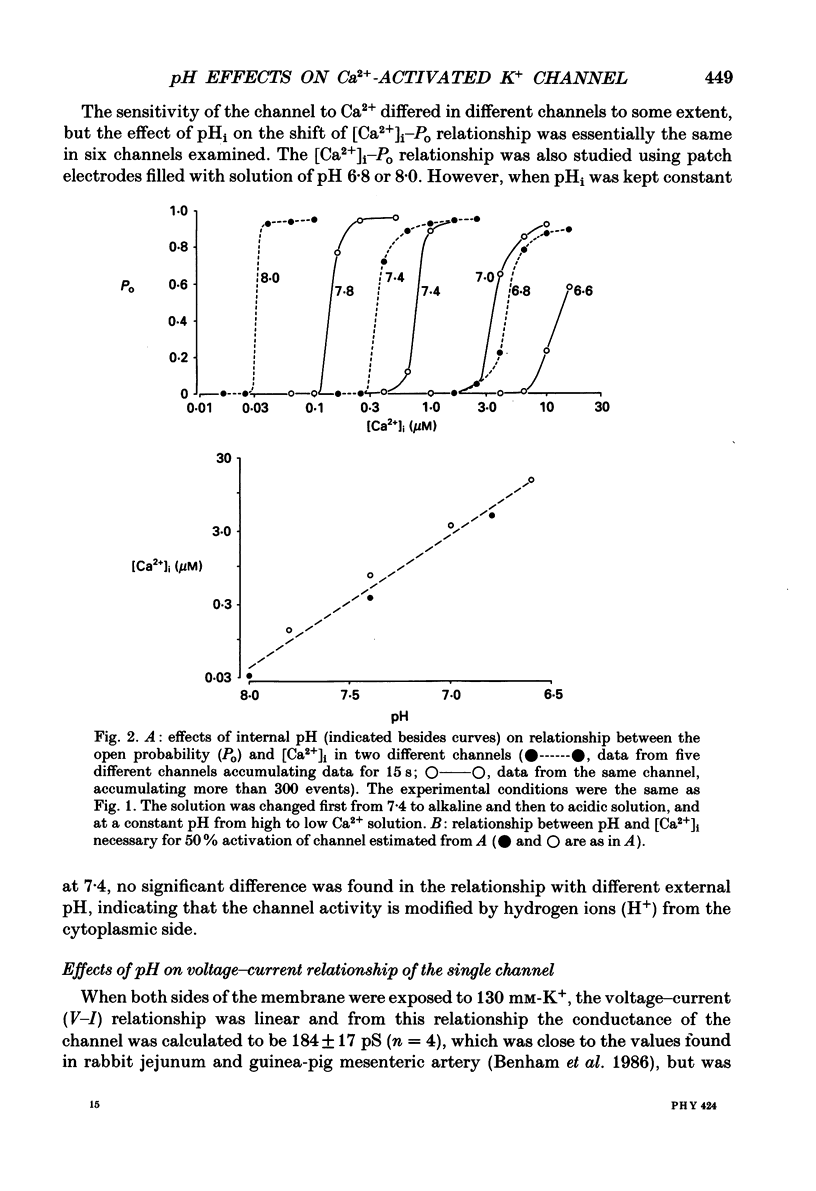
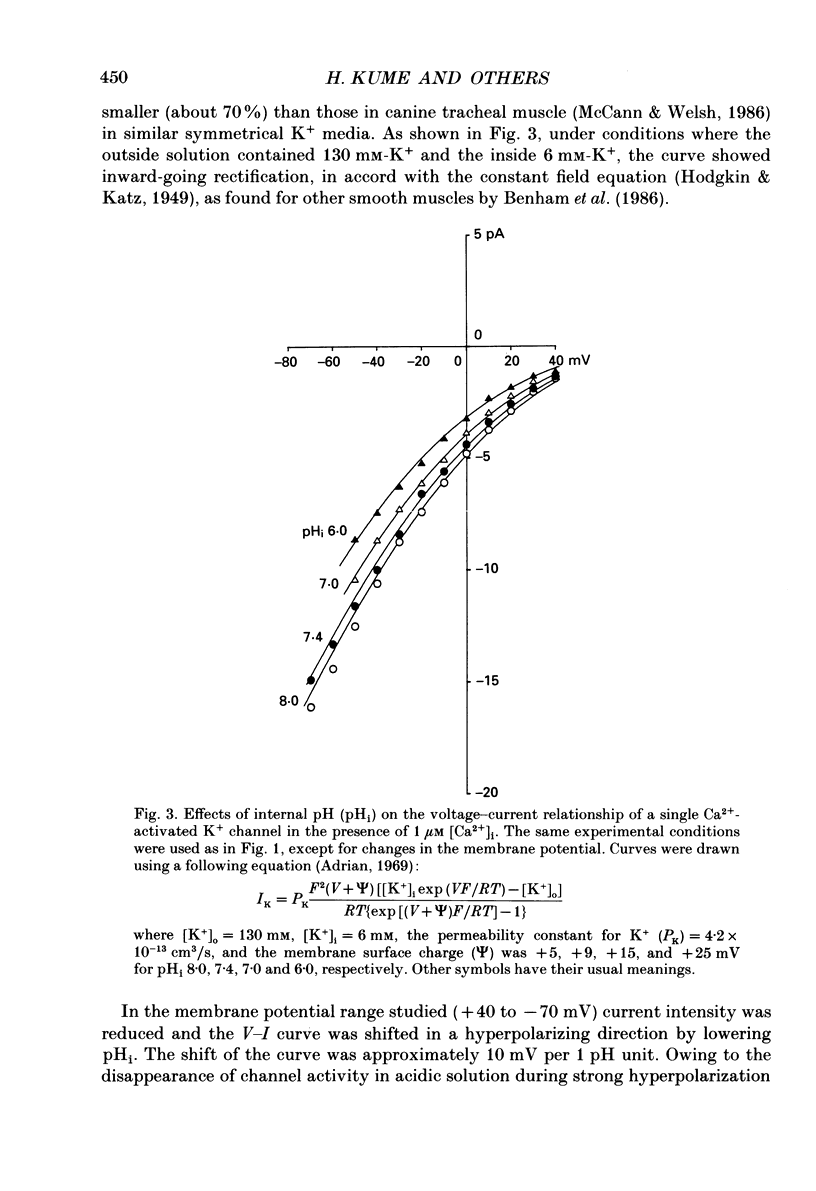
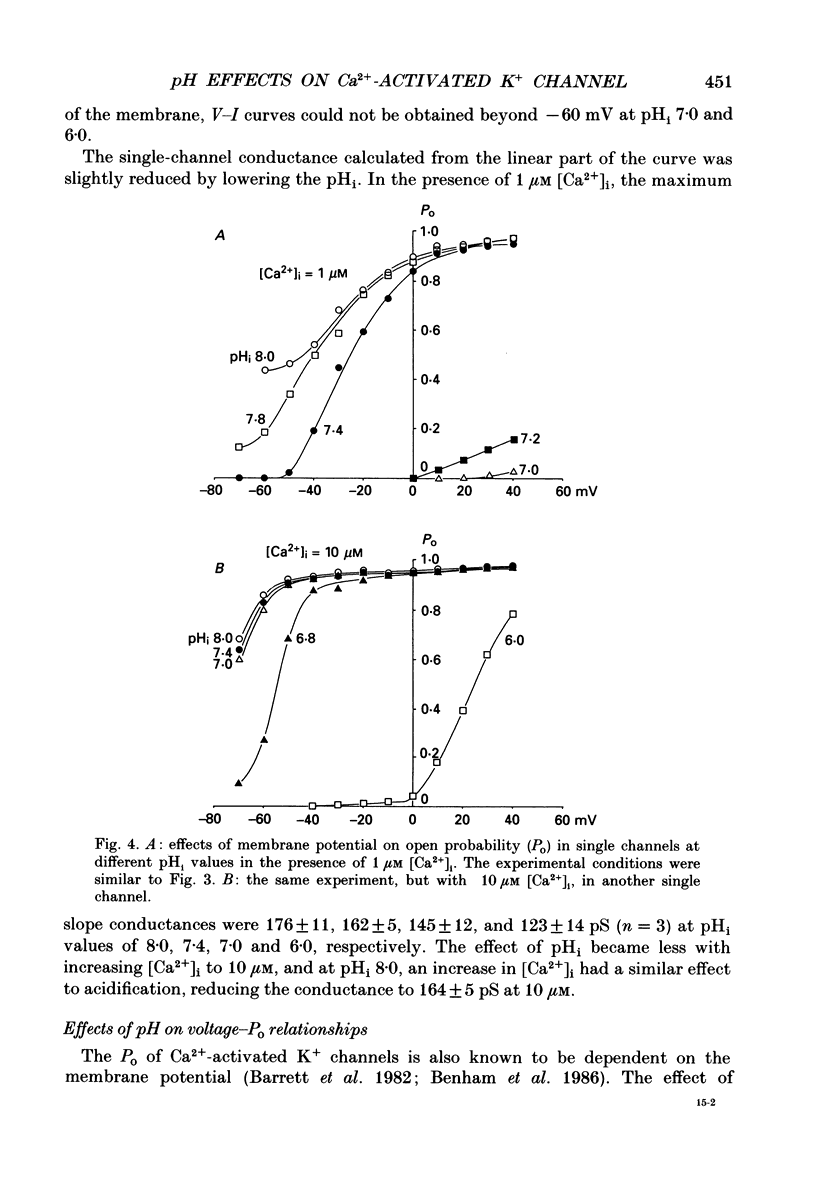
1. The effects of intracellular pH (pHi) on calcium-activated potassium channels (Ca2(+)-activated K+ channels) were studied in membrane patches of smooth muscle freshly dispersed from the rabbit trachea. Single-channel currents were recorded with an 'inside-out' patch clamp technique, mainly at 0 mV, with the external (electrode) medium containing 130 mM-K+ and the internal (bath) medium 6 mM-K+. 2. With an internal Ca2+ concentration ([Ca2+]i) of 1 microM, the fraction of time during which the channel was in an open state (the open probability, Po) was more than 0.8 at pHi 7.4. The channel activity nearly disappeared at pHi 7.0. The [Ca2+]i-Po relationship was shifted to higher [Ca2+]i by acidosis, the shift being approximately an 8-fold increase for a fall in pHi of 0.5 units. 3. The membrane potential and current intensity (V-I) relationship of single channels between +30 and -50 mV was shifted in a hyperpolarizing direction by intracellular acidosis. The shift was roughly 10 mV for 1 pH unit at 1 microM [Ca2+]i. At pHi 7.4 [Ca2+]i 1 microM, the V-Po relationship was shifted in a depolarizing direction by acidification. When [Ca2+]i was increased to 10 microM, V-Po relationship became less sensitive to V as well as pHi changes. 4. When Po was high, the probability density function of open and closed time distributions could be fitted by two exponentials. When Po was decreased to less than 0.3, either by reducing [Ca2+]i or by lowering pHi, another component having long closed times appeared. At similar Po values, the time constant of open time distribution was smaller with lower pHi. 5. It is concluded that the main effect of an increase in intracellular hydrogen ions is to decrease the open probability of the Ca2(+)-activated K+ channel, by reducing the sensitivity to Ca2+ and also shortening the open state.
Full text
PDF


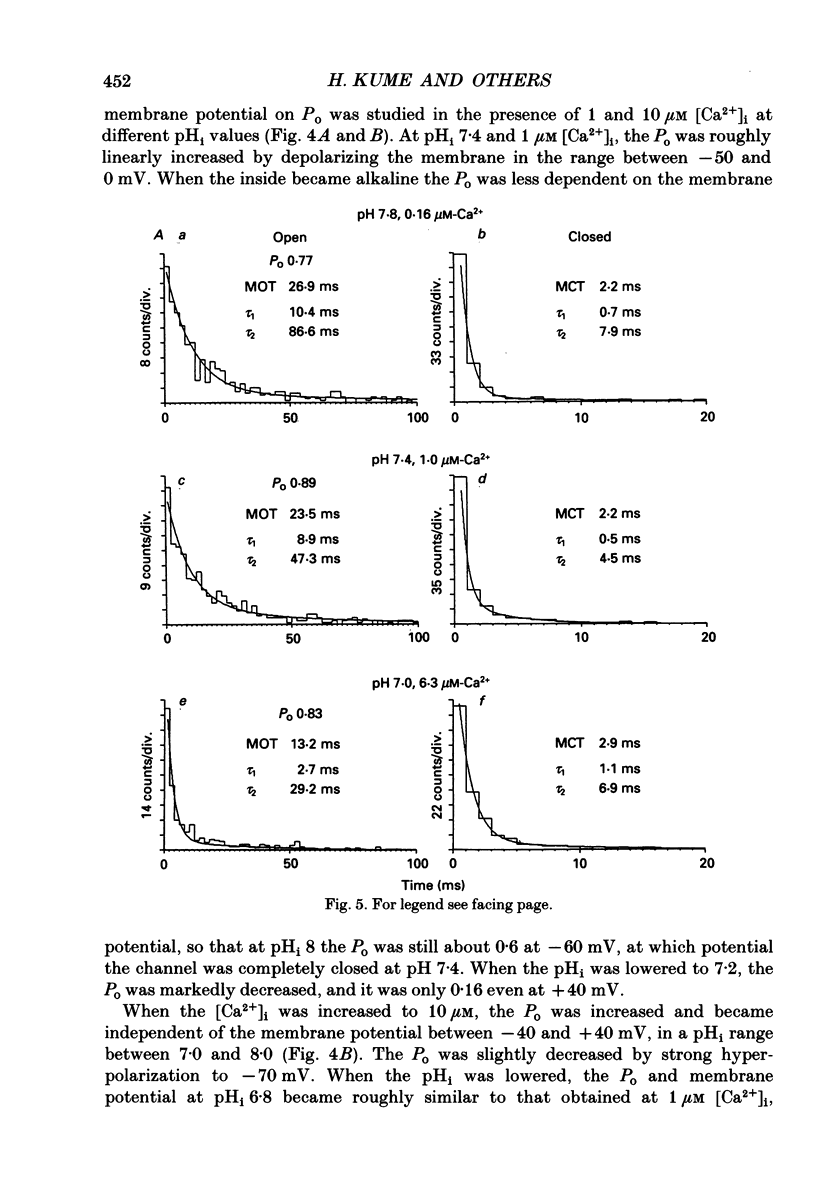
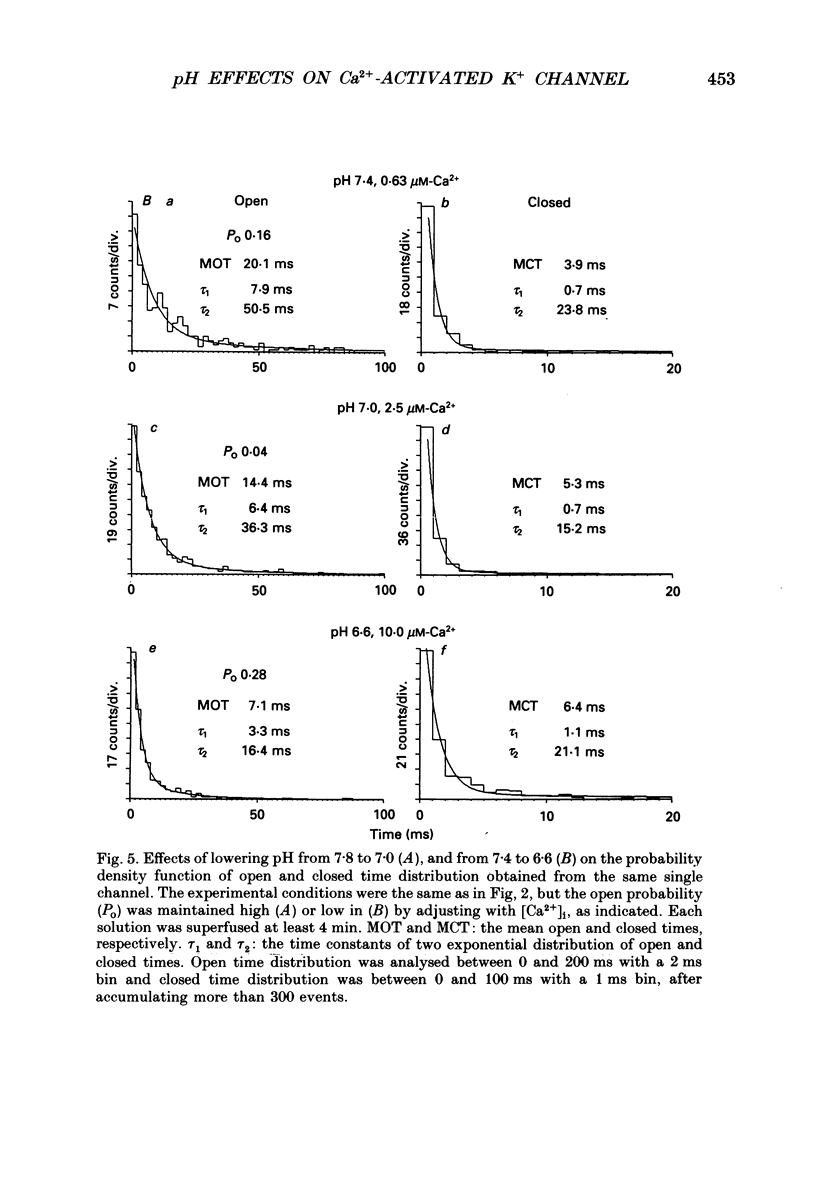




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H. Rectification in muscle membrane. Prog Biophys Mol Biol. 1969;19(2):339–369. [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen O., Zeuthen T. Maxi K+ channels in leaky epithelia are regulated by intracellular Ca2+, pH and membrane potential. Pflugers Arch. 1987 Mar;408(3):249–259. doi: 10.1007/BF02181467. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Ikeuchi M., Fujimoto W. Y. Lowering of pHi inhibits Ca2+-activated K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]
- Deutsch C., Lee S. C. Modulation of K+ currents in human lymphocytes by pH. J Physiol. 1989 Jun;413:399–413. doi: 10.1113/jphysiol.1989.sp017660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Inoue R., Okabe K., Kitamura K., Kuriyama H. A newly identified Ca2+ dependent K+ channel in the smooth muscle membrane of single cells dispersed from the rabbit portal vein. Pflugers Arch. 1986 Feb;406(2):138–143. doi: 10.1007/BF00586674. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Sato R. Intra- and extracellular actions of proton on the calcium current of isolated guinea pig ventricular cells. Circ Res. 1986 Sep;59(3):348–355. doi: 10.1161/01.res.59.3.348. [DOI] [PubMed] [Google Scholar]
- Kaibara M., Kameyama M. Inhibition of the calcium channel by intracellular protons in single ventricular myocytes of the guinea-pig. J Physiol. 1988 Sep;403:621–640. doi: 10.1113/jphysiol.1988.sp017268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi Y. The effects of intracellular protons on the electrical activity of single ventricular cells. Pflugers Arch. 1982 Sep;394(3):264–270. doi: 10.1007/BF00589102. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H., Flanagan P., Pearson G. T. Quantification of Ca2+-activated K+ channels under hormonal control in pig pancreas acinar cells. Nature. 1983 Sep 15;305(5931):228–232. doi: 10.1038/305228a0. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Calcium-activated potassium channels in canine airway smooth muscle. J Physiol. 1986 Mar;372:113–127. doi: 10.1113/jphysiol.1986.sp016000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W. J., Hagiwara S. Block of inward rectification by intracellular H+ in immature oocytes of the starfish Mediaster aequalis. J Gen Physiol. 1982 Jan;79(1):115–130. doi: 10.1085/jgp.79.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W., Jr Appearance of calcium action potentials in crayfish slow muscle fibres under conditions of low intracellular pH. J Physiol. 1980 May;302:335–346. doi: 10.1113/jphysiol.1980.sp013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W., Spalding B. C., Hille B. Low intracellular pH and chemical agents slow inactivation gating in sodium channels of muscle. Nature. 1980 Mar 27;284(5754):360–363. doi: 10.1038/284360a0. [DOI] [PubMed] [Google Scholar]
- Stampe P., Vestergaard-Bogind B. The Ca2+-sensitive K+-conductance of the human red cell membrane is strongly dependent on cellular pH. Biochim Biophys Acta. 1985 May 14;815(2):313–321. doi: 10.1016/0005-2736(85)90302-5. [DOI] [PubMed] [Google Scholar]
- Vogel S., Sperelakis N. Blockade of myocardial slow inward current at low pH. Am J Physiol. 1977 Sep;233(3):C99–103. doi: 10.1152/ajpcell.1977.233.3.C99. [DOI] [PubMed] [Google Scholar]
- Wanke E., Carbone E., Testa P. L. K+ conductance modified by a titratable group accessible to protons from the intracellular side of the squid axon membrane. Biophys J. 1979 May;26(2):319–324. doi: 10.1016/S0006-3495(79)85251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B. S., Lecar H., Adler M. Single calcium-dependent potassium channels in clonal anterior pituitary cells. Biophys J. 1982 Sep;39(3):313–317. doi: 10.1016/S0006-3495(82)84522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S. Smooth muscle intracellular pH: measurement, regulation, and function. Am J Physiol. 1988 Feb;254(2 Pt 1):C213–C225. doi: 10.1152/ajpcell.1988.254.2.C213. [DOI] [PubMed] [Google Scholar]


