Abstract
1. Calcium currents and intramembrane charge movements were measured in cut twitch muscle fibres of the frog and the time course of activation of the current was studied using various conditioning pulse protocols. 2. When a conditioning activation was produced by a depolarizing pulse which ended before inactivation occurred, a subsequent depolarization led to a faster onset of activation, indicating that the system had not completely returned to the initial state during the interval between the two pulses. 3. The interval between conditioning and test pulse was varied at different subthreshold potentials to study the time course of restoring the steady-state conditions. Complete restoration required a waiting period of about 1 min at the holding potential of -80 mV due to a very slow process but partial recovery was reached within 100 ms. This initial recovery process was strongly voltage dependent and became considerably slower when the interval potential approached the threshold for current activation. 4. Stepping to a roughly 10 mV subthreshold potential without applying a conditioning activation caused no change in the time course of the current produced by a subsequent test depolarization. Depolarizing just to the current threshold caused a slowly progressing acceleration of test current activation. 5. The peak current-voltage relation in the fast gating regime caused by a conditioning activation coincided with the current-voltage relation measured under steady-state conditions, indicating not that a new channel population had become activated but that the same channels showed a different gating behaviour. 6. Intramembrane charge movements measured in 2 mM-Cd2+ and tested at potentials between -40 and +40 mV showed negligible changes when preceded by a strong depolarization. 7. We discuss several possible models which can explain the fact that the current is speeded up by a conditioning activation while the charge movements remain unchanged. It is possible that the fast voltage-dependent transition which becomes visible after conditioning pulses reflects a rapid conformational change of the Ca2+ channel molecule which also occurs during its normal gating mode but remains undetectable in terms of conductance. In view of the hypothesis that the Ca2+ channel molecule forms a voltage sensor for excitation-contraction coupling this fast transition could be coupled to the control of Ca2+ release from the sarcoplasmic reticulum.
Full text
PDF



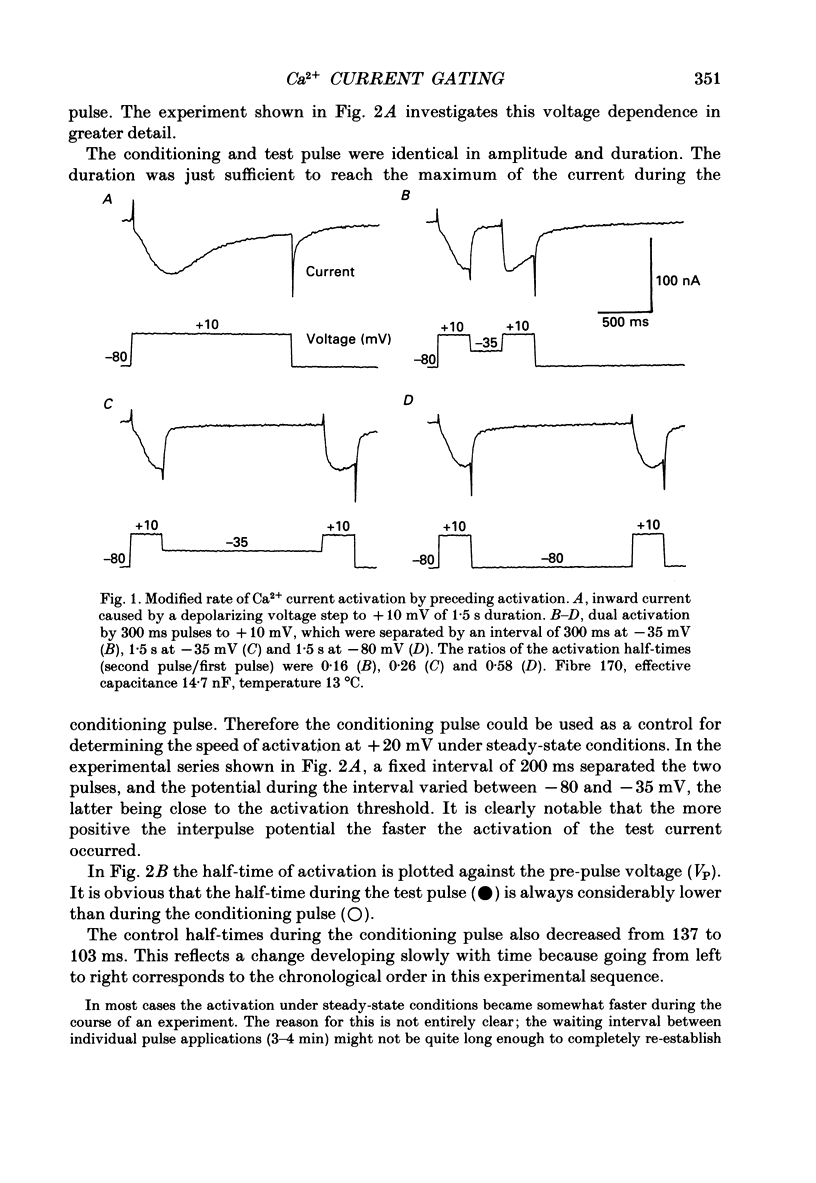
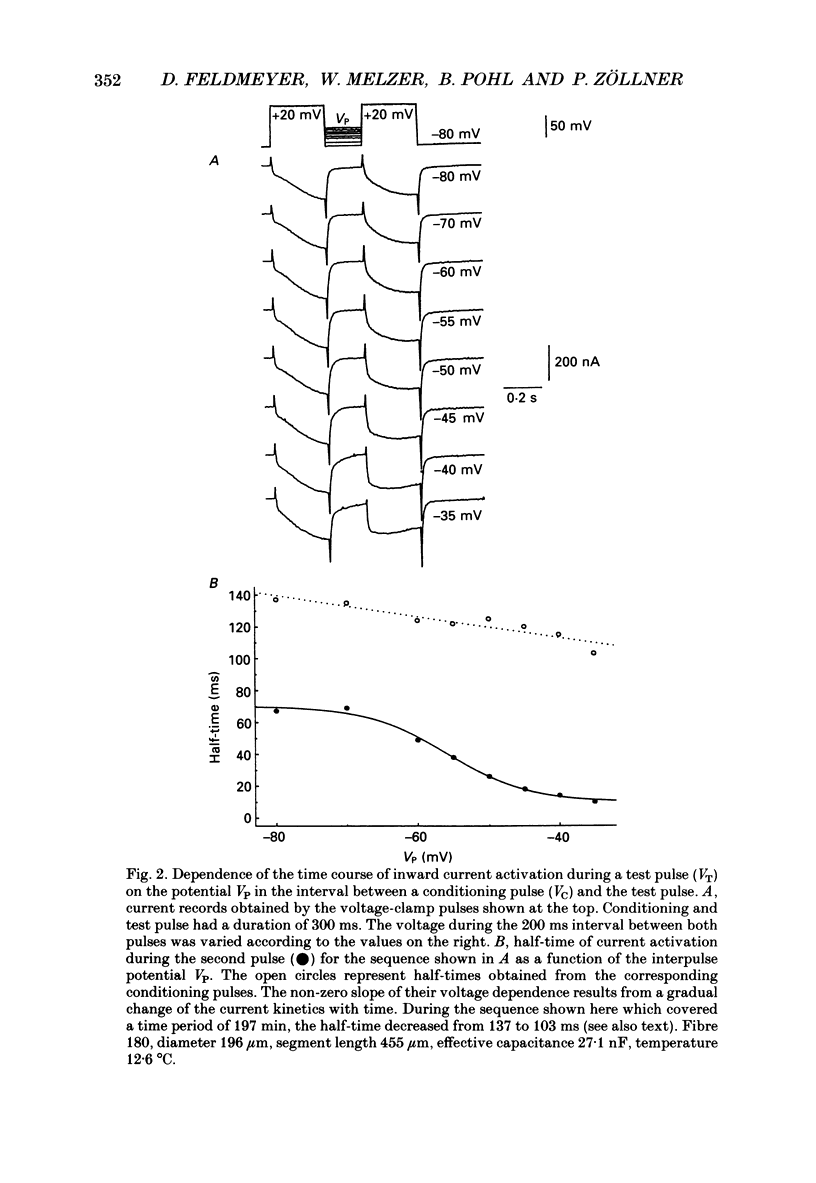
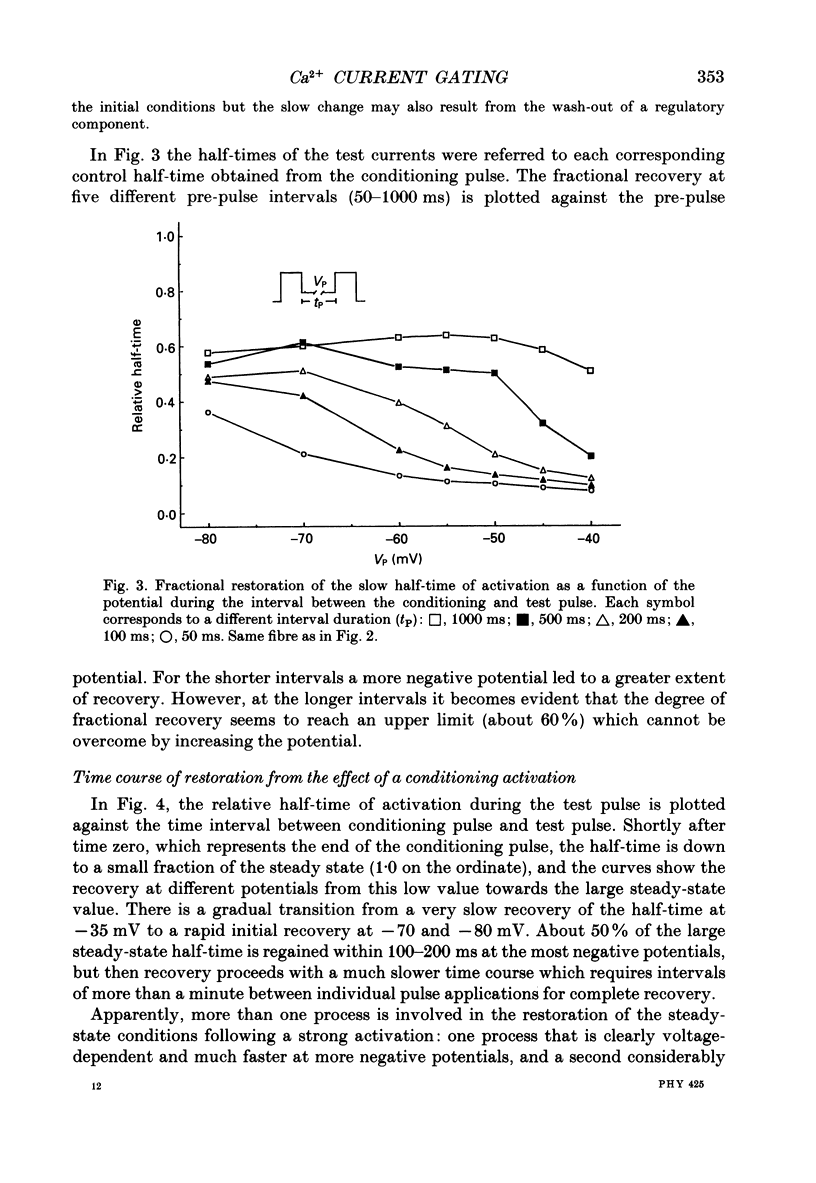
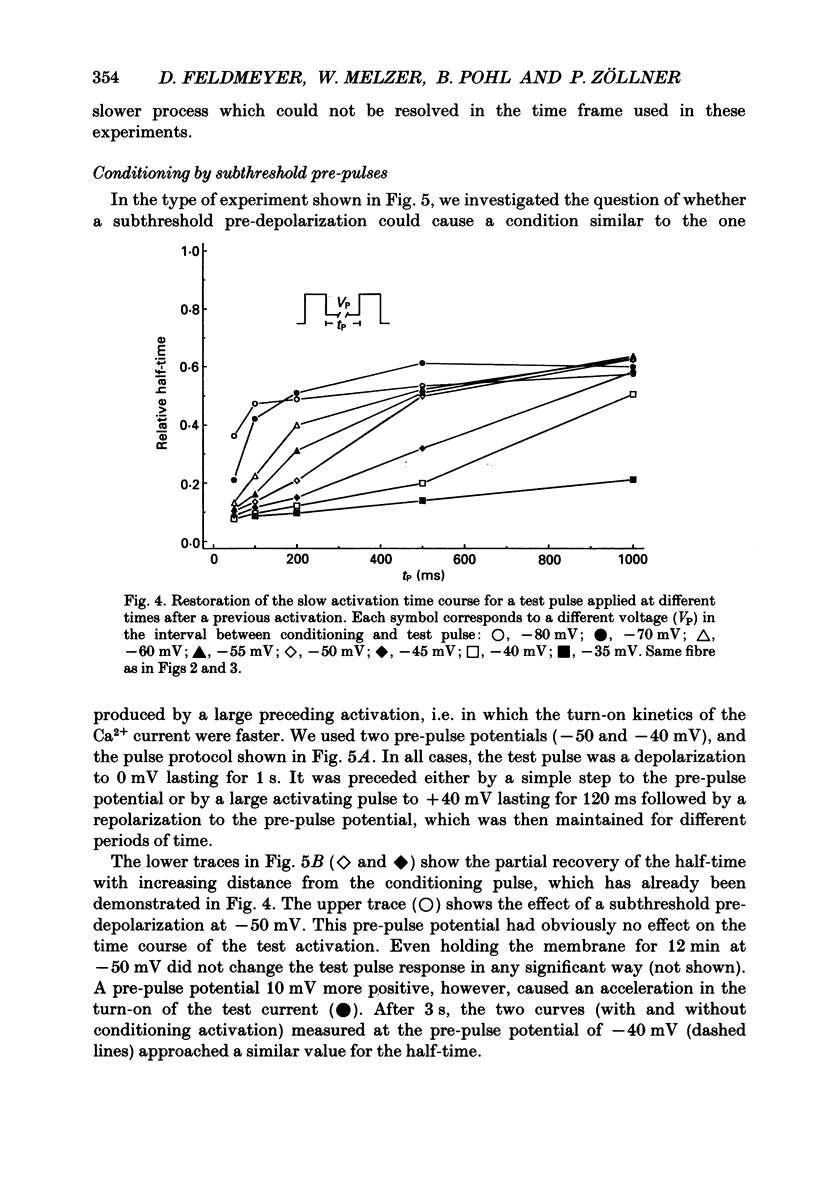








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. Charge movement in the membrane of striated muscle. J Physiol. 1976 Jan;254(2):339–360. doi: 10.1113/jphysiol.1976.sp011235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Fink R., Palade P. T. Calcium depletion in frog muscle tubules: the decline of calcium current under maintained depolarization. J Physiol. 1981 Mar;312:177–207. doi: 10.1113/jphysiol.1981.sp013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreola J., Calvo J., García M. C., Sánchez J. A. Modulation of calcium channels of twitch skeletal muscle fibres of the frog by adrenaline and cyclic adenosine monophosphate. J Physiol. 1987 Dec;393:307–330. doi: 10.1113/jphysiol.1987.sp016825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983 Nov;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. More than a Ca2+ channel? Trends Neurosci. 1989 Apr;12(4):128–130. doi: 10.1016/0166-2236(89)90050-7. [DOI] [PubMed] [Google Scholar]
- Berwe D., Gottschalk G., Lüttgau H. C. Effects of the calcium antagonist gallopamil (D600) upon excitation-contraction coupling in toe muscle fibres of the frog. J Physiol. 1987 Apr;385:693–707. doi: 10.1113/jphysiol.1987.sp016515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Fitts R., Pizarro G., Ríos E. Voltage sensors of the frog skeletal muscle membrane require calcium to function in excitation-contraction coupling. J Physiol. 1988 Apr;398:475–505. doi: 10.1113/jphysiol.1988.sp017053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Rios E. Intramembrane charge movement in frog skeletal muscle fibres. Properties of charge 2. J Physiol. 1987 Jun;387:489–517. doi: 10.1113/jphysiol.1987.sp016586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Ríos E., Stéfani E. Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres. J Physiol. 1988 Apr;398:441–473. doi: 10.1113/jphysiol.1988.sp017052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. P., Leung A. T., Sharp A. H. The biochemistry and molecular biology of the dihydropyridine-sensitive calcium channel. Trends Neurosci. 1988 Oct;11(10):425–430. doi: 10.1016/0166-2236(88)90193-2. [DOI] [PubMed] [Google Scholar]
- Cota G., Stefani E. A fast-activated inward calcium current in twitch muscle fibres of the frog (Rana montezume). J Physiol. 1986 Jan;370:151–163. doi: 10.1113/jphysiol.1986.sp015927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., McCarthy R. T., Milton R. L. Paralysis of frog skeletal muscle fibres by the calcium antagonist D-600. J Physiol. 1983 Aug;341:495–505. doi: 10.1113/jphysiol.1983.sp014819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann R., Lüttgau H. C. The effect of the phenylalkylamine D888 (devapamil) on force and Ca2+ current in isolated frog skeletal muscle fibres. J Physiol. 1989 Jun;413:521–541. doi: 10.1113/jphysiol.1989.sp017667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D., Melzer W., Pohl B. Effects of gallopamil on calcium release and intramembrane charge movements in frog skeletal muscle fibres. J Physiol. 1990 Feb;421:343–362. doi: 10.1113/jphysiol.1990.sp017948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García J., Stefani E. Appropriate conditions to record activation of fast Ca2+ channels in frog skeletal muscle (Rana pipiens). Pflugers Arch. 1987 May;408(6):646–648. doi: 10.1007/BF00581169. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hencek M., Zacharová D., Zachar J. Fast calcium currents in cut skeletal muscle fibres of the frogs Rana temporaria and Xenopus laevis. Gen Physiol Biophys. 1988 Dec;7(6):651–656. [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Intramembrane charge movements in skeletal muscle. Physiol Rev. 1988 Oct;68(4):1197–1147. doi: 10.1152/physrev.1988.68.4.1197. [DOI] [PubMed] [Google Scholar]
- Hui C. S., Milton R. L., Eisenberg R. S. Charge movement in skeletal muscle fibers paralyzed by the calcium-entry blocker D600. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2582–2585. doi: 10.1073/pnas.81.8.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs L., Rios E., Schneider M. F. Measurement and modification of free calcium transients in frog skeletal muscle fibres by a metallochromic indicator dye. J Physiol. 1983 Oct;343:161–196. doi: 10.1113/jphysiol.1983.sp014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D. Components of charge movement in rabbit skeletal muscle: the effect of tetracaine and nifedipine. J Physiol. 1986 Jul;376:85–100. doi: 10.1113/jphysiol.1986.sp016143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Walsh T. Calcium currents, charge movement and dihydropyridine binding in fast- and slow-twitch muscles of rat and rabbit. J Physiol. 1987 Dec;393:595–617. doi: 10.1113/jphysiol.1987.sp016843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Spiecker W. The effects of calcium deprivation upon mechanical and electrophysiological parameters in skeletal muscle fibres of the frog. J Physiol. 1979 Nov;296:411–429. doi: 10.1113/jphysiol.1979.sp013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Cavalié A., Trautwein W., Pelzer D. Voltage-dependent properties of macroscopic and elementary calcium channel currents in guinea pig ventricular myocytes. Pflugers Arch. 1986 May;406(5):437–448. doi: 10.1007/BF00583365. [DOI] [PubMed] [Google Scholar]
- Melzer W., Schneider M. F., Simon B. J., Szucs G. Intramembrane charge movement and calcium release in frog skeletal muscle. J Physiol. 1986 Apr;373:481–511. doi: 10.1113/jphysiol.1986.sp016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Sanchez J. A., Stefani E. Inward calcium current in twitch muscle fibres of the frog. J Physiol. 1978 Oct;283:197–209. doi: 10.1113/jphysiol.1978.sp012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Simon B. J. Inactivation of calcium release from the sarcoplasmic reticulum in frog skeletal muscle. J Physiol. 1988 Nov;405:727–745. doi: 10.1113/jphysiol.1988.sp017358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. M., McCleskey E. W., Almers W. Dihydropyridine receptors in muscle are voltage-dependent but most are not functional calcium channels. 1985 Apr 25-May 1Nature. 314(6013):747–751. doi: 10.1038/314747a0. [DOI] [PubMed] [Google Scholar]
- Simon B. J., Schneider M. F. Time course of activation of calcium release from sarcoplasmic reticulum in skeletal muscle. Biophys J. 1988 Dec;54(6):1159–1163. doi: 10.1016/S0006-3495(88)83050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez J. A., Stefani E. Kinetic properties of calcium channels of twitch muscle fibres of the frog. J Physiol. 1983 Apr;337:1–17. doi: 10.1113/jphysiol.1983.sp014607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- White M. M., Bezanilla F. Activation of squid axon K+ channels. Ionic and gating current studies. J Gen Physiol. 1985 Apr;85(4):539–554. doi: 10.1085/jgp.85.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]


