Abstract
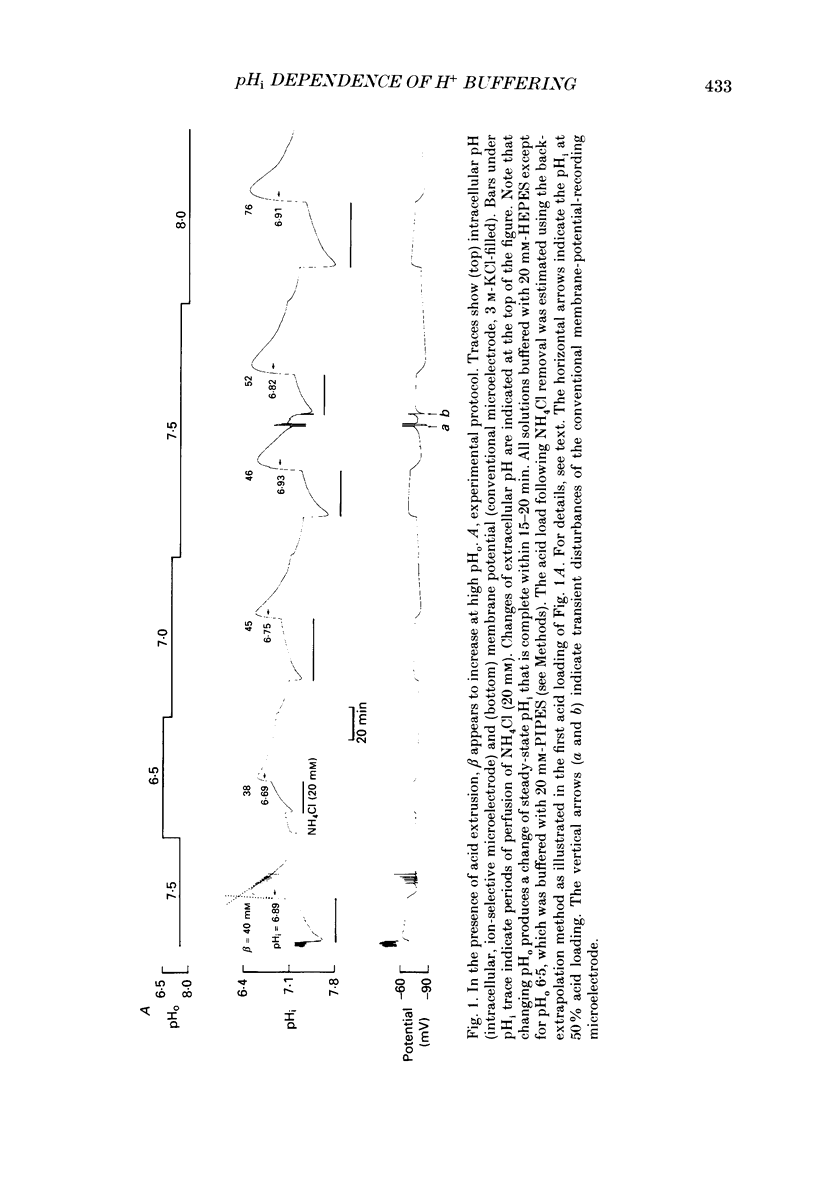
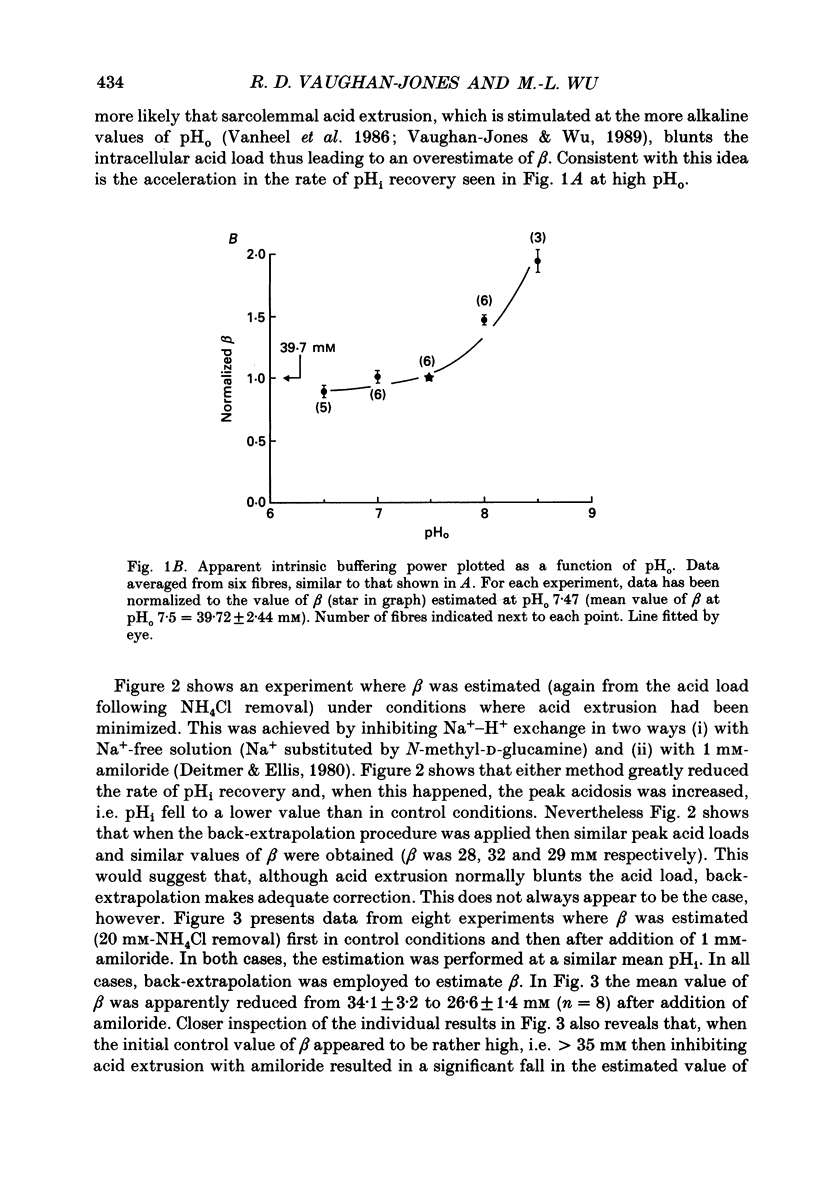
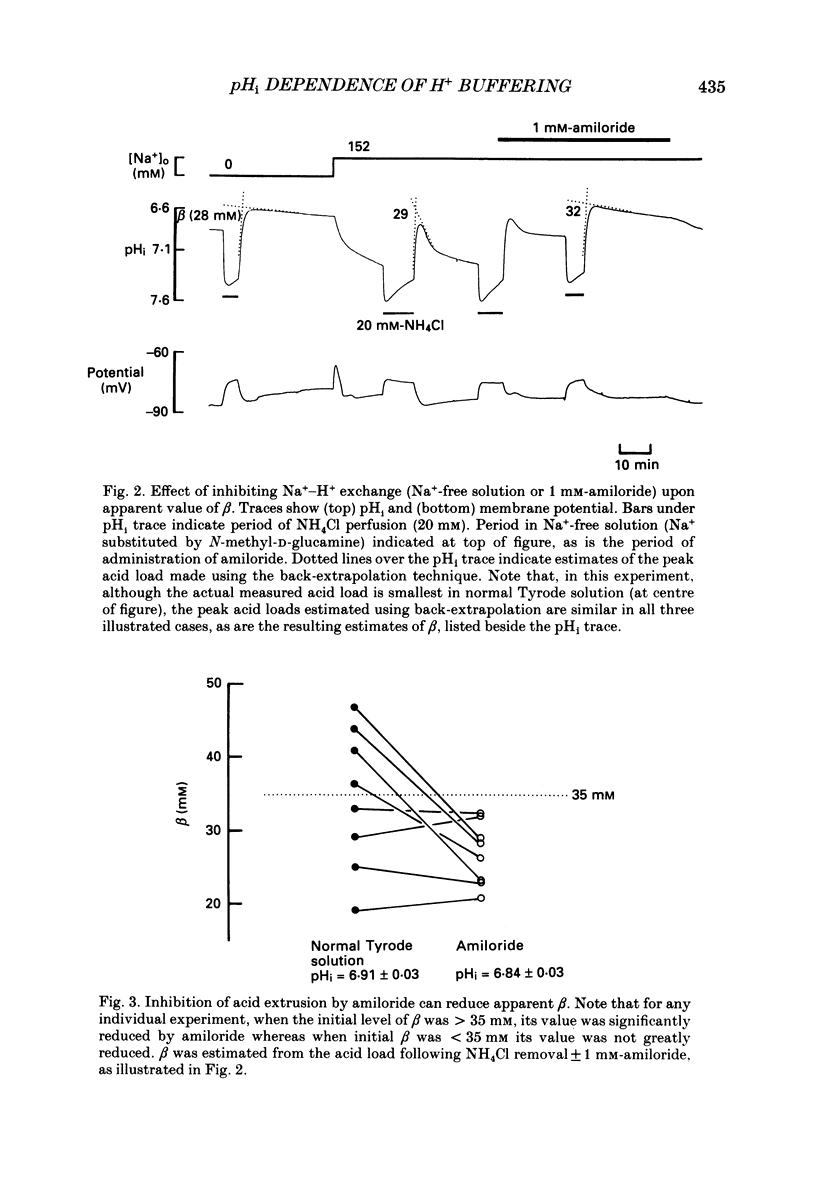
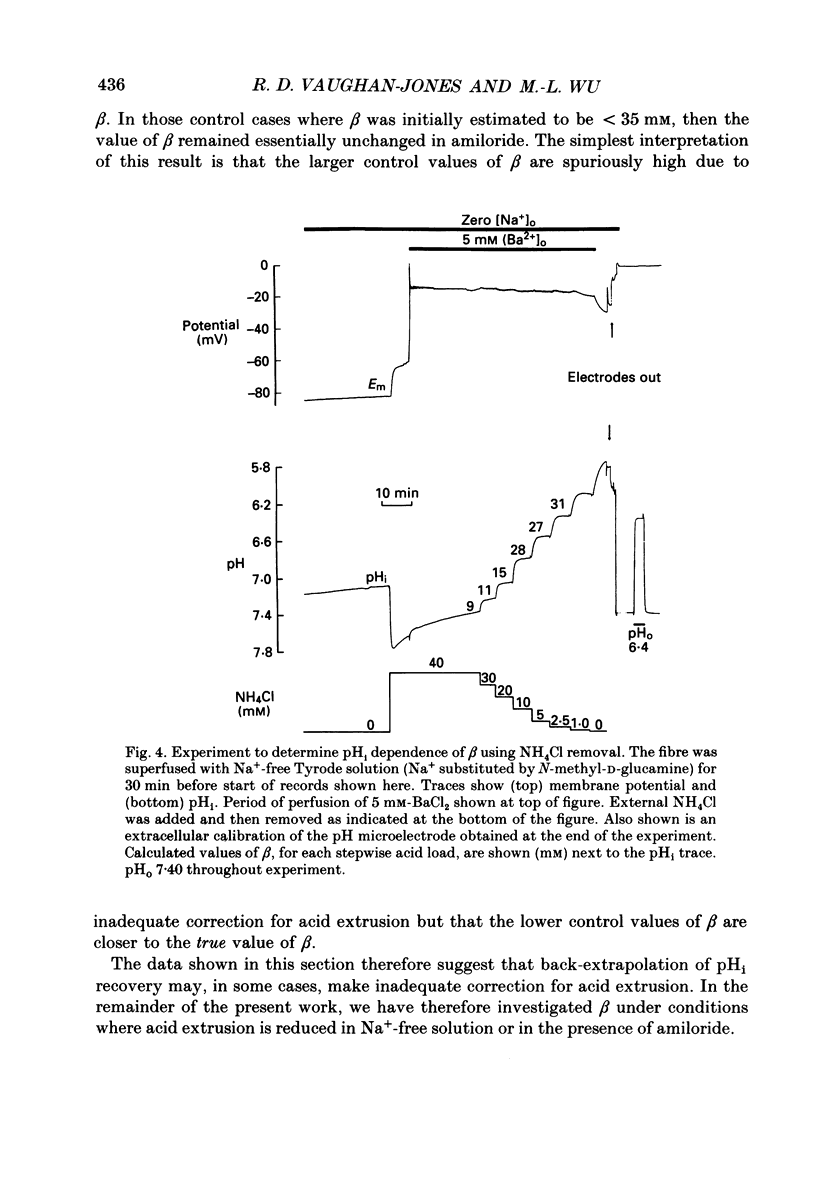
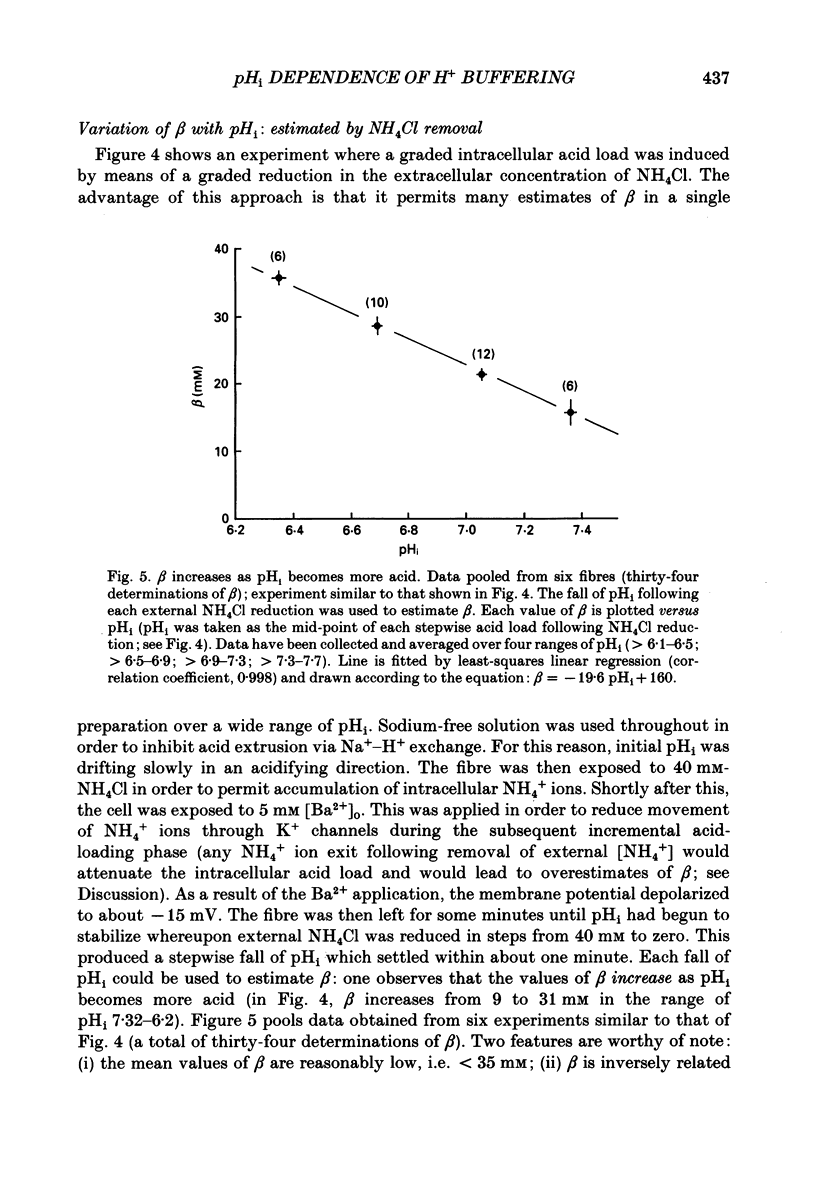
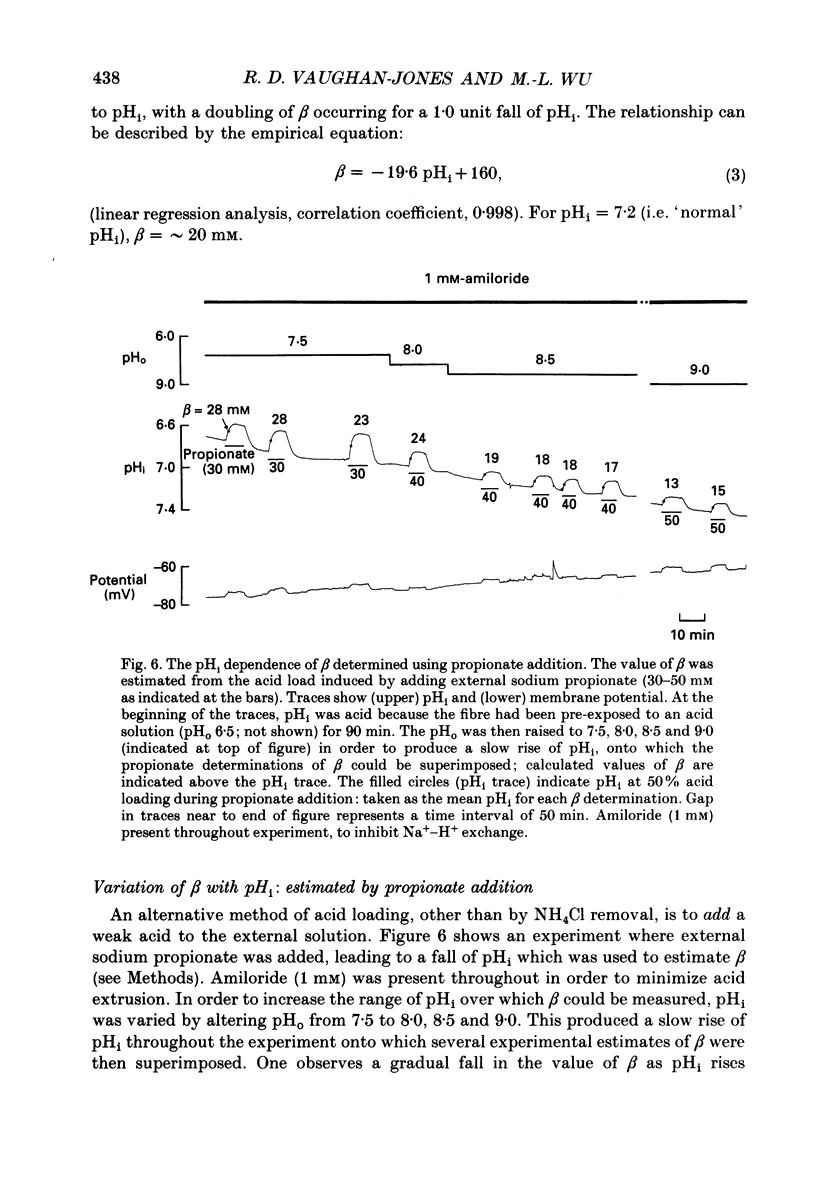
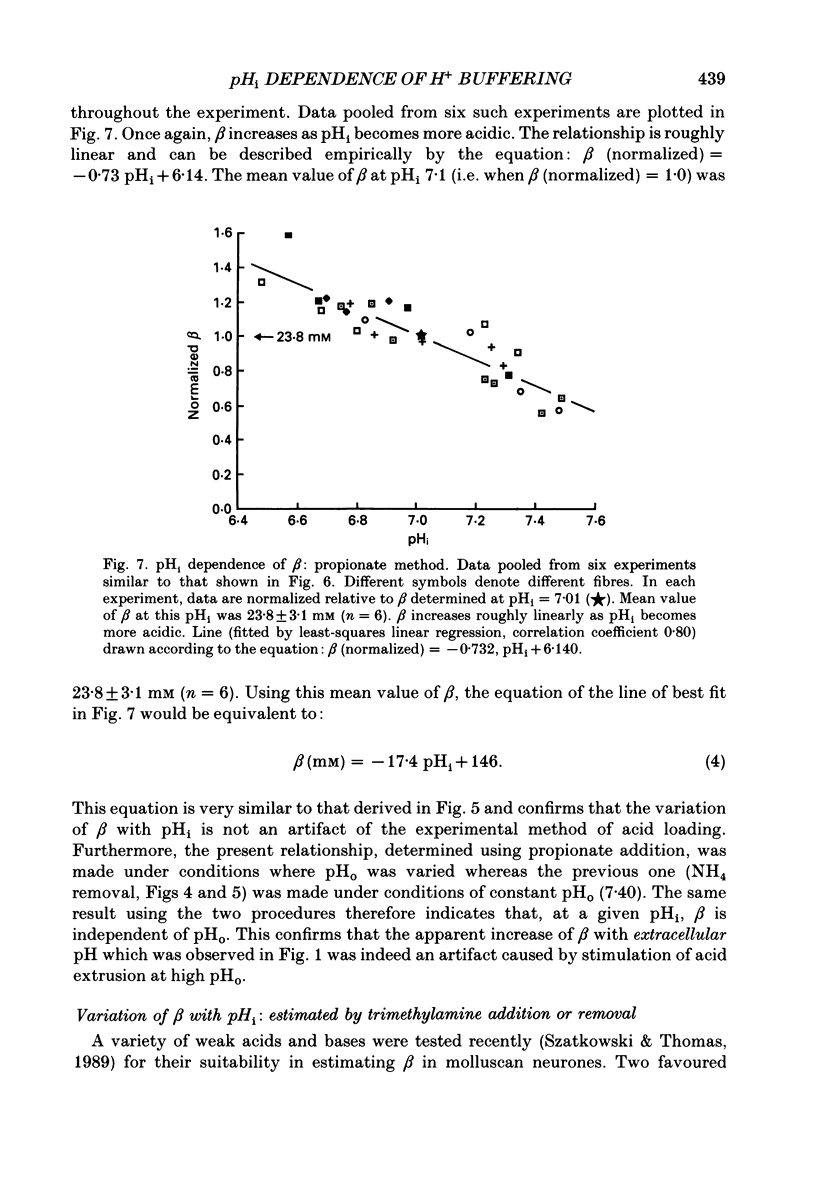
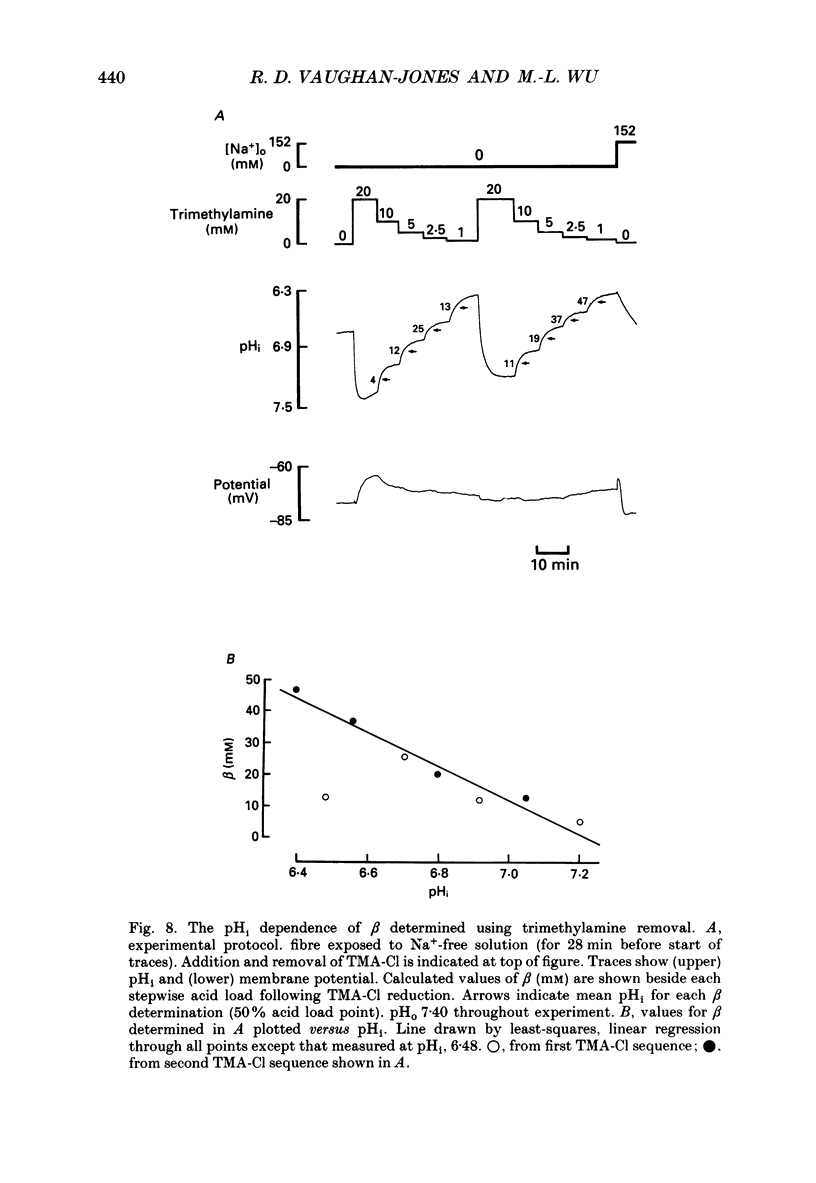
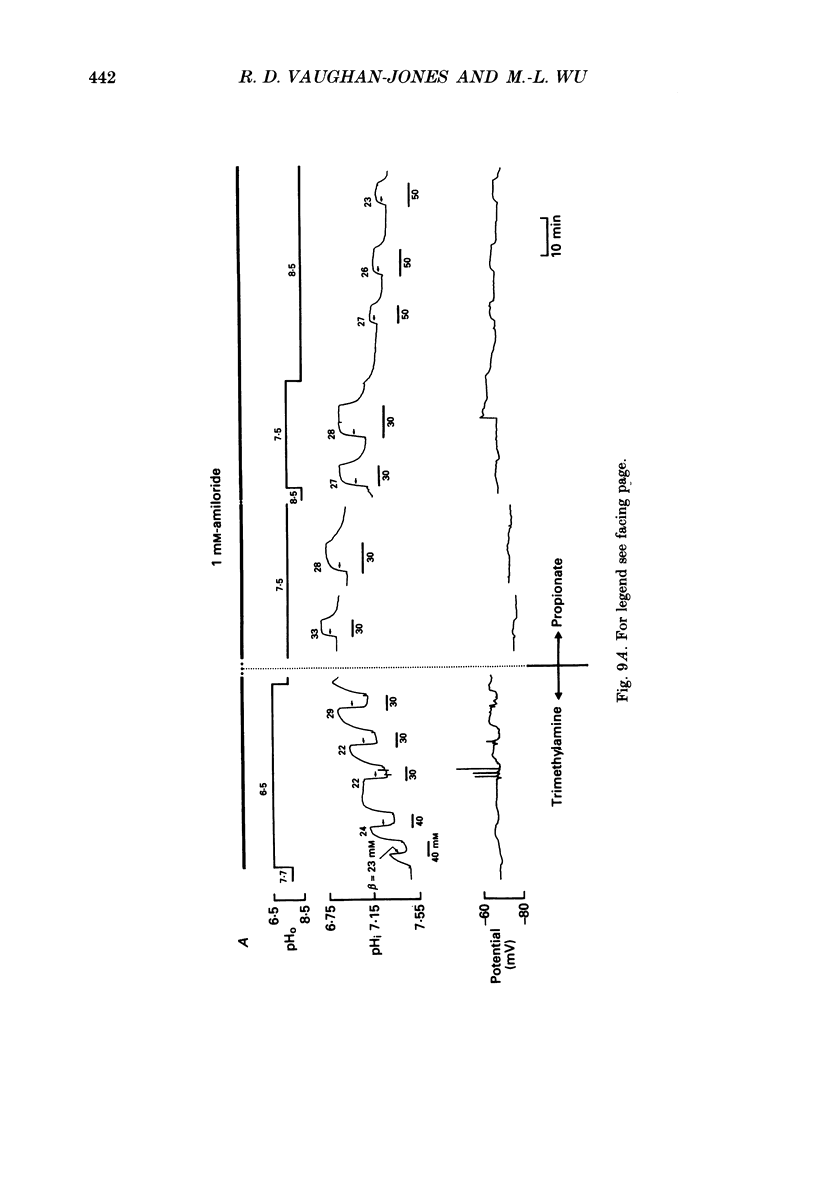
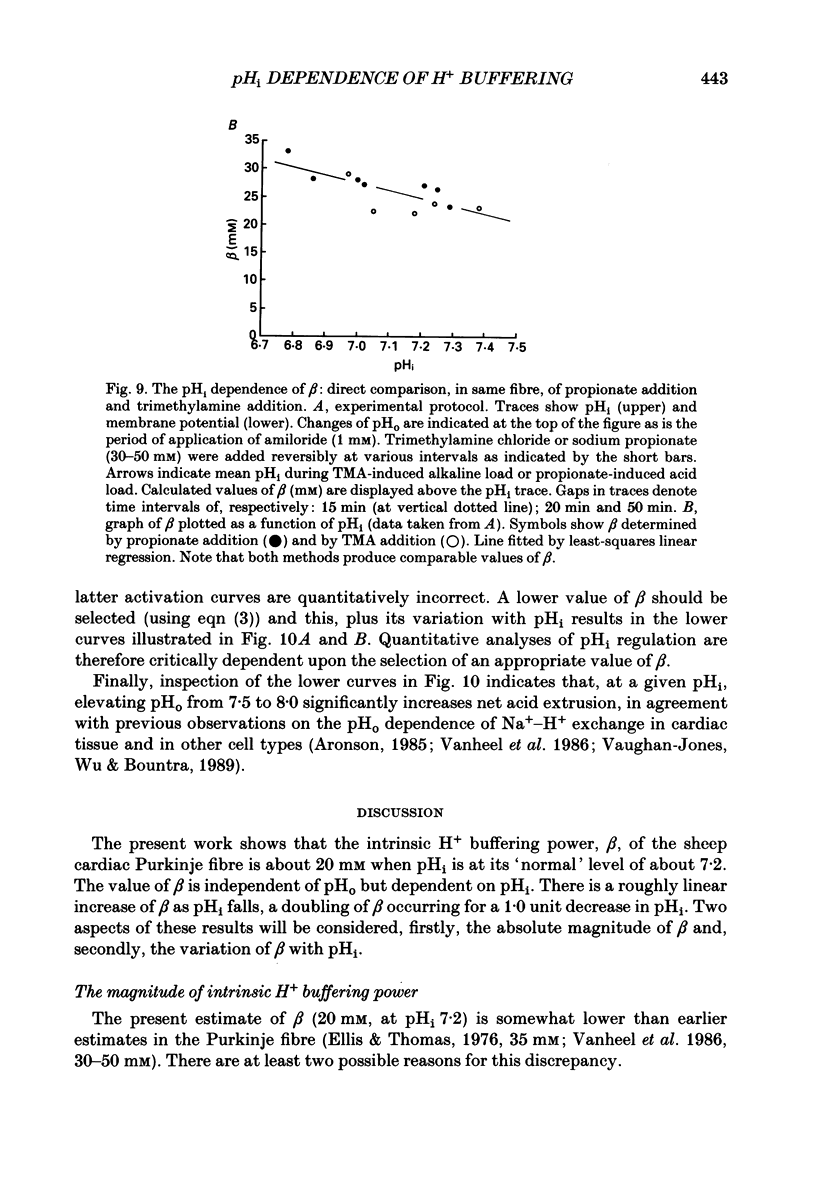
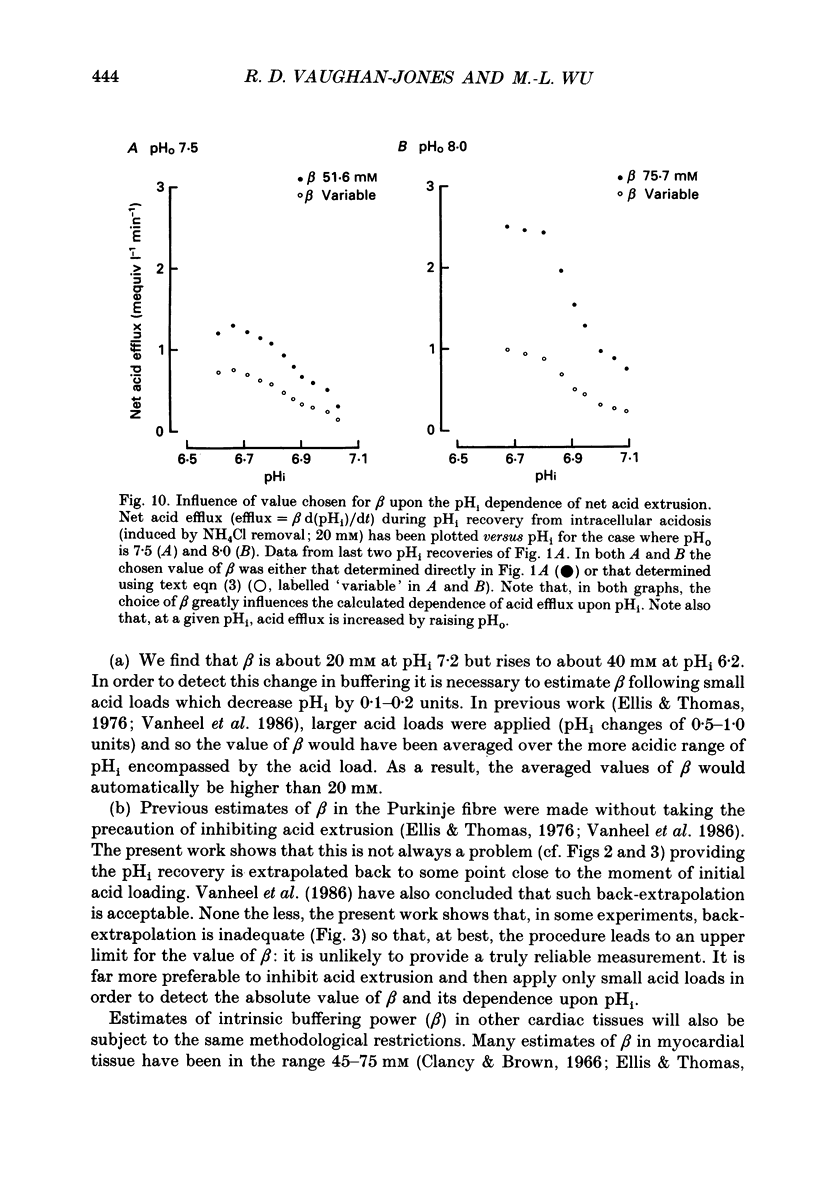
1. Intrinsic, intracellular H+ buffering power (beta) was estimated in the isolated sheep cardiac Purkinje fibre at various values of intracellular pH (pHi) in the range 6.2-7.5 and for various values of extracellular pH (pHo) in the range 6.5-8.5. Buffering power was calculated from the fall of pHi (recorded with an intracellular pH-selective microelectrode) induced by addition and removal of extracellular, permeant weak acids and bases (NH4Cl, trimethylamine chloride, sodium propionate). Experiments were performed under conditions nominally free of CO2-HCO3. 2. beta was estimated firstly following acid loads induced by NH4Cl removal (10-20 mM) under conditions where Na(+)-H+ exchange was operational (i.e. in Na(+)-containing Tyrode solution). At constant pHi, the value of beta appeared to double (from a control level of 39.7 mM) as pHo was increased from 7.5 to 8.5. Notably, raising pHo in this range greatly accelerated pHi recovery from an intracellular acid load, indicating stimulation of acid extrusion. It is likely that this stimulation results in an overestimation of beta because it blunts the intracellular acid load. The apparent elevation of beta at high pHo may therefore be an artifact. 3. Estimates of beta were compared (NH4Cl removal) before and after inhibiting Na(+)-H+ exchange in Na(+)-free solution or with amiloride (1 mM). The acid load was larger and in many (but not all) cases the apparent value of beta decreased after inhibition of acid extrusion. This indicates that, if Na(+)-H+ exchange is operational, it can result in an overestimate of beta. In amiloride, beta was 26.6 +/- 1.4 mM (n = 8) at a mean pHi of 6.84 +/- 0.03. 4. Small stepwise reductions of external NH4Cl (from 40 to 0 mM), in the presence of Na(+)-free solution plus 5 mM-BaCl2 at constant pHo, resulted in small stepwise reductions of pHi (approximately 0.1 units). When these were used to calculate beta, we observed that beta increased roughly linearly as pHi became more acid. For a pHi of 7.2, beta approximately 20 mM. 5. An almost identical relationship between beta and pHi was found when using the method of sodium propionate addition (10-50 mM): amiloride (1 mM) was present and pHi was manipulated to various test levels by changing pHo. This confirms that beta varies inversely with pHi and also that it is independent of pHo. We conclude that the apparent variation of beta with pHo observed earlier was indeed an artifact.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C. Direct measurement of intracellular pH and buffering power in smooth muscle cells of guinea-pig vas deferens. J Physiol. 1984 Apr;349:571–585. doi: 10.1113/jphysiol.1984.sp015174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C. Movement of acid equivalents across the mammalian smooth muscle cell membrane. Ciba Found Symp. 1988;139:3–22. doi: 10.1002/9780470513699.ch2. [DOI] [PubMed] [Google Scholar]
- Aronson P. S. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Bountra C., Kaila K., Vaughan-Jones R. D. Effect of repetitive activity upon intracellular pH, sodium and contraction in sheep cardiac Purkinje fibres. J Physiol. 1988 Apr;398:341–360. doi: 10.1113/jphysiol.1988.sp017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C., Powell T., Vaughan-Jones R. D. Comparison of intracellular pH transients in single ventricular myocytes and isolated ventricular muscle of guinea-pig. J Physiol. 1990 May;424:343–365. doi: 10.1113/jphysiol.1990.sp018071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3-. Am J Physiol. 1988 Dec;255(6 Pt 1):C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Lederer W. J. A novel experimental chamber for single-cell voltage-clamp and patch-clamp applications with low electrical noise and excellent temperature and flow control. Pflugers Arch. 1986 May;406(5):536–539. doi: 10.1007/BF00583378. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Verdonck F. Reduction of potassium permeability by chloride substitution in cardiac cells. J Physiol. 1977 Feb;265(1):193–206. doi: 10.1113/jphysiol.1977.sp011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy R. L., Brown E. B., Jr In vivo CO-2 buffer curves of skeletal and cardiac muscle. Am J Physiol. 1966 Dec;211(6):1309–1312. doi: 10.1152/ajplegacy.1966.211.6.1309. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980 Jul;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D., Thomas R. C. Direct measurement of the intracellular pH of mammalian cardiac muscle. J Physiol. 1976 Nov;262(3):755–771. doi: 10.1113/jphysiol.1976.sp011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean T., Frelin C., Vigne P., Barbry P., Lazdunski M. Biochemical properties of the Na+/H+ exchange system in rat brain synaptosomes. Interdependence of internal and external pH control of the exchange activity. J Biol Chem. 1985 Aug 15;260(17):9678–9684. [PubMed] [Google Scholar]
- Micro-electrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. J Physiol. 1977 Jun;267(3):791–810. doi: 10.1113/jphysiol.1977.sp011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms D., Jacob R., Horres C. R., Lieberman M. Na/H exchange in cultured chick heart cells. pHi regulation. J Gen Physiol. 1985 Jan;85(1):43–64. doi: 10.1085/jgp.85.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Szatkowski M. S., Thomas R. C. The intrinsic intracellular H+ buffering power of snail neurones. J Physiol. 1989 Feb;409:89–101. doi: 10.1113/jphysiol.1989.sp017486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A., Leusen I. Influence of surface pH on intracellular pH regulation in cardiac and skeletal muscle. Am J Physiol. 1986 May;250(5 Pt 1):C748–C760. doi: 10.1152/ajpcell.1986.250.5.C748. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. An investigation of chloride-bicarbonate exchange in the sheep cardiac Purkinje fibre. J Physiol. 1986 Oct;379:377–406. doi: 10.1113/jphysiol.1986.sp016259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Kaila K. The sensitivity of liquid sensor, ion-selective microelectrodes to changes in temperature and solution level. Pflugers Arch. 1986 Jun;406(6):641–644. doi: 10.1007/BF00584033. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of chloride in quiescent sheep-heart Purkinje fibres studied using intracellular chloride and pH-sensitive micro-electrodes. J Physiol. 1979 Oct;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of intracellular pH in cardiac muscle. Ciba Found Symp. 1988;139:23–46. doi: 10.1002/9780470513699.ch3. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L., Bountra C. Sodium-hydrogen exchange and its role in controlling contractility during acidosis in cardiac muscle. Mol Cell Biochem. 1989 Sep 7;89(2):157–162. doi: 10.1007/BF00220769. [DOI] [PubMed] [Google Scholar]
- Wolfe C. L., Gilbert H. F., Brindle K. M., Radda G. K. Determination of buffering capacity of rat myocardium during ischemia. Biochim Biophys Acta. 1988 Aug 19;971(1):9–20. doi: 10.1016/0167-4889(88)90156-5. [DOI] [PubMed] [Google Scholar]


