Abstract
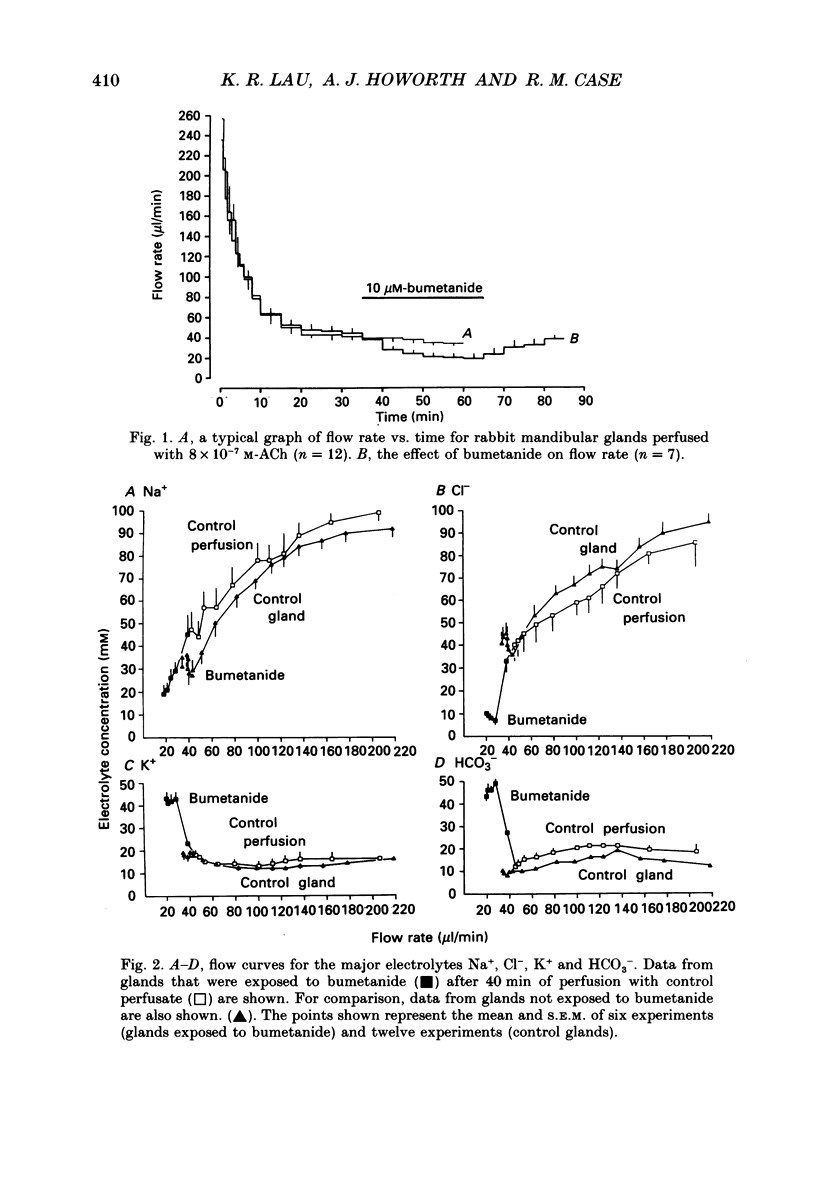
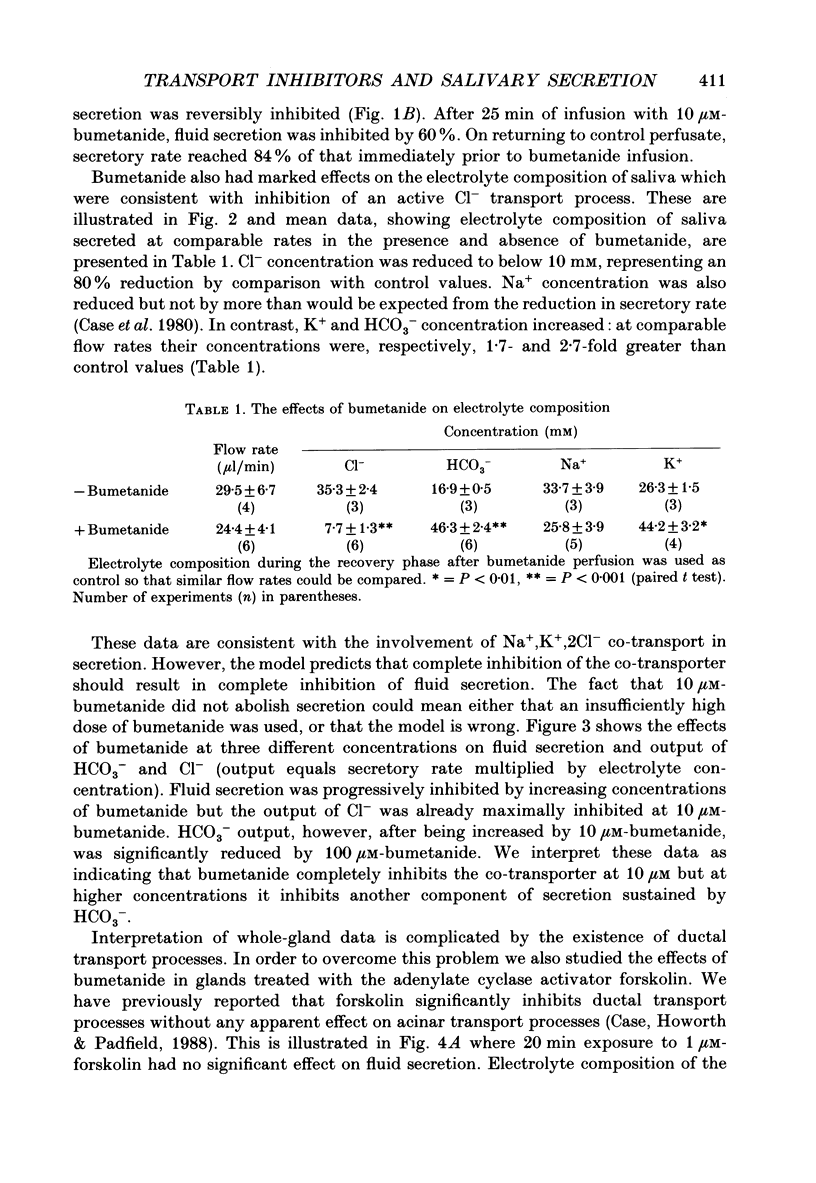
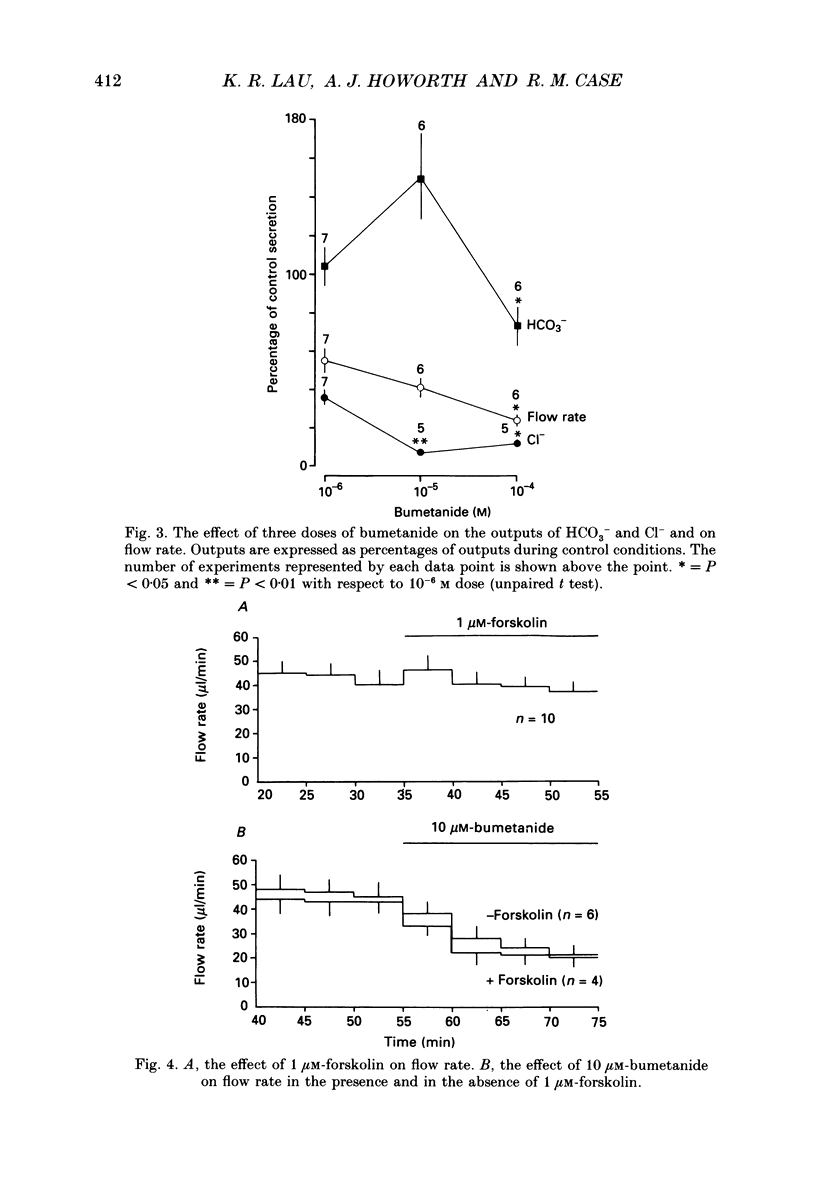
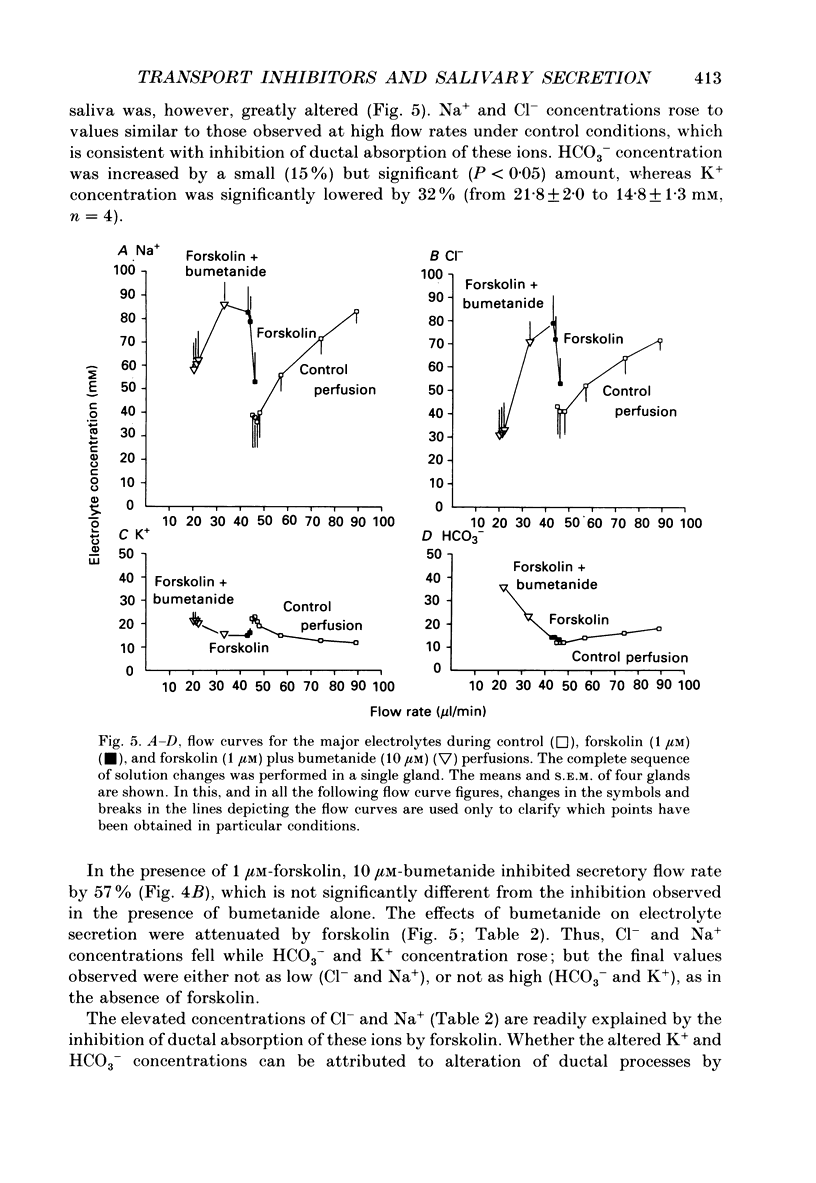
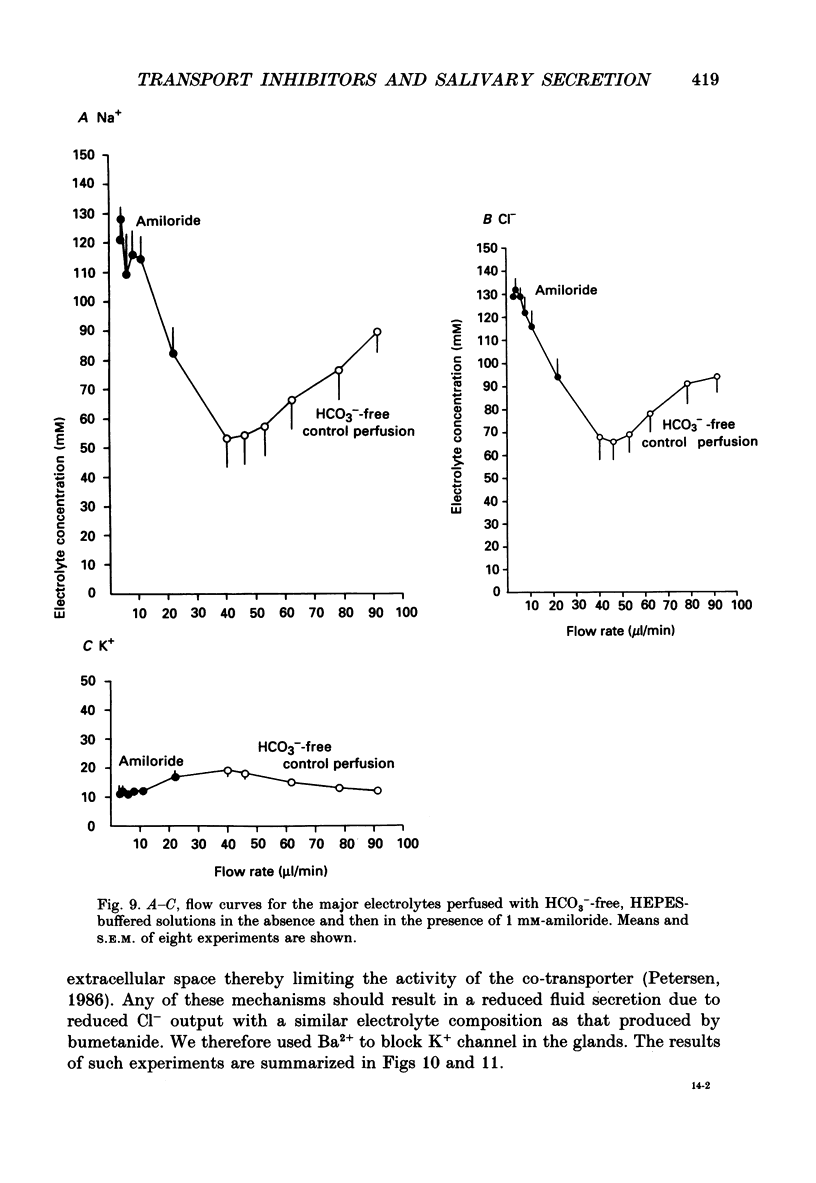
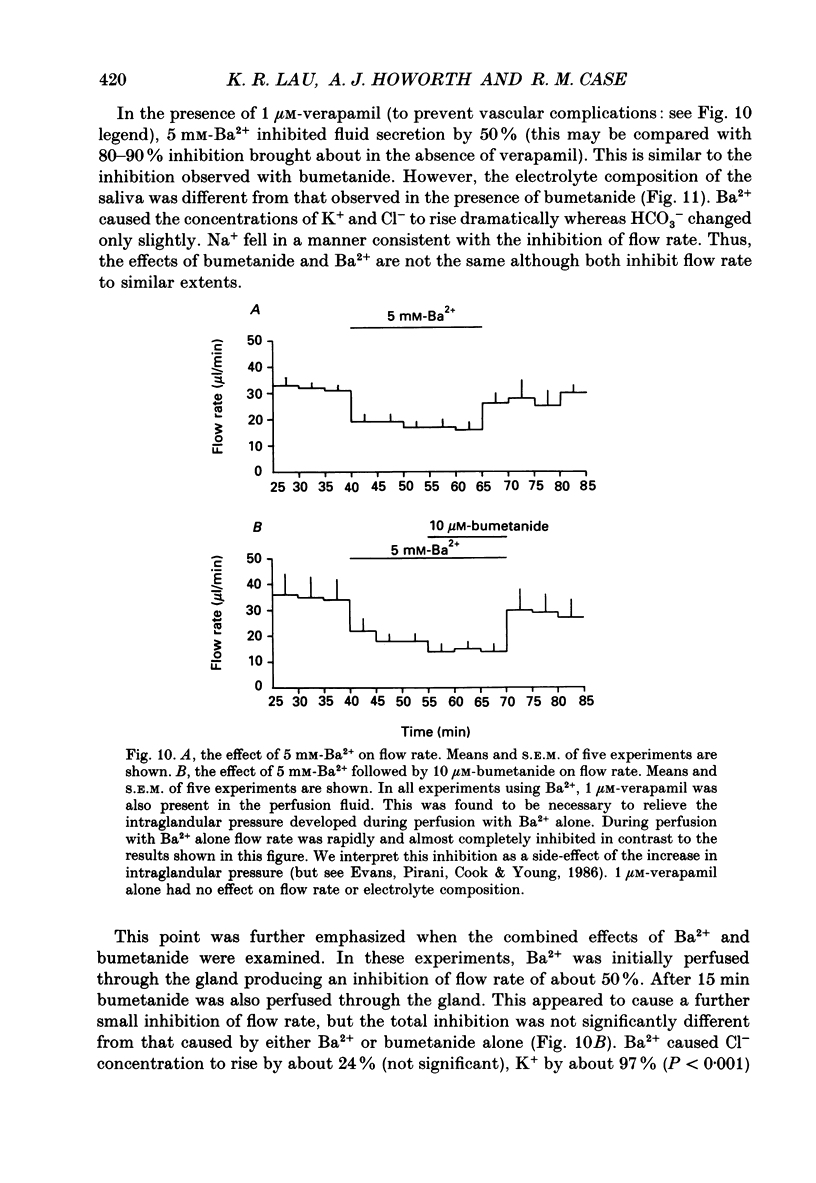
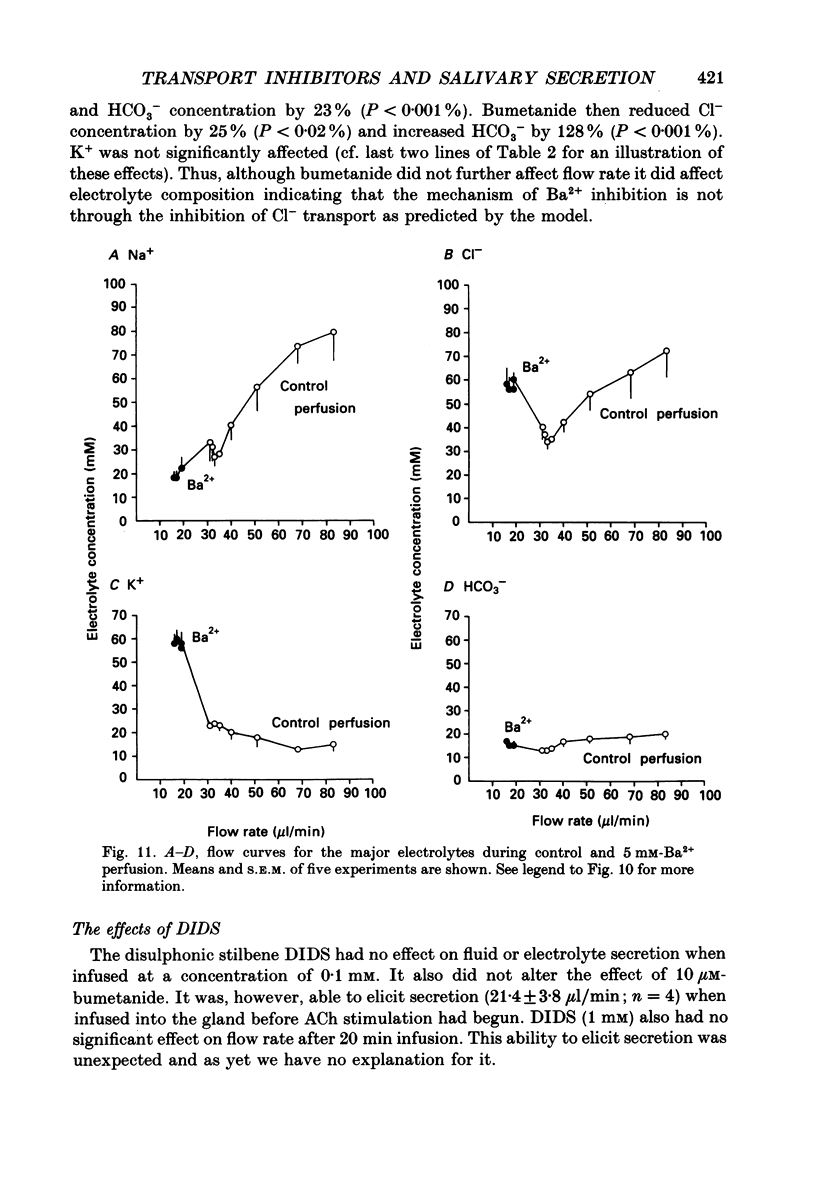
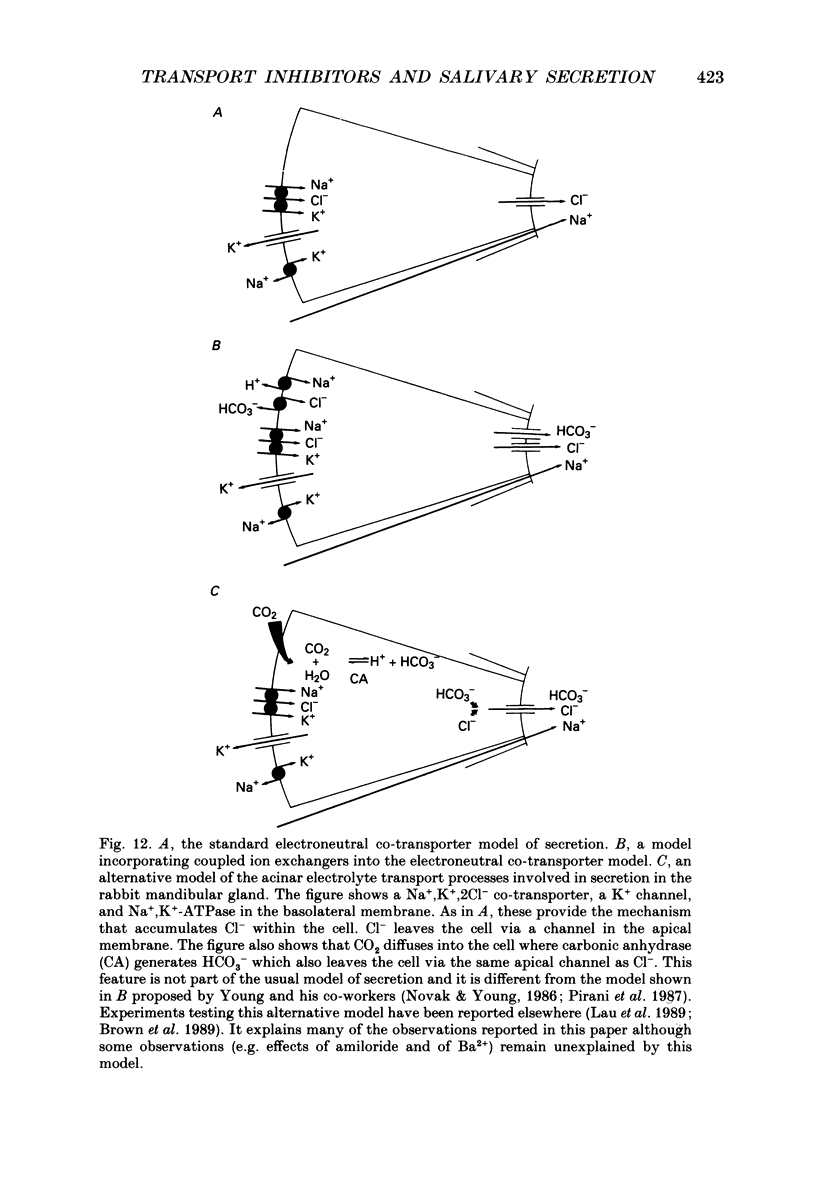
1. In order to distinguish between models of anion secretion, the effects of transport inhibitors on saliva flow rate and electrolyte composition were studied during the plateau phase of secretion in rabbit mandibular salivary glands. 2. Bumetanide, an inhibitor of Na+,K+,2Cl- co-transport, inhibited flow rate (by 60%) and reduced Cl- concentration. K+ and HCO3- concentrations were increased. Forskolin, an adenylate cyclase activator which inhibits ductal transport, did not significantly affect this pattern of changes. 3. Amiloride, used at concentrations that would inhibit Na(+)-H+ exchange, inhibited flow rate (by 30%). Cl- concentration was initially increased before subsequently decreasing at the same time as HCO3- concentration increased. These concentration changes can probably be attributed to ductal transport. When amiloride was applied to glands perfused with nominally HCO3- -free solutions, inhibition of flow rate was rapid and almost complete. 4. When amiloride and bumetanide were both present in the perfusate, flow rate was inhibited by 92%. The pattern of electrolyte changes was not significantly different from that observed in the presence of bumetanide alone. 5. Inhibition of K+ channel activity using Ba2+ also inhibited flow rate. Cl- concentration was increased as was K+ concentration. HCO3- concentration was not increased. 6. The anion exchange inhibitor DIDS (4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid) had no effect on either flow rate or electrolyte concentration. It did, however, elicit secretion in the absence of acetylcholine. 7. The data suggest that Na(+)-H+ and Cl- -HCO3- exchangers are unlikely to be involved in fluid and electrolyte secretion in these glands as suggested by some authors. Most of the data can be explained by postulating the existence of non-specific anion channels in the apical membranes of the acinar cells.
Full text
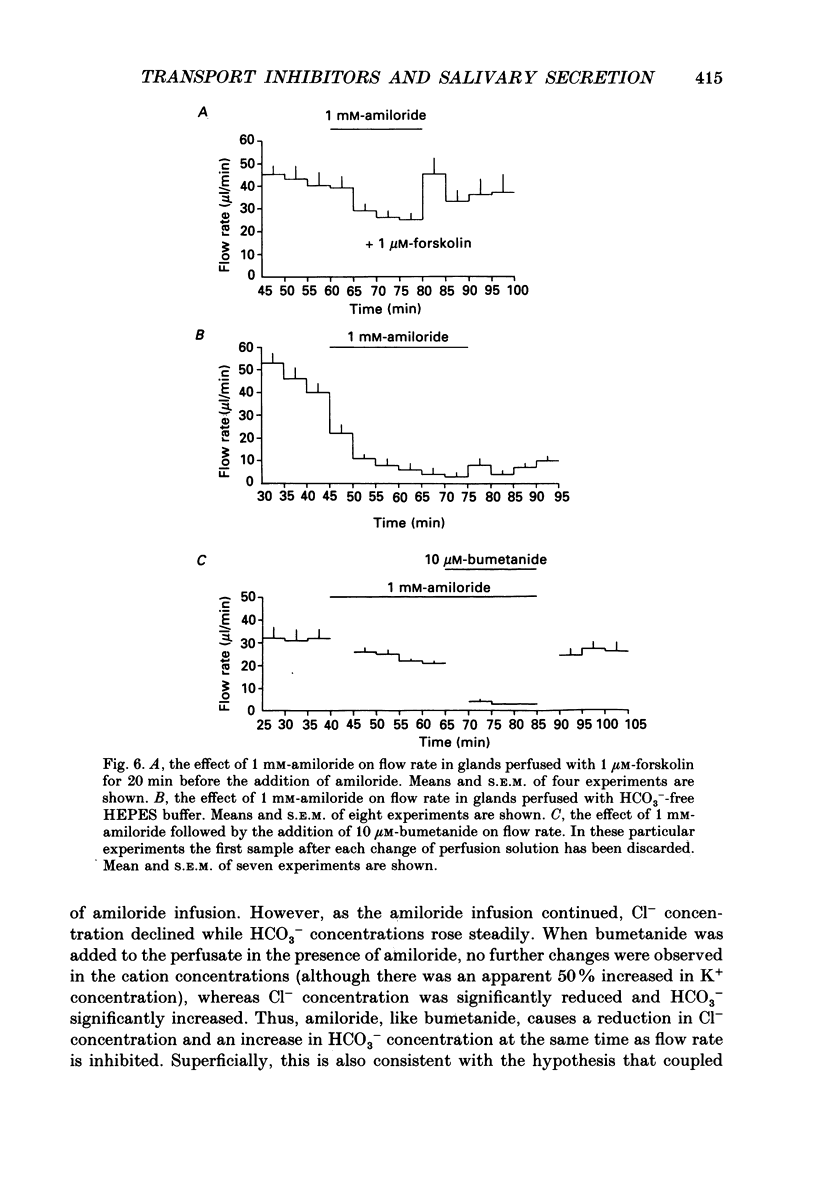
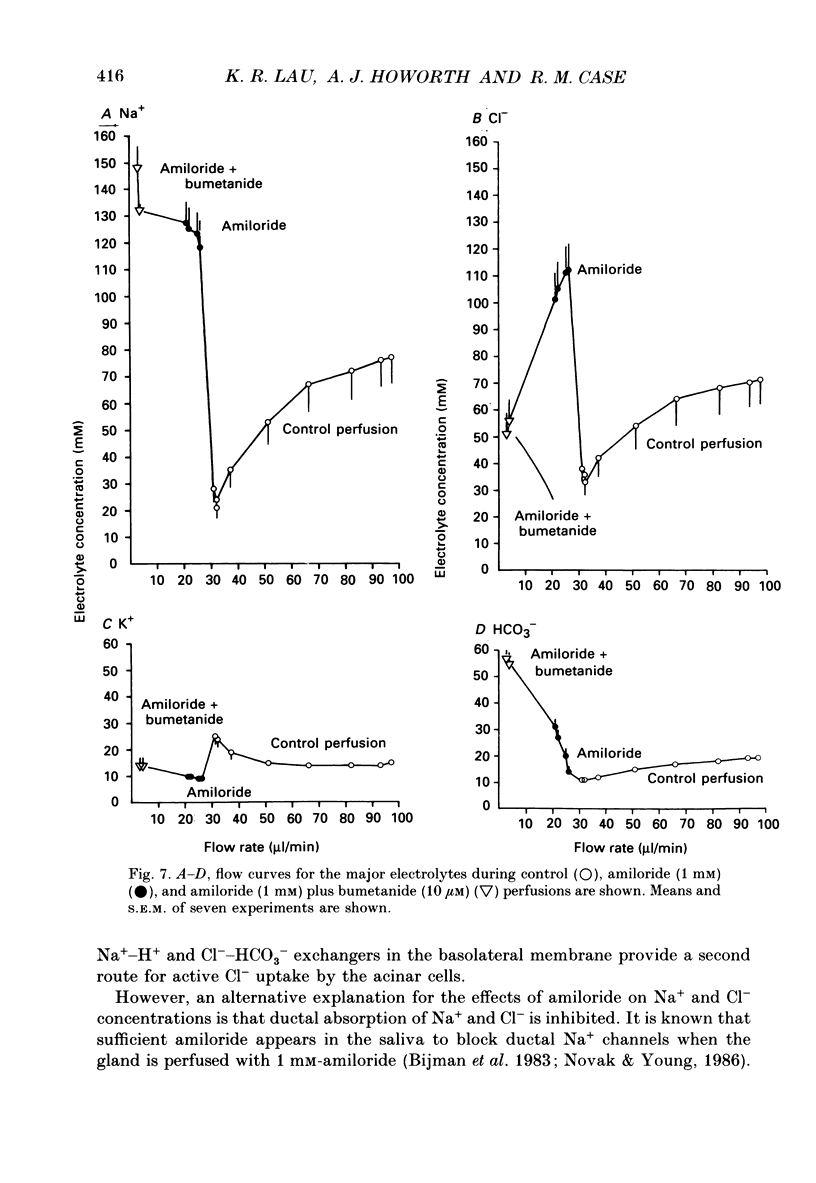
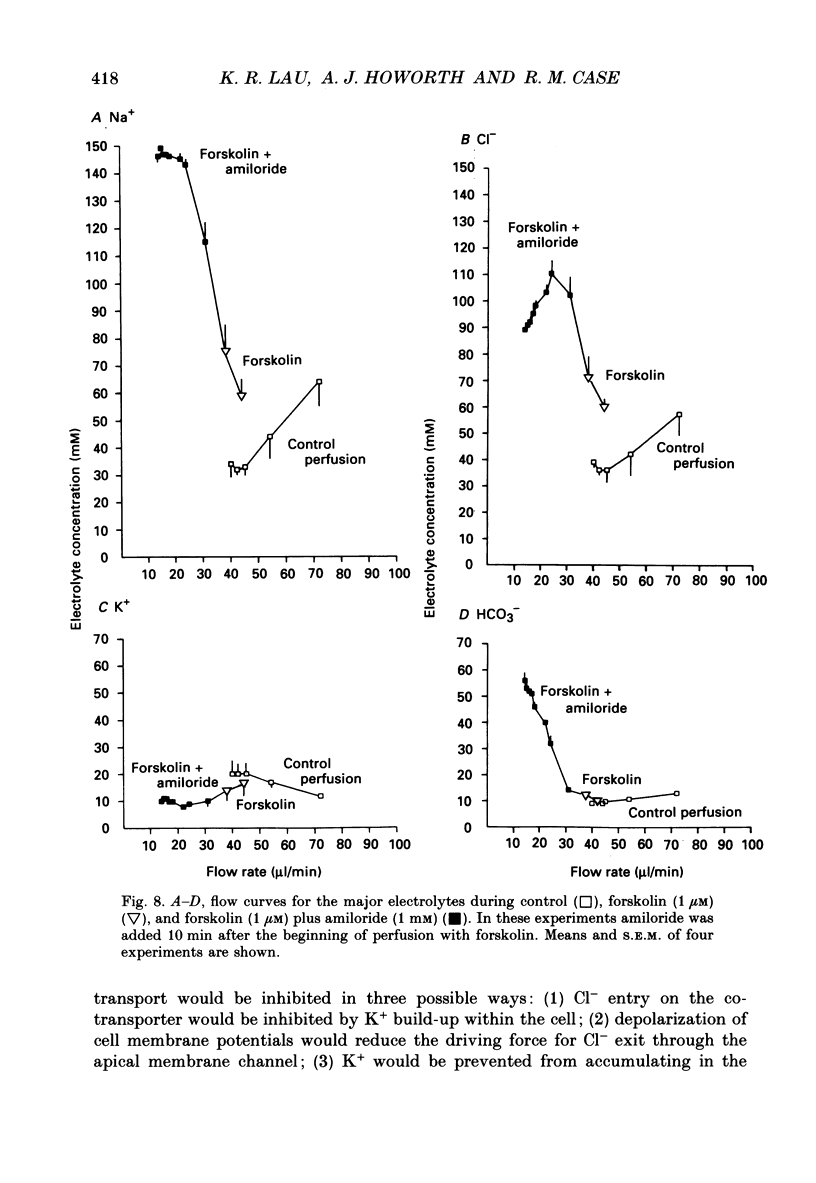
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGEN A. S. The secretion of potassium in saliva. J Physiol. 1956 Apr 27;132(1):20–39. doi: 10.1113/jphysiol.1956.sp005500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijman J., Cook D. I., van Os C. H. Effect of amiloride on electrolyte transport parameters of the main duct of the rabbit mandibular salivary gland. Pflugers Arch. 1983 Jul;398(2):96–102. doi: 10.1007/BF00581055. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Elliott A. C., Lau K. R. Indirect evidence for the presence of non-specific anion channels in rabbit mandibular salivary gland acinar cells. J Physiol. 1989 Jul;414:415–431. doi: 10.1113/jphysiol.1989.sp017696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. D., Loo D. D., Wright E. M. Ca2+-activated K+ channels in the apical membrane of Necturus choroid plexus. J Membr Biol. 1988 Nov;105(3):207–219. doi: 10.1007/BF01870998. [DOI] [PubMed] [Google Scholar]
- Case R. M., Conigrave A. D., Favaloro E. J., Novak I., Thompson C. H., Young J. A. The role of buffer anions and protons in secretion by the rabbit mandibular salivary gland. J Physiol. 1982 Jan;322:273–286. doi: 10.1113/jphysiol.1982.sp014037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Conigrave A. D., Novak I., Young J. A. Electrolyte and protein secretion by the perfused rabbit mandibular gland stimulated with acetylcholine or catecholamines. J Physiol. 1980 Mar;300:467–487. doi: 10.1113/jphysiol.1980.sp013173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Howorth A. J., Padfield P. J. Effects of acetylcholine, isoprenaline and forskolin on electrolyte and protein composition of rabbit mandibular saliva. J Physiol. 1988 Dec;406:411–430. doi: 10.1113/jphysiol.1988.sp017388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Hunter M., Novak I., Young J. A. The anionic basis of fluid secretion by the rabbit mandibular salivary gland. J Physiol. 1984 Apr;349:619–630. doi: 10.1113/jphysiol.1984.sp015177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. A., Pirani D., Cook D. I., Young J. A. Intraepithelial current flow in rat pancreatic secretory epithelia. Pflugers Arch. 1986;407 (Suppl 2):S107–S111. doi: 10.1007/BF00584938. [DOI] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A., Tan Y. P., Trautmann A. Blockage of Ca-activated Cl conductance by furosemide in rat lacrimal glands. Pflugers Arch. 1986 Jan;406(1):65–68. doi: 10.1007/BF00582955. [DOI] [PubMed] [Google Scholar]
- Exley P. M., Fuller C. M., Gallacher D. V. Potassium uptake in the mouse submandibular gland is dependent on chloride and sodium and abolished by piretanide. J Physiol. 1986 Sep;378:97–108. doi: 10.1113/jphysiol.1986.sp016209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers G. A., De Pont J. J., Van Nooy I. G., Fleuren-Jakobs A. M., Bonting S. L., Rodrigues de Miranda J. F. Amiloride is a cholinergic antagonist in the rabbit pancreas. Biochim Biophys Acta. 1984 Jun 19;804(2):237–244. doi: 10.1016/0167-4889(84)90155-1. [DOI] [PubMed] [Google Scholar]
- Lau K. R., Case R. M. Evidence for apical chloride channels in rabbit mandibular salivary glands. A chloride-selective microelectrode study. Pflugers Arch. 1988 Jun;411(6):670–675. doi: 10.1007/BF00580864. [DOI] [PubMed] [Google Scholar]
- Lau K. R., Elliott A. C., Brown P. D. Acetylcholine-induced intracellular acidosis in rabbit salivary gland acinar cells. Am J Physiol. 1989 Feb;256(2 Pt 1):C288–C295. doi: 10.1152/ajpcell.1989.256.2.C288. [DOI] [PubMed] [Google Scholar]
- Manganel M., Turner R. J. Coupled Na+/H+ exchange in rat parotid basolateral membrane vesicles. J Membr Biol. 1988 Jun;102(3):247–254. doi: 10.1007/BF01925718. [DOI] [PubMed] [Google Scholar]
- Martinez J. R., Cassity N. Effects of 4,4'-diisothiocyano-2,2'-stilbene disulphonic acid and amiloride on salivary secretion by isolated, perfused rat submandibular glands. Arch Oral Biol. 1985;30(11-12):797–803. doi: 10.1016/0003-9969(85)90134-7. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak I., Young J. A. Two independent anion transport systems in rabbit mandibular salivary glands. Pflugers Arch. 1986 Dec;407(6):649–656. doi: 10.1007/BF00582647. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Saito Y., Nishiyama A. Evidence for an anion exchanger in the mouse lacrimal gland acinar cell membrane. J Membr Biol. 1988 Nov;105(3):273–280. doi: 10.1007/BF01871004. [DOI] [PubMed] [Google Scholar]
- Patarca R., Candia O. A., Reinach P. S. Mode of inhibition of active chloride transport in the frog cornea by furosemide. Am J Physiol. 1983 Dec;245(6):F660–F669. doi: 10.1152/ajprenal.1983.245.6.F660. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Calcium-activated potassium channels and fluid secretion by exocrine glands. Am J Physiol. 1986 Jul;251(1 Pt 1):G1–13. doi: 10.1152/ajpgi.1986.251.1.G1. [DOI] [PubMed] [Google Scholar]
- Pirani D., Evans L. A., Cook D. I., Young J. A. Intracellular pH in the rat mandibular salivary gland: the role of Na-H and Cl-HCO3 antiports in secretion. Pflugers Arch. 1987 Feb;408(2):178–184. doi: 10.1007/BF00581349. [DOI] [PubMed] [Google Scholar]
- Reeves W. B., McDonald G. A., Mehta P., Andreoli T. E. Activation of K+ channels in renal medullary vesicles by cAMP-dependent protein kinase. J Membr Biol. 1989 Jul;109(1):65–72. doi: 10.1007/BF01870791. [DOI] [PubMed] [Google Scholar]
- Saito Y., Ozawa T., Hayashi H., Nishiyama A. The effect of acetylcholine on chloride transport across the mouse lacrimal gland acinar cell membranes. Pflugers Arch. 1987 Jul;409(3):280–288. doi: 10.1007/BF00583477. [DOI] [PubMed] [Google Scholar]
- Saito Y., Ozawa T., Nishiyama A. Acetylcholine-induced Na+ influx in the mouse lacrimal gland acinar cells: demonstration of multiple Na+ transport mechanisms by intracellular Na+ activity measurements. J Membr Biol. 1987;98(2):135–144. doi: 10.1007/BF01872126. [DOI] [PubMed] [Google Scholar]
- Saito Y., Ozawa T., Suzuki S., Nishiyama A. Intracellular pH regulation in the mouse lacrimal gland acinar cells. J Membr Biol. 1988;101(1):73–81. doi: 10.1007/BF01872822. [DOI] [PubMed] [Google Scholar]
- Silva P., Stoff J., Field M., Fine L., Forrest J. N., Epstein F. H. Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Physiol. 1977 Oct;233(4):F298–F306. doi: 10.1152/ajprenal.1977.233.4.F298. [DOI] [PubMed] [Google Scholar]
- Steward M. C., Seo Y., Case R. M. Intracellular pH during secretion in the perfused rabbit mandibular salivary gland measured by 31P NMR spectroscopy. Pflugers Arch. 1989 Jun;414(2):200–207. doi: 10.1007/BF00580964. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Petersen O. H. The effect of Na+ and Cl- removal and of loop diuretics on acetylcholine-evoked membrane potential changes in mouse lacrimal acinar cells. Q J Exp Physiol. 1985 Jul;70(3):437–445. doi: 10.1113/expphysiol.1985.sp002927. [DOI] [PubMed] [Google Scholar]
- THAYSEN J. H., THORN N. A., SCHWARTZ I. L. Excretion of sodium, potassium, chloride and carbon dioxide in human parotid saliva. Am J Physiol. 1954 Jul;178(1):155–159. doi: 10.1152/ajplegacy.1954.178.1.155. [DOI] [PubMed] [Google Scholar]
- Turner R. J., George J. N., Baum B. J. Evidence for a Na+/K+/Cl- cotransport system in basolateral membrane vesicles from the rabbit parotid. J Membr Biol. 1986;94(2):143–152. doi: 10.1007/BF01871194. [DOI] [PubMed] [Google Scholar]


