Abstract
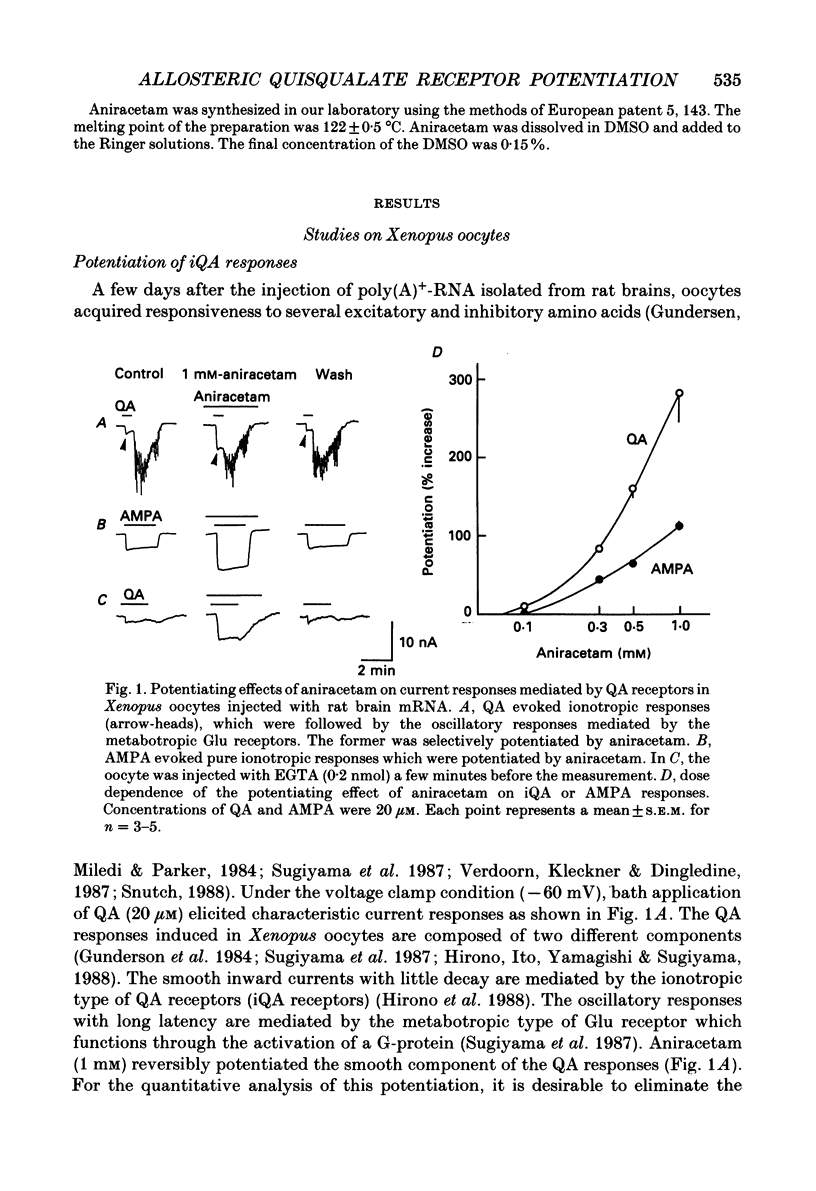
1. Allosteric potentiation of the ionotropic quisqualate (iQA) receptor by a nootropic drug aniracetam (1-p-anisoyl-2-pyrrolidinone) was investigated using Xenopus oocytes injected with rat brain mRNA and rat hippocampal slices. 2. Aniracetam potentiates the iQA responses induced in Xenopus oocytes by rat brain mRNA in a reversible manner. This effect was observed above the concentrations of 0.1 mM. Kainate. N-methyl-D-aspartate and gamma-aminobutyric acid responses induced in the same oocytes were not affected. 3. The specific potentiation of iQA responses was accompanied by an increase in the conductance change of iQA and alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) responses, but the affinity of receptors for agonist and the ion-selectivity of the channels (reversal potentials) were not changed. 4. Aniracetam reversibly potentiated the iQA responses recorded intracellularly from the pyramidal cells in the CA1 region of rat hippocampal slices. The excitatory postsynaptic potentials (EPSPs) in Schaffer collateral-commissural-CA1 synapses were also potentiated by aniracetam. 5. Population EPSPs recorded in the mossy fibre-CA3 synapses as well as Schaffer-commissural synapses were also potentiated by aniracetam. The amplitudes of the potentiation were not changed by the formation of long-term potentiation.
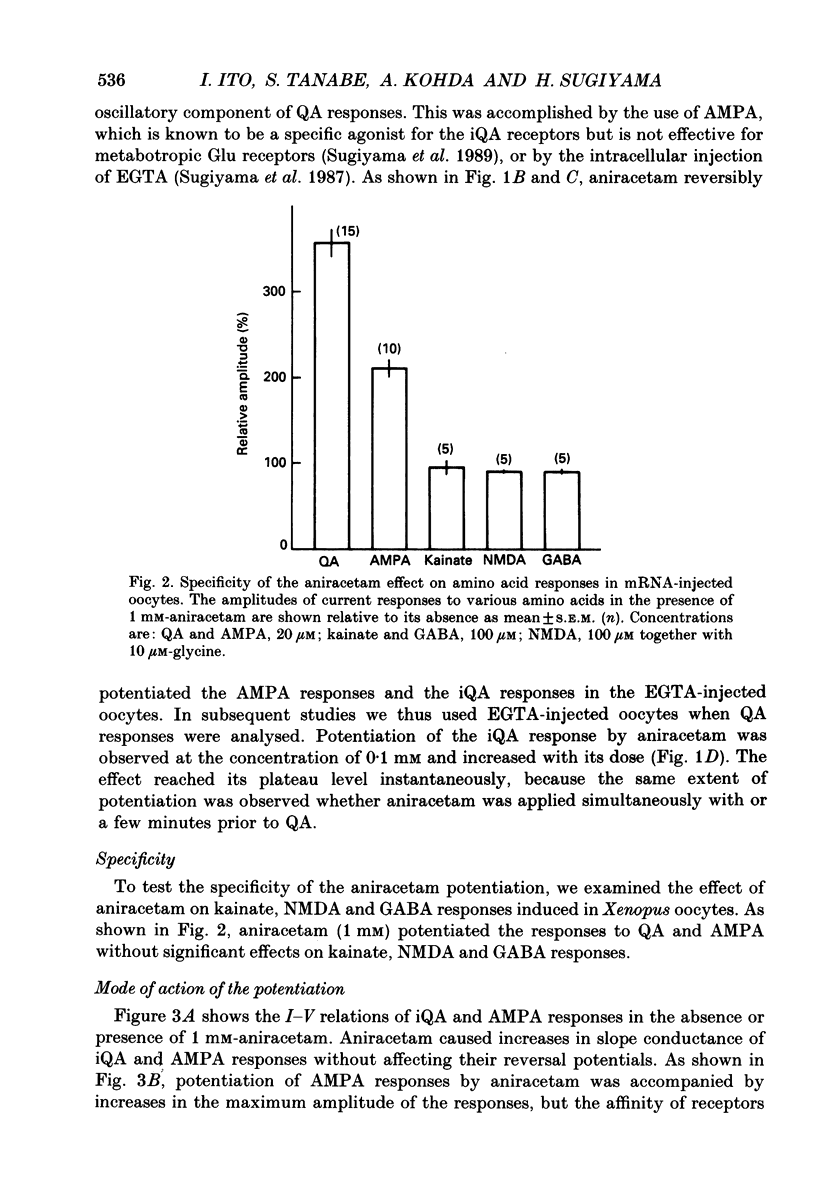
Full text
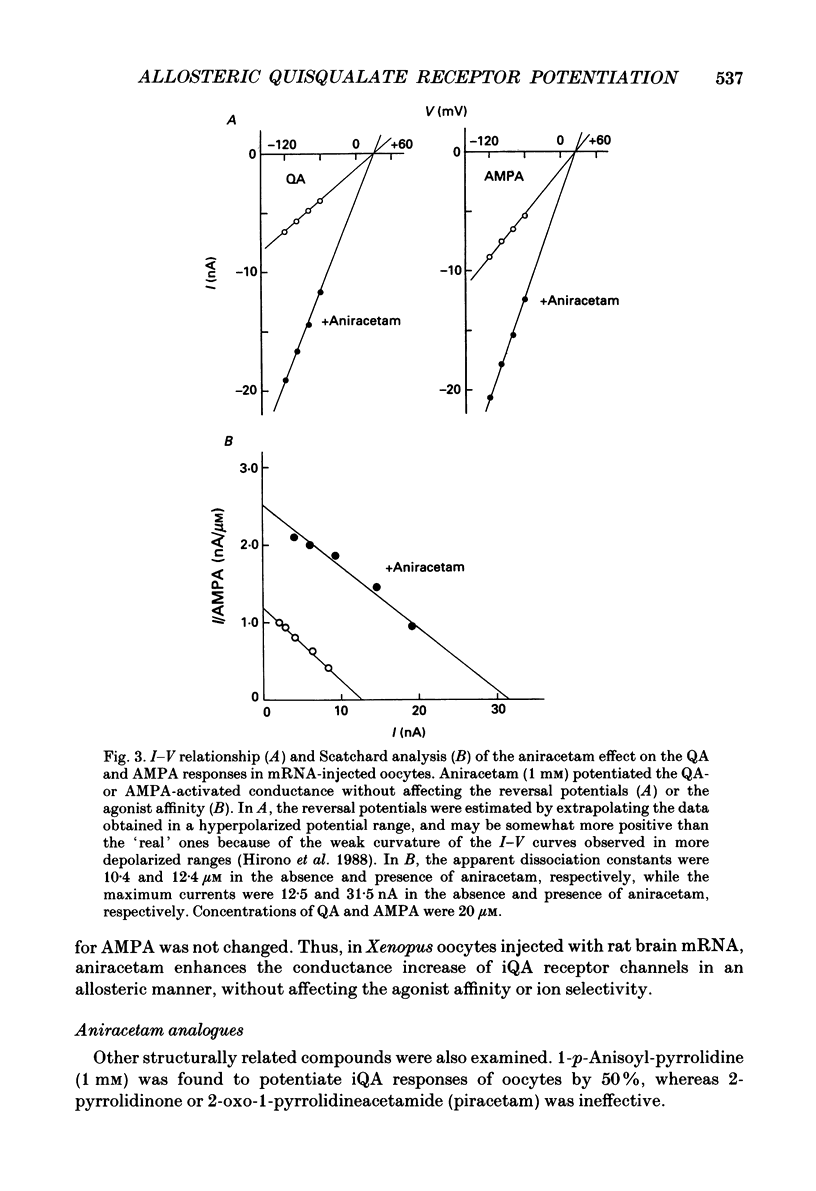
PDF

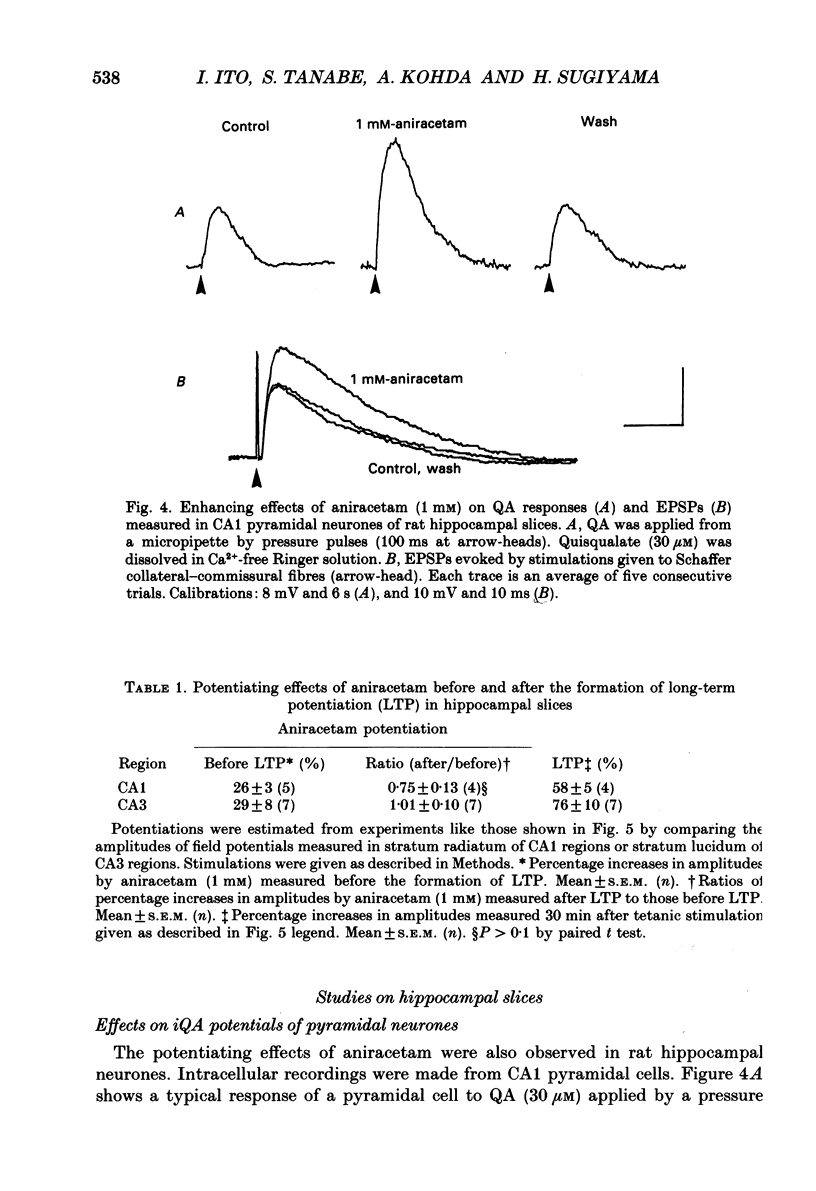




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988 Mar;11(3):112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Cumin R., Bandle E. F., Gamzu E., Haefely W. E. Effects of the novel compound aniracetam (Ro 13-5057) upon impaired learning and memory in rodents. Psychopharmacology (Berl) 1982;78(2):104–111. doi: 10.1007/BF00432244. [DOI] [PubMed] [Google Scholar]
- Davies S. N., Lester R. A., Reymann K. G., Collingridge G. L. Temporally distinct pre- and post-synaptic mechanisms maintain long-term potentiation. Nature. 1989 Apr 6;338(6215):500–503. doi: 10.1038/338500a0. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Glutamate and kainate receptors induced by rat brain messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):127–143. doi: 10.1098/rspb.1984.0027. [DOI] [PubMed] [Google Scholar]
- Hirono C., Ito I., Yamagishi S., Sugiyama H. Characterization of glutamate receptors induced in Xenopus oocytes after injection of rat brain mRNA. Neurosci Res. 1988 Dec;6(2):106–114. doi: 10.1016/0168-0102(88)90012-0. [DOI] [PubMed] [Google Scholar]
- Ito I., Okada D., Sugiyama H. Pertussis toxin suppresses long-term potentiation of hippocampal mossy fiber synapses. Neurosci Lett. 1988 Jul 19;90(1-2):181–185. doi: 10.1016/0304-3940(88)90808-7. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kauer J. A., Malenka R. C., Nicoll R. A. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988 Dec;1(10):911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- Kiskin N. I., Krishtal O. A., Tsyndrenko AYa Excitatory amino acid receptors in hippocampal neurons: kainate fails to desensitize them. Neurosci Lett. 1986 Jan 30;63(3):225–230. doi: 10.1016/0304-3940(86)90360-5. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr Concanavalin A selectively reduces desensitization of mammalian neuronal quisqualate receptors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1411–1415. doi: 10.1073/pnas.86.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Muller D., Joly M., Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988 Dec 23;242(4886):1694–1697. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. J., Fischbach G. D. Characterization of excitatory amino acid receptors expressed by embryonic chick motoneurons in vitro. J Neurosci. 1986 Nov;6(11):3275–3283. doi: 10.1523/JNEUROSCI.06-11-03275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada D., Yamagishi S., Sugiyama H. Differential effects of phospholipase inhibitors in long-term potentiation in the rat hippocampal mossy fiber synapses and Schaffer/commissural synapses. Neurosci Lett. 1989 May 22;100(1-3):141–146. doi: 10.1016/0304-3940(89)90674-5. [DOI] [PubMed] [Google Scholar]
- Rassendren F. A., Lory P., Pin J. P., Bockaert J., Nargeot J. A specific quisqualate agonist inhibits kainate responses induced in Xenopus oocytes injected with rat brain RNA. Neurosci Lett. 1989 May 8;99(3):333–339. doi: 10.1016/0304-3940(89)90469-2. [DOI] [PubMed] [Google Scholar]
- Snutch T. P. The use of Xenopus oocytes to probe synaptic communication. Trends Neurosci. 1988 Jun;11(6):250–256. doi: 10.1016/0166-2236(88)90102-6. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Wong R. K. GABAA responses in hippocampal neurons are potentiated by glutamate. Nature. 1989 Jan 12;337(6203):170–173. doi: 10.1038/337170a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Watanabe M. Glutamate receptor subtypes may be classified into two major categories: a study on Xenopus oocytes injected with rat brain mRNA. Neuron. 1989 Jul;3(1):129–132. doi: 10.1016/0896-6273(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Dingledine R. Excitatory amino acid receptors expressed in Xenopus oocytes: agonist pharmacology. Mol Pharmacol. 1988 Sep;34(3):298–307. [PubMed] [Google Scholar]
- Verdoorn T. A., Kleckner N. W., Dingledine R. Rat brain N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Science. 1987 Nov 20;238(4830):1114–1116. doi: 10.1126/science.2825347. [DOI] [PubMed] [Google Scholar]
- Vincent G., Verderese A., Gamzu E. The effects of aniracetam (Ro 13-5057) on the enhancement and protection of memory. Ann N Y Acad Sci. 1985;444:489–491. doi: 10.1111/j.1749-6632.1985.tb37620.x. [DOI] [PubMed] [Google Scholar]


