Abstract
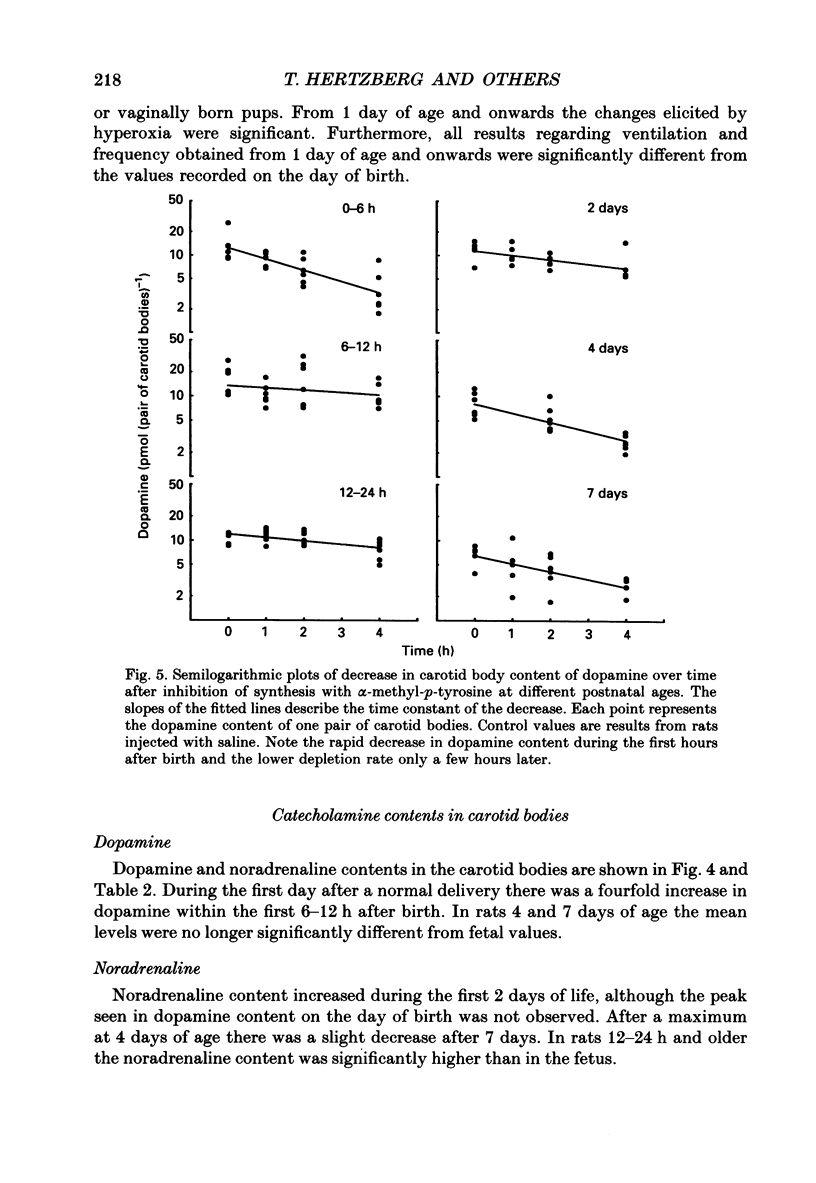
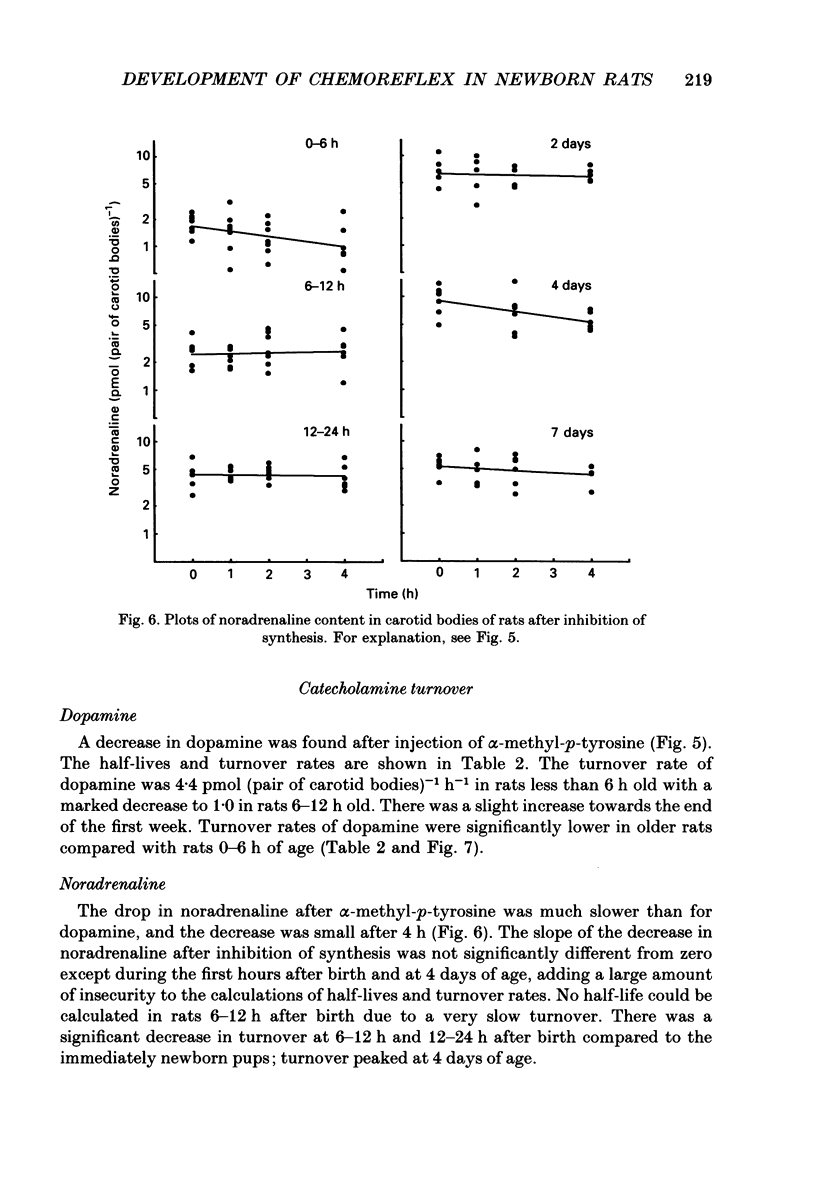
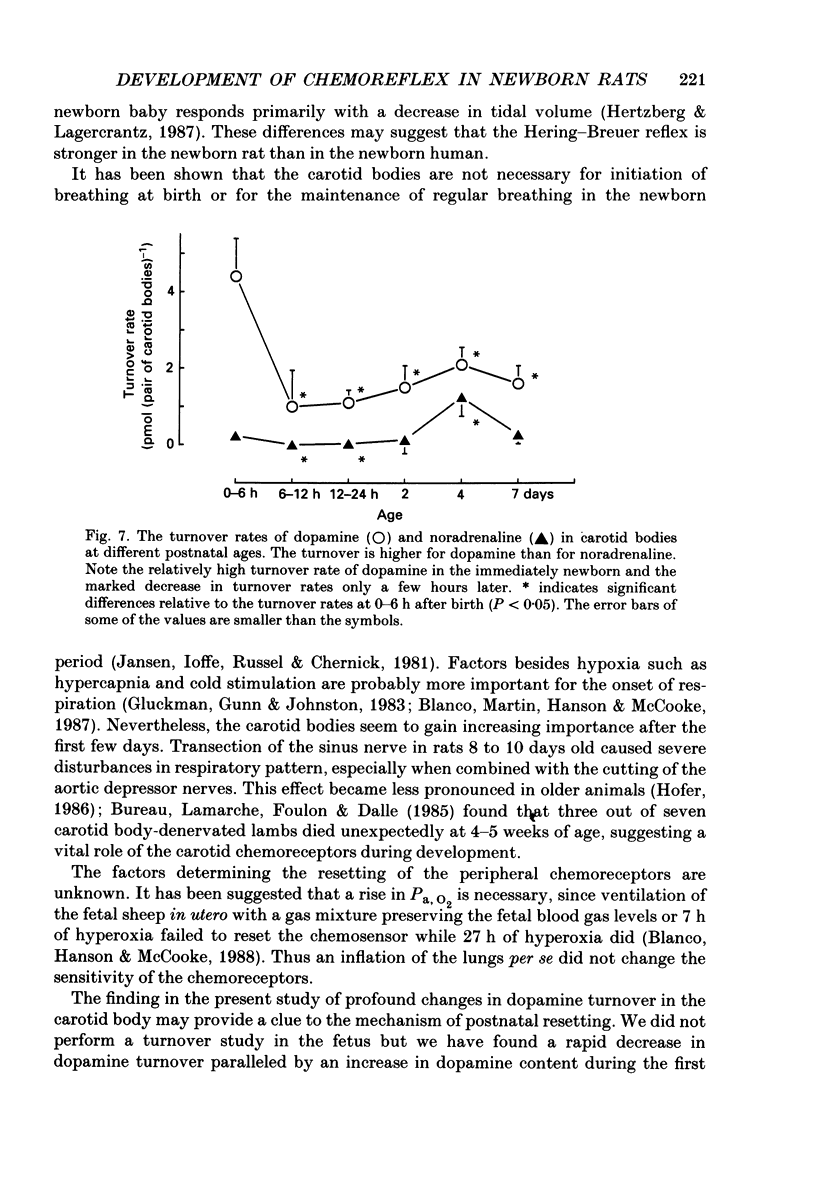
1. The peripheral, arterial chemoreceptors in the carotid body are active and responsive in the fetus. At birth, when oxygenation increases, the chemoreceptors are silenced. Over the next few days the sensitivity is reset toward the adult level and the chemoreceptors influence breathing during normal conditions. In order to investigate the underlying mechanisms of this resetting we examined the strength of the chemoreflex in newborn rats and correlated this to the contents of dopamine and noradrenaline in the carotid bodies of the newborn pups and near-term fetuses. Furthermore, turnover rates of dopamine and noradrenaline were determined in newborn rats up to 1 week of age by analysis of catecholamine decreases after inhibition of synthesis with alpha-methyl-p-tyrosine. 2. Chemoreceptor influence was assessed by the method of 'physiological chemodenervation' with hyperoxia of 15-20 s duration in unanaesthetized rat pups. Relative changes in ventilation elicited by hyperoxia were determined by body plethysmography. We found no change in ventilation on the day of birth either in vaginally born rats or in near-term pups delivered by Caesarean section. After 1 day there was a significant decrease in ventilation of -19.4 +/- 2.3% (mean +/- S.E.M.) and at 7 days of age the decrease was -28.8 +/- 2.2%, suggesting an increasing influence from the peripheral chemoreceptors. 3. The contents of dopamine and noradrenaline were measured by high-performance liquid chromatography. Dopamine increased from 3.7 +/- 0.4 pmol (pair of carotid bodies)-1 in the fetus to a peak of 15.9 +/- 2.6, 6-12 h after birth followed by a decline to 7.1 +/- 0.7 at 7 days of age. Noradrenaline levels increased from 1.3 +/- 0.3 in the fetus to 9.6 +/- 1.1 pmol (pair of carotid bodies)-1 after 4 days. The turnover rate of dopamine decreased from 4.4 pmol (pair of carotid bodies)-1 h-1 0-6 h after birth to 1.0 at 6-12 h of age. The turnover rate of noradrenaline also decreased over the first hours following delivery. 4. Since dopamine is an inhibitory neuromodulator in this system, we suggest that the increase in sensitivity seen after the first day of life is, at least in part, due to a decrease in the release of dopamine and thus a removal of an inhibitory mechanism.
Full text
PDF


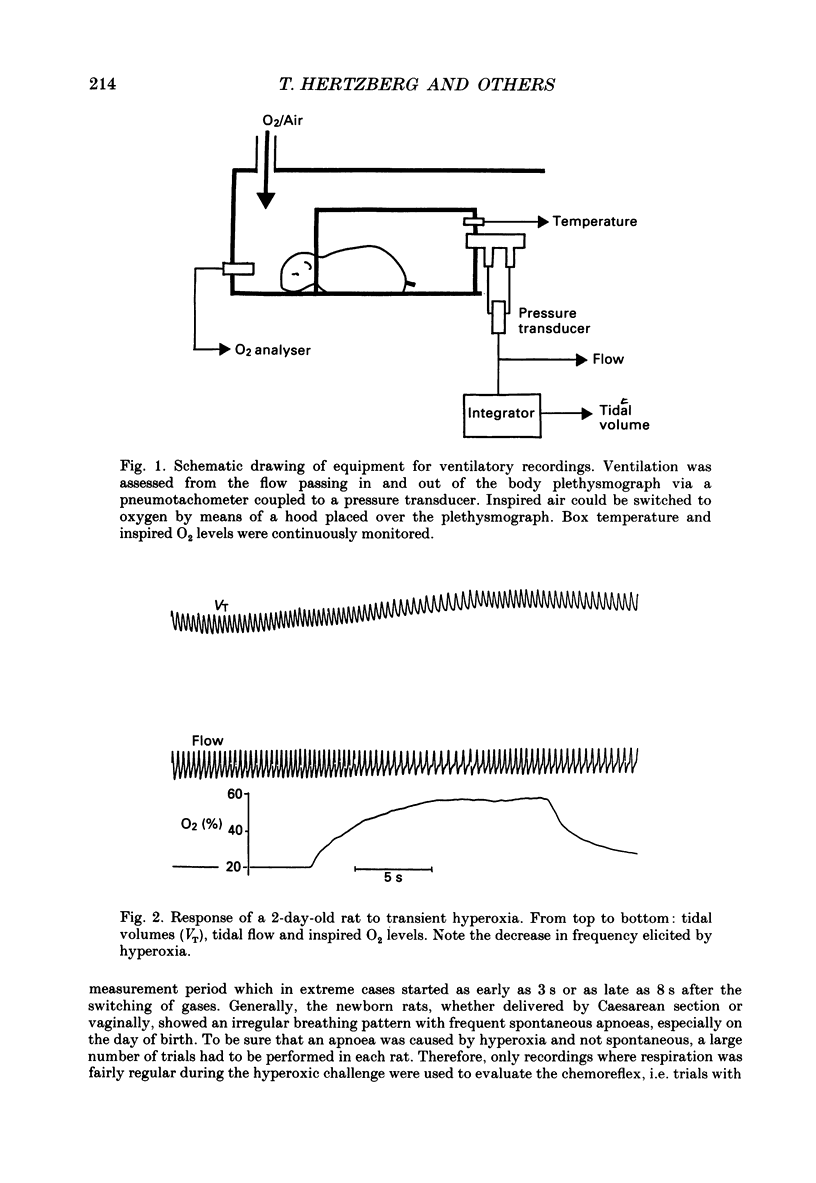
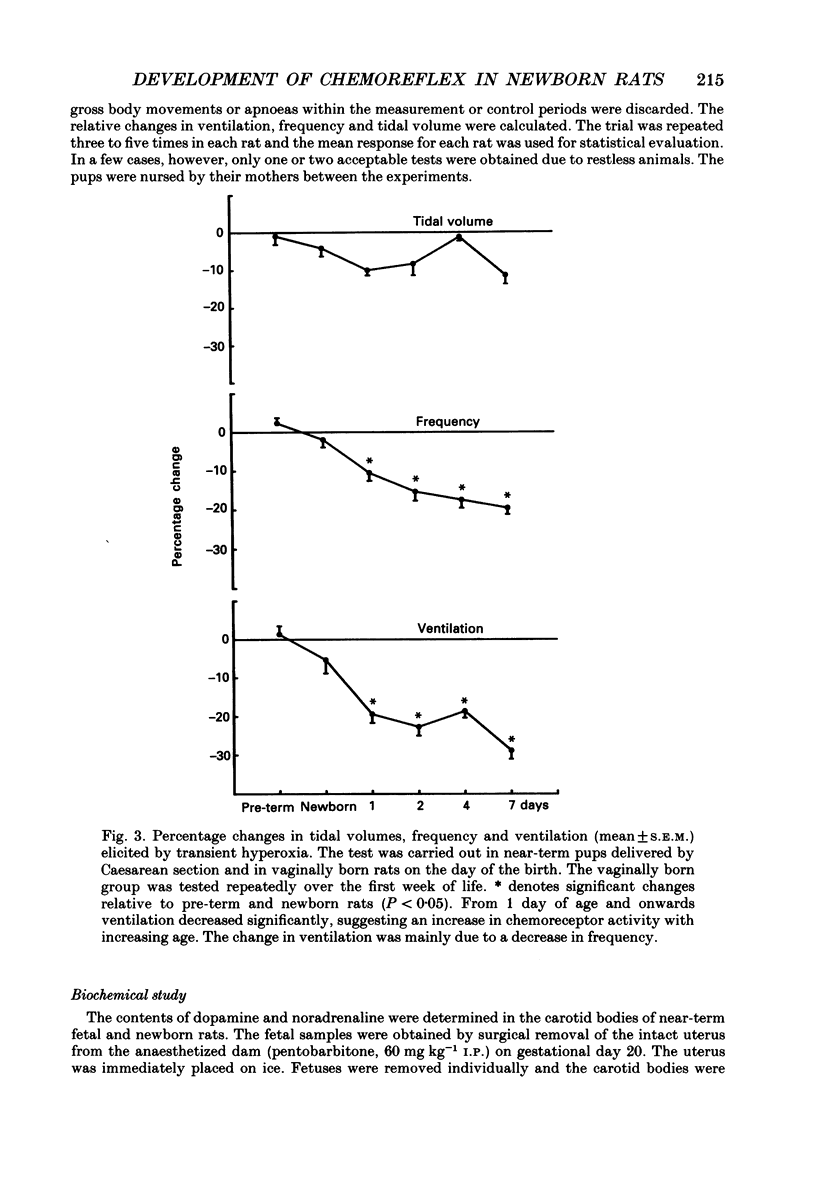
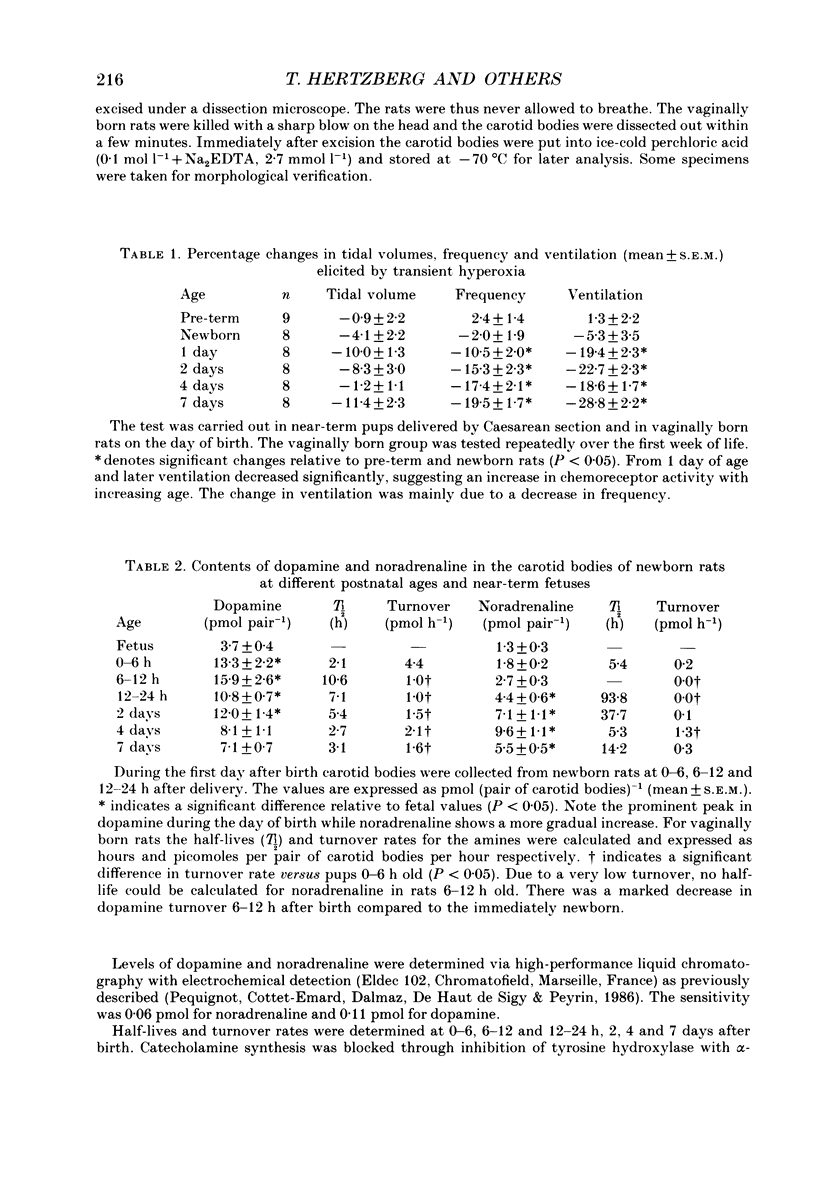
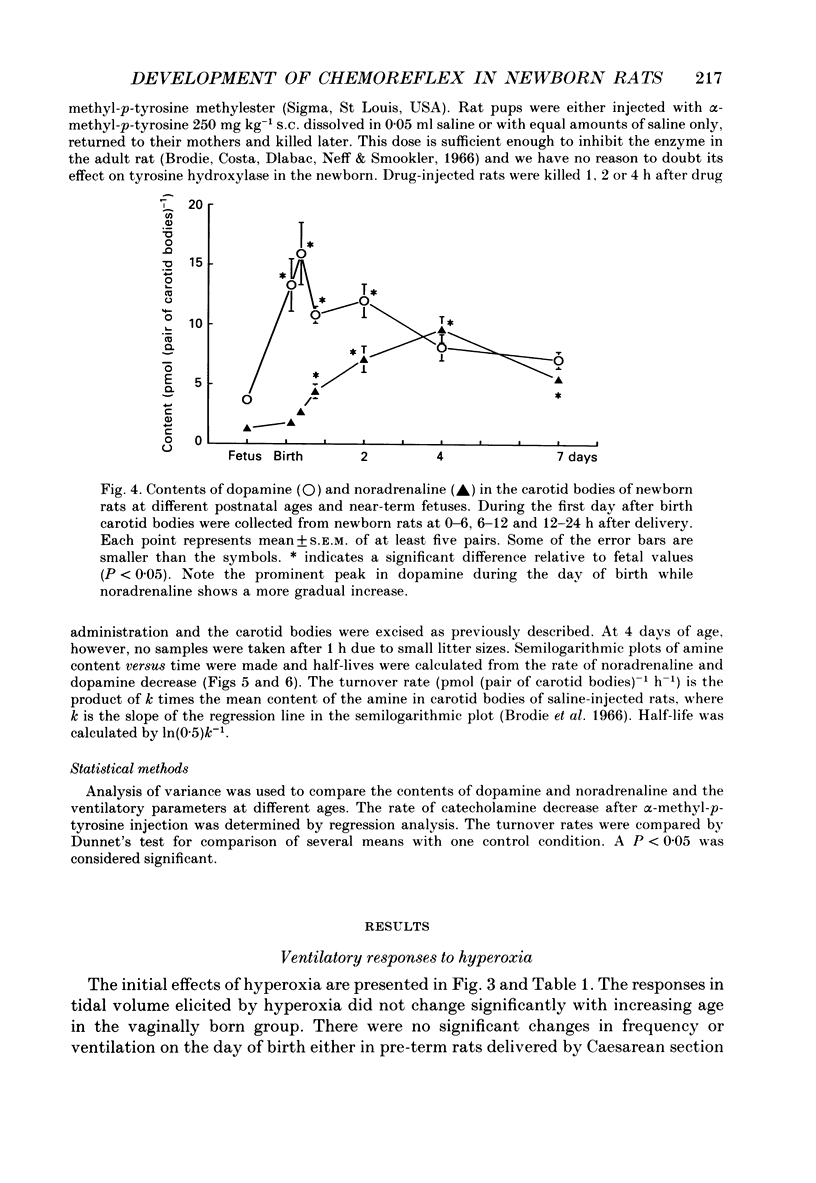





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biscoe T. J., Purves M. J. Carotid body chemoreceptor activity in the new-born lamb. J Physiol. 1967 Jun;190(3):443–454. doi: 10.1113/jphysiol.1967.sp008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgard G. E., Kressin N. A., Nielsen A. M., Daristotle L., Smith C. A., Forster H. V. Dopamine blockade alters ventilatory acclimatization to hypoxia in goats. Respir Physiol. 1987 Aug;69(2):245–255. doi: 10.1016/0034-5687(87)90031-4. [DOI] [PubMed] [Google Scholar]
- Blanco C. E., Dawes G. S., Hanson M. A., McCooke H. B. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol. 1984 Jun;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C. E., Hanson M. A., McCooke H. B. Effects on carotid chemoreceptor resetting of pulmonary ventilation in the fetal lamb in utero. J Dev Physiol. 1988 Apr;10(2):167–174. [PubMed] [Google Scholar]
- Blanco C. E., Martin C. B., Jr, Hanson M. A., McCooke H. B. Determinants of the onset of continuous air breathing at birth. Eur J Obstet Gynecol Reprod Biol. 1987 Oct;26(2):183–192. doi: 10.1016/0028-2243(87)90055-4. [DOI] [PubMed] [Google Scholar]
- Brodie B. B., Costa E., Dlabac A., Neff N. H., Smookler H. H. Application of steady state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. J Pharmacol Exp Ther. 1966 Dec;154(3):493–498. [PubMed] [Google Scholar]
- Bureau M. A., Lamarche J., Foulon P., Dalle D. Postnatal maturation of respiration in intact and carotid body-chemodenervated lambs. J Appl Physiol (1985) 1985 Sep;59(3):869–874. doi: 10.1152/jappl.1985.59.3.869. [DOI] [PubMed] [Google Scholar]
- Cardenas H., Zapata P. Dopamine-induced ventilatory depression in the rat, mediated by carotid nerve afferents. Neurosci Lett. 1981 Jun 12;24(1):29–33. doi: 10.1016/0304-3940(81)90354-2. [DOI] [PubMed] [Google Scholar]
- DEJOURS P. Chemoreflexes in breathing. Physiol Rev. 1962 Jul;42:335–358. doi: 10.1152/physrev.1962.42.3.335. [DOI] [PubMed] [Google Scholar]
- Davis J. N., Carlsson A. Effect of hypoxia on tyrosine and tryptophan hydroxylation in unanaesthetized rat brain. J Neurochem. 1973 Mar;20(3):913–915. doi: 10.1111/j.1471-4159.1973.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Docherty R. J., McQueen D. S. Inhibitory action of dopamine on cat carotid chemoreceptors. J Physiol. 1978 Jun;279:425–436. doi: 10.1113/jphysiol.1978.sp012354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman N. H., Lahiri S., Braudo L., Cherniack N. S., Fishman A. P. The blunted ventilatory response to hypoxia in cyanotic congenital heart disease. N Engl J Med. 1970 Feb 19;282(8):405–411. doi: 10.1056/NEJM197002192820801. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre C., Fidone S. J. Transduction mechanisms in carotid body: glomus cells, putative neurotransmitters, and nerve endings. Am J Physiol. 1980 Nov;239(5):C135–C152. doi: 10.1152/ajpcell.1980.239.5.C135. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre C., Zapata P. Perspectives in carotid body research. J Appl Physiol Respir Environ Exerc Physiol. 1984 Oct;57(4):931–957. doi: 10.1152/jappl.1984.57.4.931. [DOI] [PubMed] [Google Scholar]
- Fidone S., Gonzalez C., Yoshizaki K. Effects of hypoxia on catecholamine synthesis in rabbit carotid body in vitro. J Physiol. 1982 Dec;333:81–91. doi: 10.1113/jphysiol.1982.sp014440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidone S., Gonzalez C., Yoshizaki K. Effects of low oxygen on the release of dopamine from the rabbit carotid body in vitro. J Physiol. 1982 Dec;333:93–110. doi: 10.1113/jphysiol.1982.sp014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgering H., Ponte J., Sadig T. Adrenergic mechanisms and chemoreception in the carotid body of the cat and rabbit. J Physiol. 1982 Apr;325:1–21. doi: 10.1113/jphysiol.1982.sp014131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARD F., LACAISSE A., DEJOURS P. [Ventilatory O2 stimulus in the neonatal period in man]. J Physiol (Paris) 1960 Jan-Feb;52:108–109. [PubMed] [Google Scholar]
- Gluckman P. D., Gunn T. R., Johnston B. M. The effect of cooling on breathing and shivering in unanaesthetized fetal lambs in utero. J Physiol. 1983 Oct;343:495–506. doi: 10.1113/jphysiol.1983.sp014905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanbauer I., Hellstrom S. The regulation of dopamine and noradrenaline in the rat carotid body and its modification by denervation and by hypoxia. J Physiol. 1978 Sep;282:21–34. doi: 10.1113/jphysiol.1978.sp012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanbauer I., Karoum F., Hellstrom S., Lahiri S. Effects of hypoxia lasting up to one month on the catecholamine content in rat carotid body. Neuroscience. 1981;6(1):81–86. doi: 10.1016/0306-4522(81)90245-1. [DOI] [PubMed] [Google Scholar]
- Hertzberg T., Lagercrantz H. Postnatal sensitivity of the peripheral chemoreceptors in newborn infants. Arch Dis Child. 1987 Dec;62(12):1238–1241. doi: 10.1136/adc.62.12.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A. H., Ioffe S., Russell B. J., Chernick V. Effect of carotid chemoreceptor denervation on breathing in utero and after birth. J Appl Physiol Respir Environ Exerc Physiol. 1981 Sep;51(3):630–633. doi: 10.1152/jappl.1981.51.3.630. [DOI] [PubMed] [Google Scholar]
- Kressin N. A., Nielsen A. M., Laravuso R., Bisgard G. E. Domperidone-induced potentiation of ventilatory responses in awake goats. Respir Physiol. 1986 Aug;65(2):169–180. doi: 10.1016/0034-5687(86)90048-4. [DOI] [PubMed] [Google Scholar]
- Lahiri S., Brody J. S., Motoyama E. K., Velasquez T. M. Regulation of breathing in newborns at high altitude. J Appl Physiol Respir Environ Exerc Physiol. 1978 May;44(5):673–678. doi: 10.1152/jappl.1978.44.5.673. [DOI] [PubMed] [Google Scholar]
- Llados F., Zapata P. Effects of dopamine analogues and antagonists on carotid body chemosensors in situ. J Physiol. 1978 Jan;274:487–499. doi: 10.1113/jphysiol.1978.sp012162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER H. C., BEHRLE F. C. The effects of hypoxia on the respiration of newborn infants. Pediatrics. 1954 Aug;14(2):93–103. [PubMed] [Google Scholar]
- Mayock D. E., Standaert T. A., Guthrie R. D., Woodrum D. E. Dopamine and carotid body function in the newborn lamb. J Appl Physiol Respir Environ Exerc Physiol. 1983 Mar;54(3):814–820. doi: 10.1152/jappl.1983.54.3.814. [DOI] [PubMed] [Google Scholar]
- McCooke H. B., Hanson M. A. Respiration of conscious kittens in acute hypoxia and effect of almitrine bismesylate. J Appl Physiol (1985) 1985 Jul;59(1):18–23. doi: 10.1152/jappl.1985.59.1.18. [DOI] [PubMed] [Google Scholar]
- Mir A. K., Al-Neamy K., Pallot D. J., Nahorski S. R. Catecholamines in the carotid body of several mammalian species: effects of surgical and chemical sympathectomy. Brain Res. 1982 Dec 9;252(2):335–342. doi: 10.1016/0006-8993(82)90401-2. [DOI] [PubMed] [Google Scholar]
- Modesto J. P., da Silva K. M. Drift-free electronic integrator for respiration studies. Med Biol Eng Comput. 1982 Jul;20(4):457–460. doi: 10.1007/BF02442406. [DOI] [PubMed] [Google Scholar]
- Mortola J. P. Breathing pattern in newborns. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jun;56(6):1533–1540. doi: 10.1152/jappl.1984.56.6.1533. [DOI] [PubMed] [Google Scholar]
- Pallot D. J. The mammalian carotid body. Adv Anat Embryol Cell Biol. 1987;102:1–91. doi: 10.1007/978-3-642-71857-1. [DOI] [PubMed] [Google Scholar]
- Pequignot J. M., Cottet-Emard J. M., Dalmaz Y., De Haut de Sigy M., Peyrin L. Biochemical evidence for norepinephrine stores outside the sympathetic nerves in rat carotid body. Brain Res. 1986 Mar 5;367(1-2):238–243. doi: 10.1016/0006-8993(86)91597-0. [DOI] [PubMed] [Google Scholar]
- Pequignot J. M., Cottet-Emard J. M., Dalmaz Y., Peyrin L. Dopamine and norepinephrine dynamics in rat carotid body during long-term hypoxia. J Auton Nerv Syst. 1987 Nov;21(1):9–14. doi: 10.1016/0165-1838(87)90087-7. [DOI] [PubMed] [Google Scholar]
- Prabhakar N. R., Runold M., Yamamoto Y., Lagercrantz H., von Euler C. Effect of substance P antagonist on the hypoxia-induced carotid chemoreceptor activity. Acta Physiol Scand. 1984 Jul;121(3):301–303. doi: 10.1111/j.1748-1716.1984.tb07460.x. [DOI] [PubMed] [Google Scholar]
- Purves M. J. Chemoreceptors and their reflexes with special reference to the fetus and newborn. J Dev Physiol. 1981 Feb;3(1):21–57. [PubMed] [Google Scholar]
- Purves M. J. Onset of respiration at birth. Arch Dis Child. 1974 May;49(5):333–343. doi: 10.1136/adc.49.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigual R., Gonzalez E., Gonzalez C., Fidone S. Synthesis and release of catecholamines by the cat carotid body in vitro: effects of hypoxic stimulation. Brain Res. 1986 May 21;374(1):101–109. doi: 10.1016/0006-8993(86)90398-7. [DOI] [PubMed] [Google Scholar]
- Smolen A. J., Beaston-Wimmer P., Wright L. L., Lindley T., Cader C. Neurotransmitter synthesis, storage, and turnover in neonatally deafferented sympathetic neurons. Brain Res. 1985 Dec;355(2):211–218. doi: 10.1016/0165-3806(85)90043-4. [DOI] [PubMed] [Google Scholar]
- Sorensen S. C., Severinghaus J. W. Irreversible respiratory insensitivity to acute hypoxia in man born at high altitude. J Appl Physiol. 1968 Sep;25(3):217–220. doi: 10.1152/jappl.1968.25.3.217. [DOI] [PubMed] [Google Scholar]
- Zapata P. Effects of dopamine on carotid chemo- and baroreceptors in vitro. J Physiol. 1975 Jan;244(1):235–251. doi: 10.1113/jphysiol.1975.sp010794. [DOI] [PMC free article] [PubMed] [Google Scholar]


