Abstract
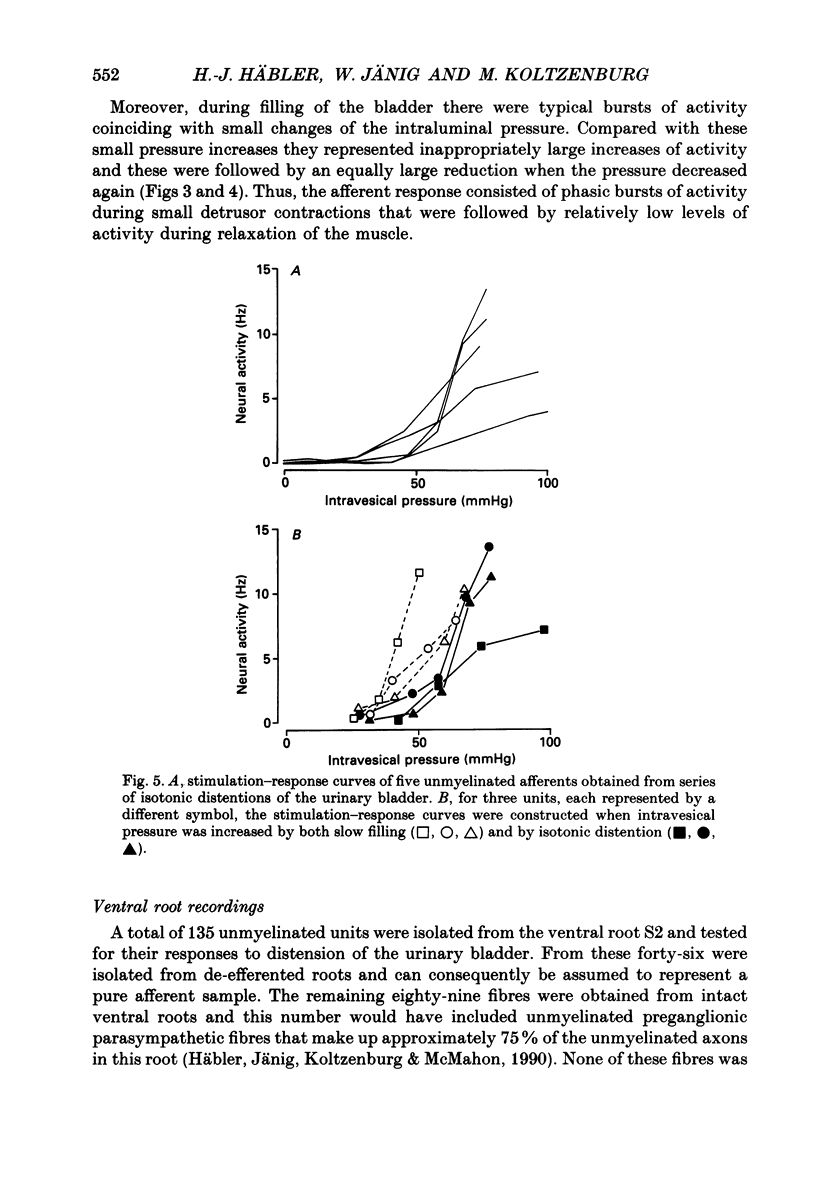
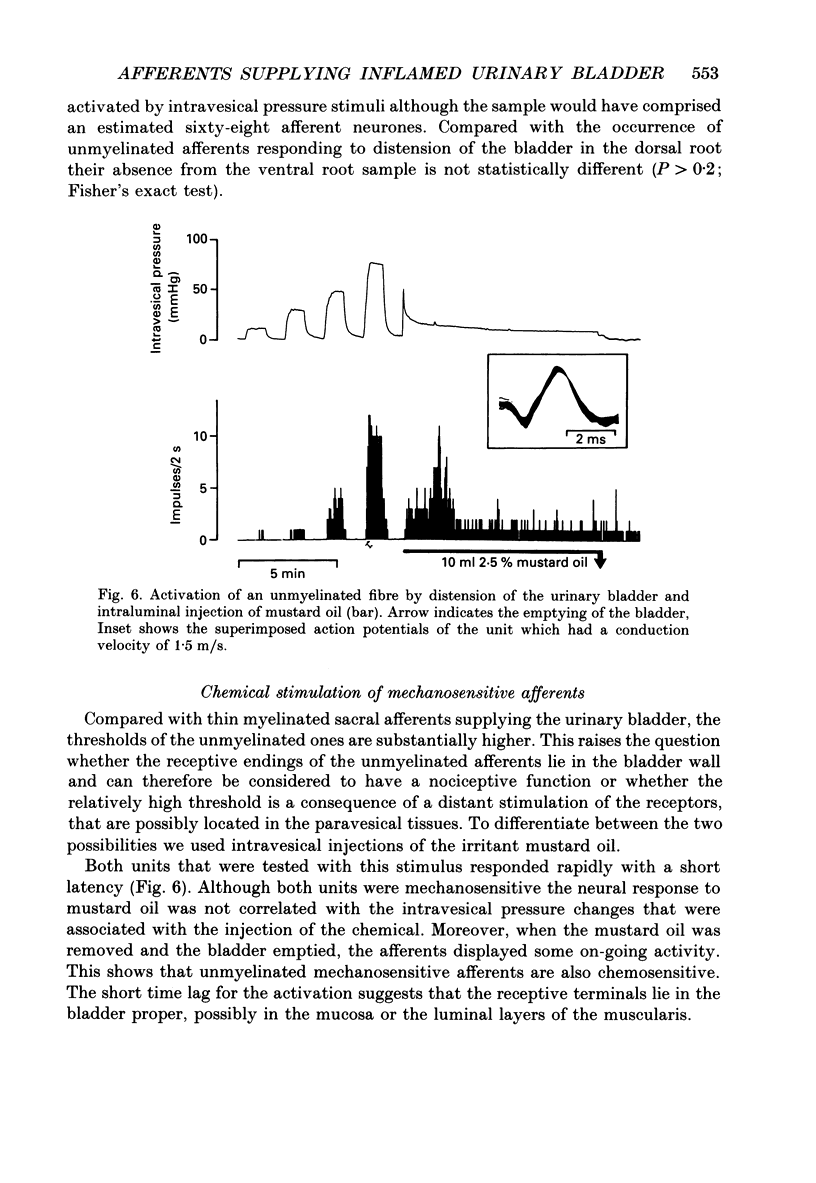
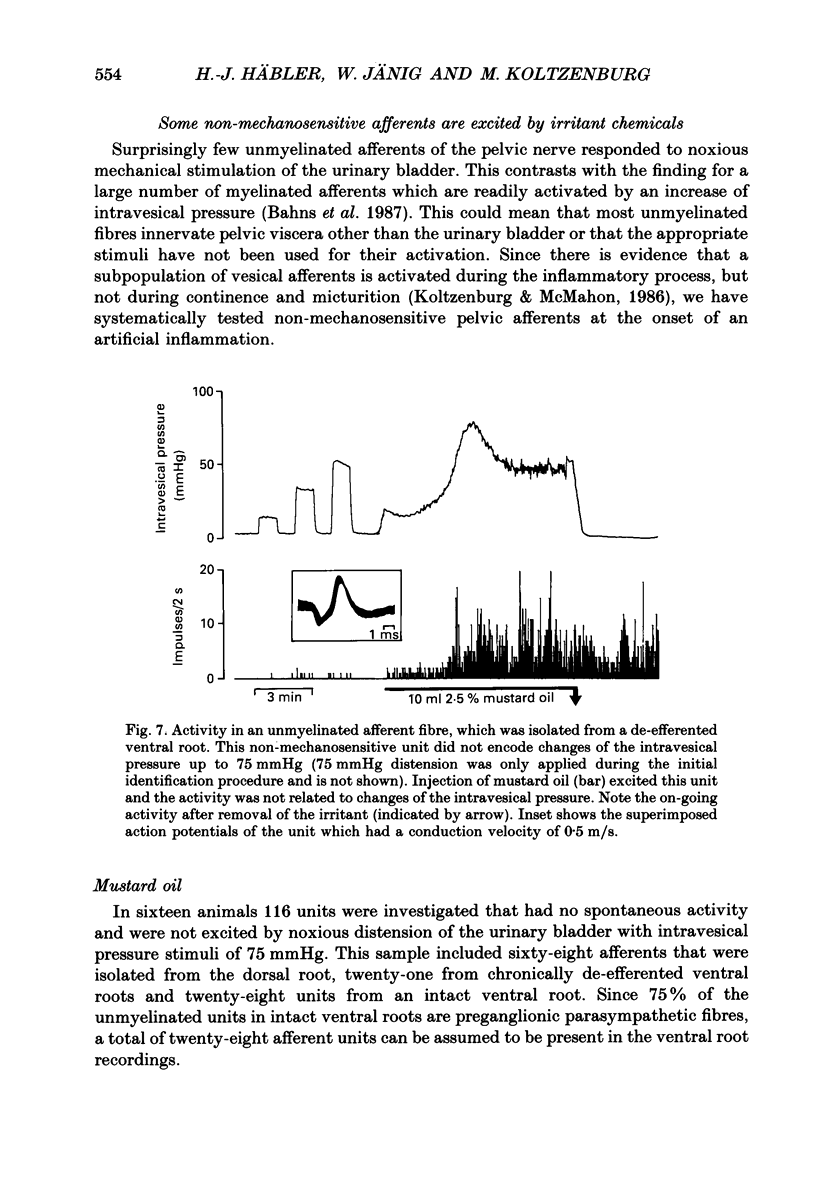
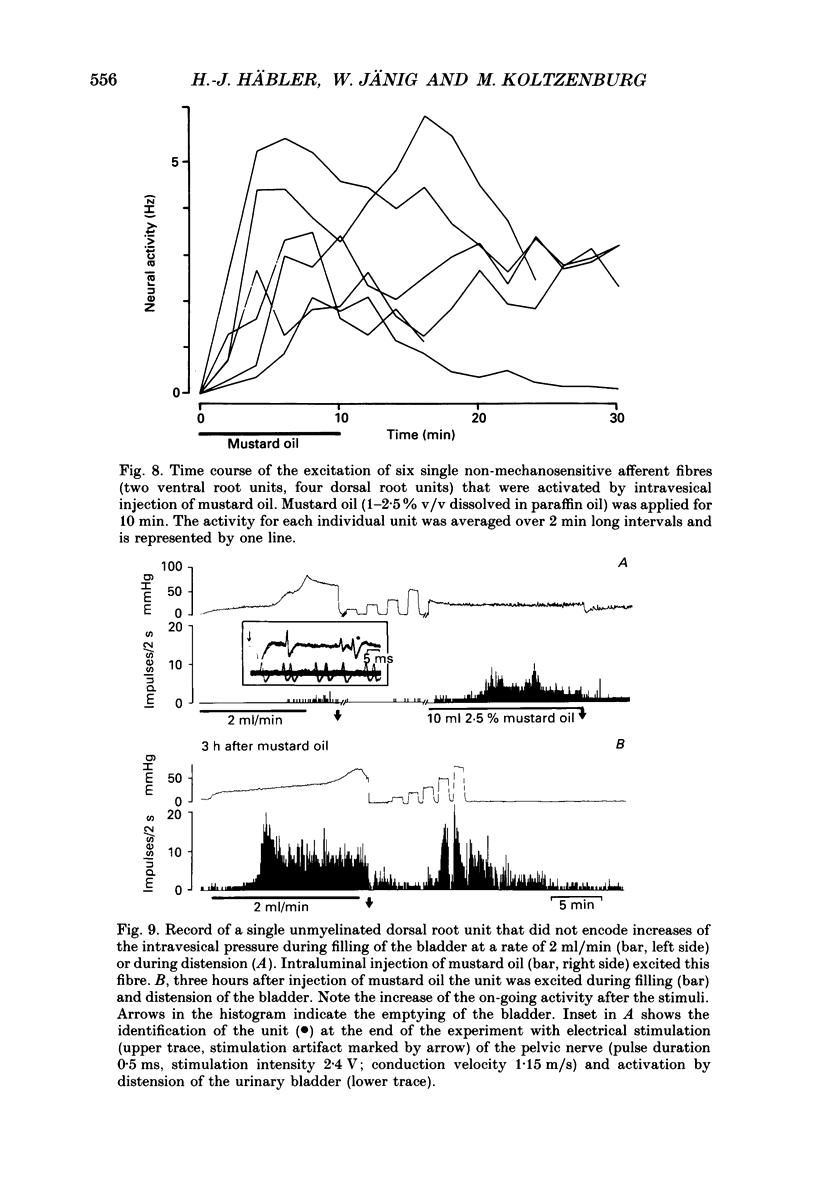
1. We examined the functional properties of unmyelinated primary afferent neurones innervating the pelvic viscera in twenty-five anaesthetized cats. The axons were isolated from the intact dorsal root and the intact or chronically de-efferented ventral root of the segment S2. All units were electrically identified with electrical stimulation of the pelvic nerve. 2. The responses of the neurones were studied with natural stimulation of the urinary bladder using innocuous and noxious increases of intravesical pressure and at the onset of an acute artificial inflammation induced by intraluminal injection of mustard or turpentine oil. 3. Out of 297 unmyelinated afferent units isolated from the dorsal root, seven were excited by an increase of the intravesical pressure during contractions and distension of the urinary bladder. These units were silent when the bladder was empty and had thresholds of 30-50 mmHg which are presumed to be noxious. Further increases of the intravesical pressure were accurately encoded by the discharge rate of the fibres. Out of sixty-eight unmyelinated afferent units isolated from the ventral root none was activated by these stimuli. 4. Intraluminal injection of mustard oil excited mechanosensitive units at short latency. The discharge was not closely related to changes of the intravesical pressure and the units displayed on-going activity after the irritant had been removed. This observation suggests that the units had also chemosensitive properties and that the receptive endings were located in the bladder wall. 5. In sixteen cats ninety-five afferent fibres that were not activated by noxious mechanical stimuli of the urinary bladder were systematically tested with intraluminal injections of mustard oil. This excited 7/67 dorsal root units and 4/28 ventral root units with short latency. Intraluminal application of turpentine oil, tested on twenty-six afferents in four animals, did not produce a rapid excitation. 6. Following the induction of an inflammation some previously non-mechanosensitive units started to respond to changes of intravesical pressure in the biologically relevant pressure range of the urinary bladder. 7. In conclusion, a small subpopulation (2.4%) of unmyelinated visceral afferents responds to high, presumably noxious, intravesical pressure and intraluminal application of chemical irritants. Acute inflammation excites a larger proportion of afferents (9.5%) that are not activated by acute noxious mechanical stimulation of the normal urinary bladder. In the inflamed bladder some previously non-mechanosensitive units started to respond to increases of intravesical pressure. These novel types of chemosensitive receptors may contribute considerably to the pathogenesis of visceral pain states.
Full text
PDF


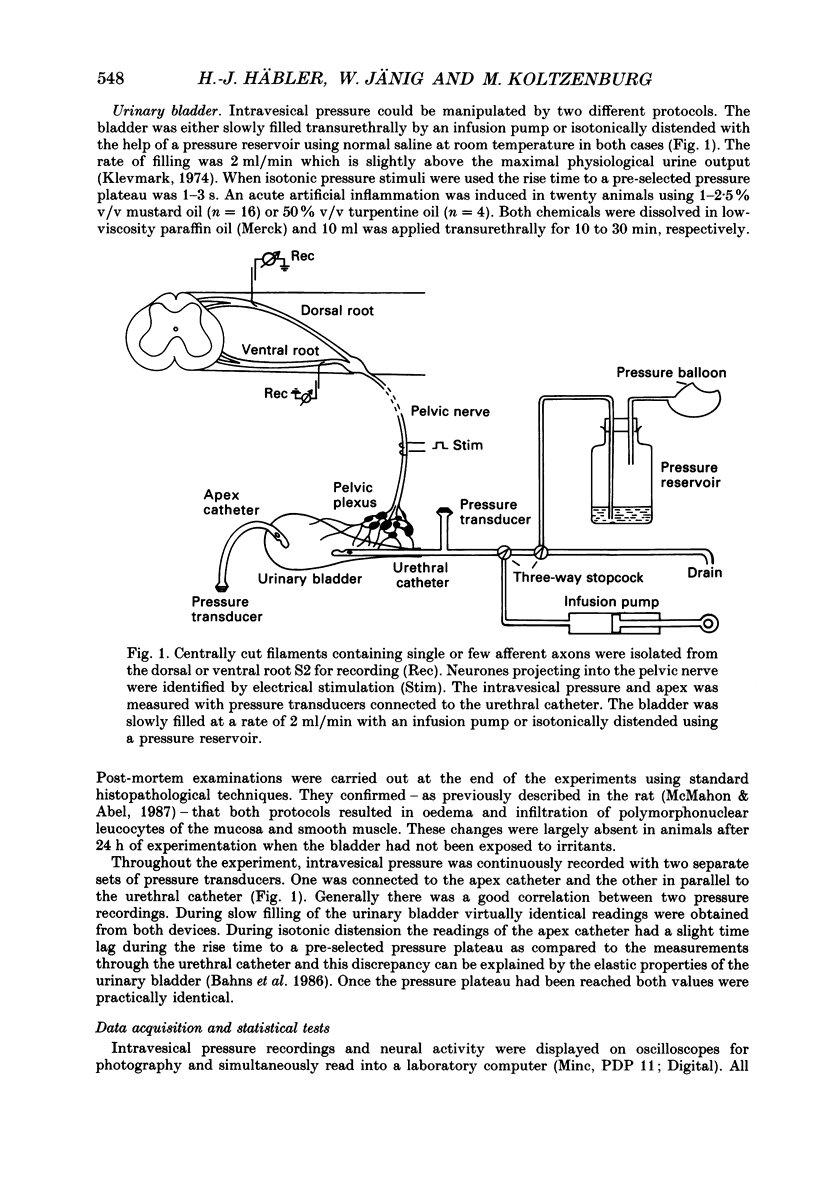
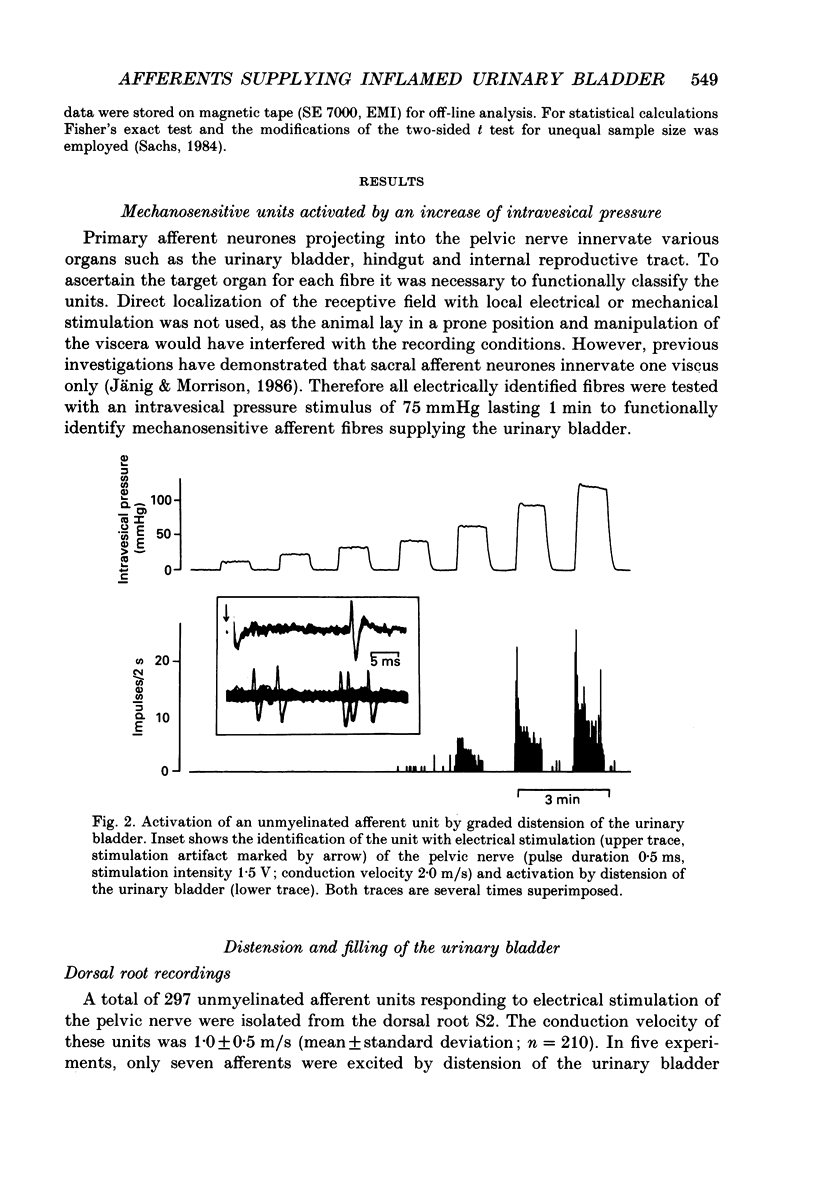
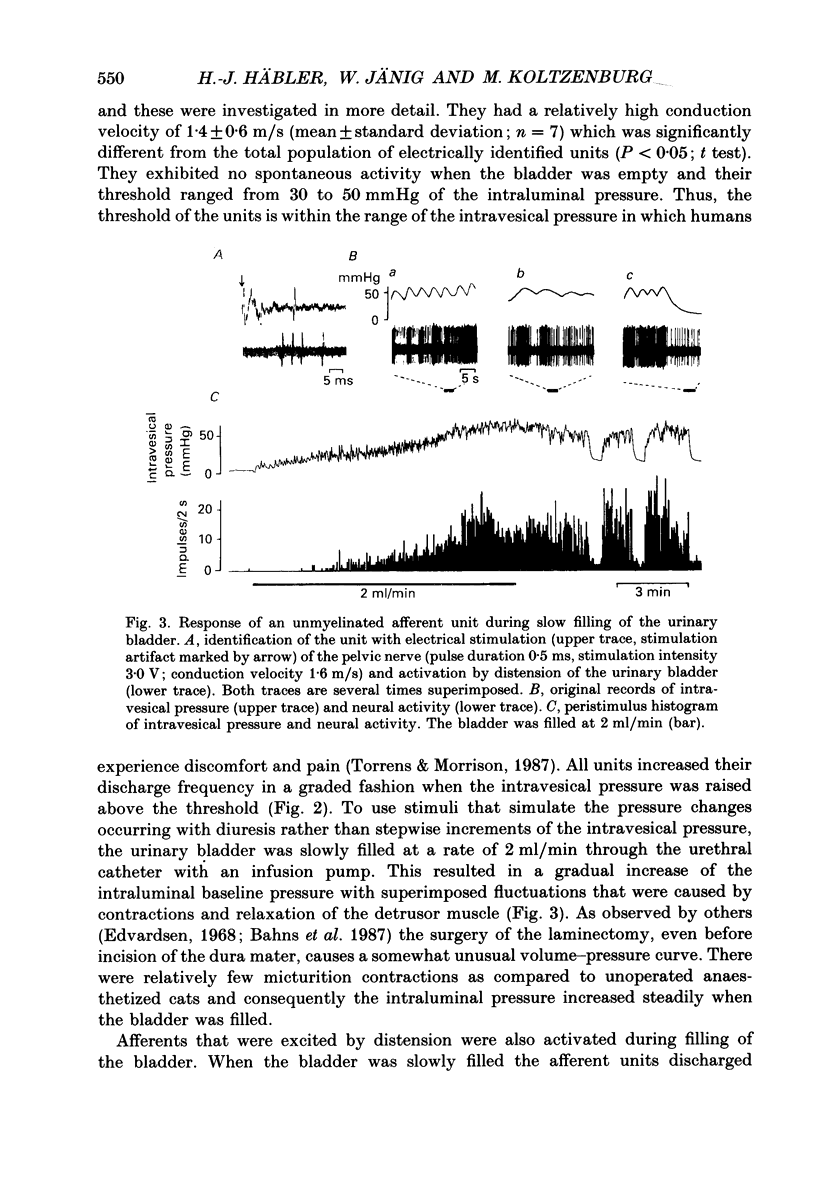
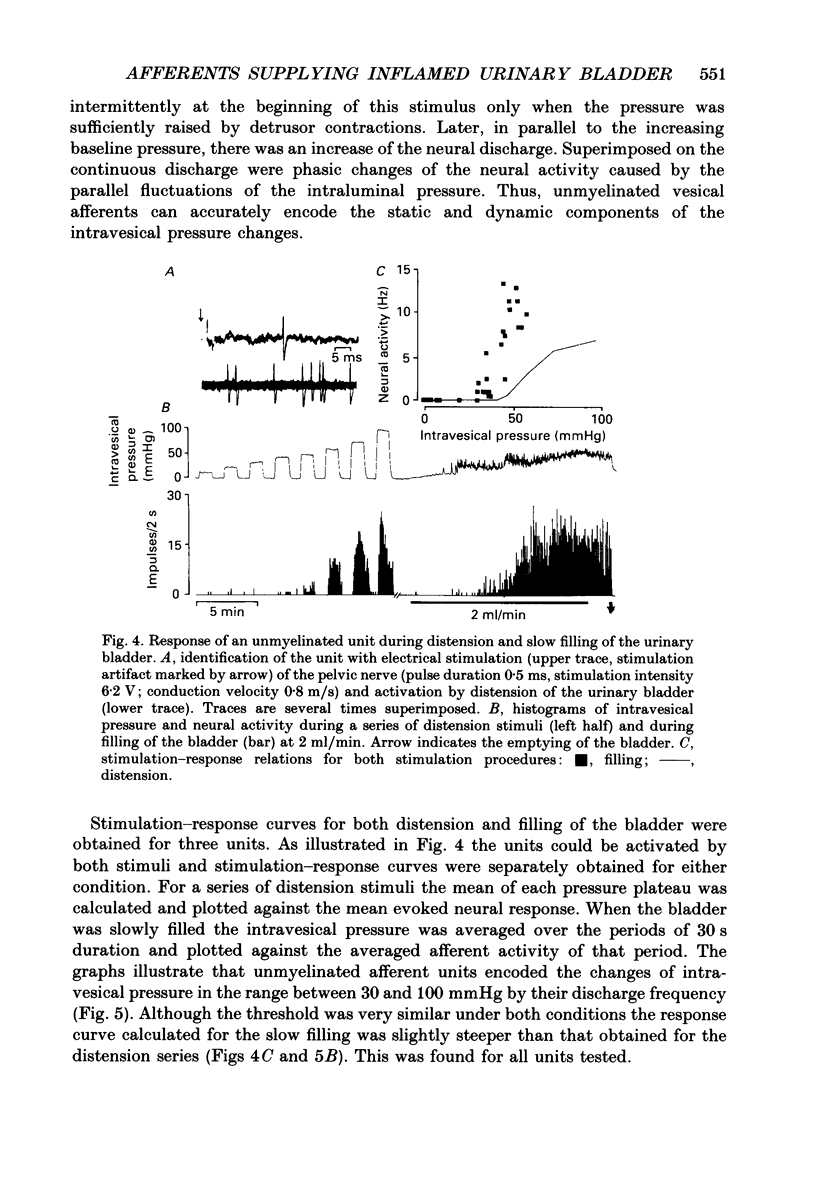

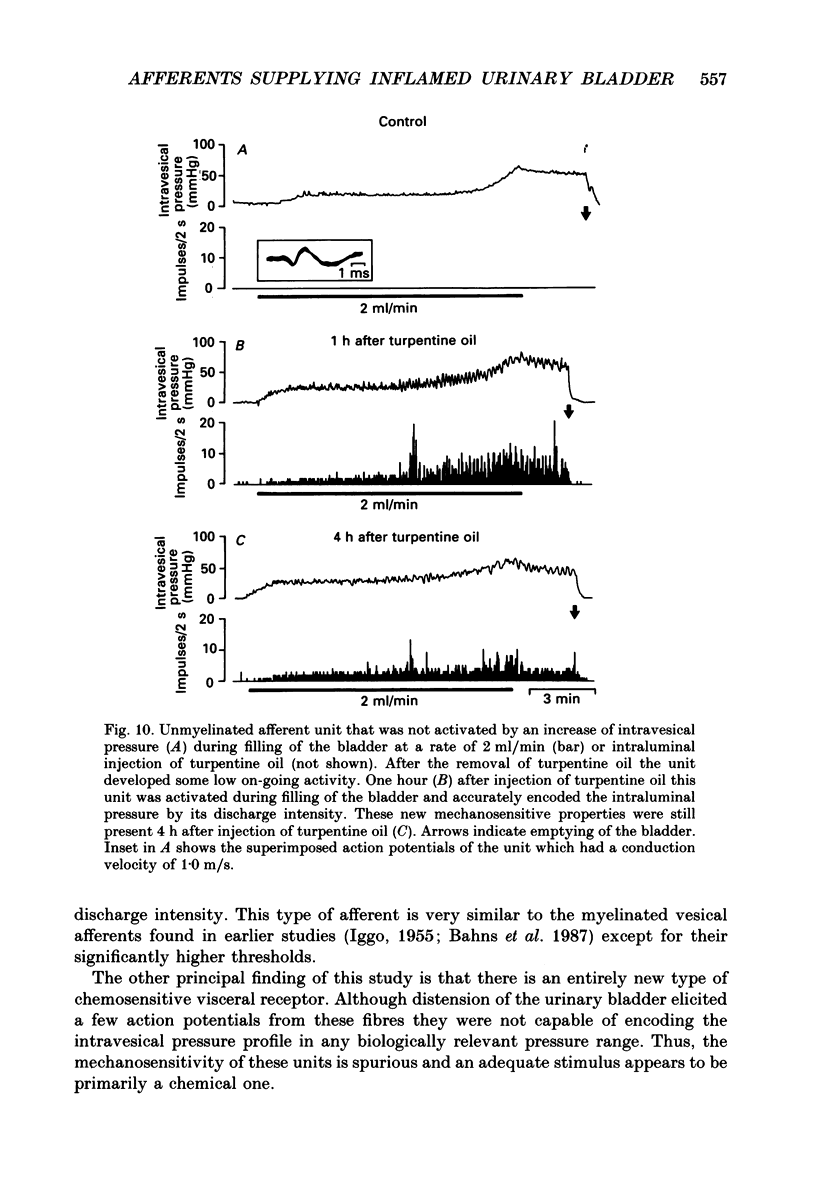





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelli L., Conte B., Somma V., Maggi C. A., Giuliani S., Geppetti P., Alessandri M., Theodorsson E., Meli A. The contribution of capsaicin-sensitive sensory nerves to xylene-induced visceral pain in conscious, freely moving rats. Naunyn Schmiedebergs Arch Pharmacol. 1988 May;337(5):545–551. doi: 10.1007/BF00182729. [DOI] [PubMed] [Google Scholar]
- Bahns E., Ernsberger U., Jänig W., Nelke A. Functional characteristics of lumbar visceral afferent fibres from the urinary bladder and the urethra in the cat. Pflugers Arch. 1986 Nov;407(5):510–518. doi: 10.1007/BF00657509. [DOI] [PubMed] [Google Scholar]
- Bahns E., Halsband U., Jänig W. Responses of sacral visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflugers Arch. 1987 Oct;410(3):296–303. doi: 10.1007/BF00580280. [DOI] [PubMed] [Google Scholar]
- Clifton G. L., Coggeshall R. E., Vance W. H., Willis W. D. Receptive fields of unmyelinated ventral root afferent fibres in the cat. J Physiol. 1976 Apr;256(3):573–600. doi: 10.1113/jphysiol.1976.sp011340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsen P. Nervous control of urinary bladder in cats. I. The collecting phase. Acta Physiol Scand. 1968 Jan-Feb;72(1):157–171. doi: 10.1111/j.1748-1716.1968.tb03838.x. [DOI] [PubMed] [Google Scholar]
- Floyd K., Hick V. E., Morrison J. F. Mechanosensitive afferent units in the hypogastric nerve of the cat. J Physiol. 1976 Jul;259(2):457–471. doi: 10.1113/jphysiol.1976.sp011476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg P., Schaible H. G., Schmidt R. F. Mechanical sensitivity of group III and IV afferents from posterior articular nerve in normal and inflamed cat knee. J Neurophysiol. 1986 Apr;55(4):635–643. doi: 10.1152/jn.1986.55.4.635. [DOI] [PubMed] [Google Scholar]
- HARDY J. D., WOLFF H. G., GOODELL H. Experimental evidence on the nature of cutaneous hyperalgesia. J Clin Invest. 1950 Jan;29(1):115–140. doi: 10.1172/JCI102227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häbler H. J., Jänig W., Koltzenburg M. A novel type of unmyelinated chemosensitive nociceptor in the acutely inflamed urinary bladder. Agents Actions. 1988 Dec;25(3-4):219–221. doi: 10.1007/BF01965016. [DOI] [PubMed] [Google Scholar]
- Häbler H. J., Jänig W., Koltzenburg M., McMahon S. B. A quantitative study of the central projection patterns of unmyelinated ventral root afferents in the cat. J Physiol. 1990 Mar;422:265–287. doi: 10.1113/jphysiol.1990.sp017983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955 Jun 28;128(3):593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., McLachlan E. M. Organization of lumbar spinal outflow to distal colon and pelvic organs. Physiol Rev. 1987 Oct;67(4):1332–1404. doi: 10.1152/physrev.1987.67.4.1332. [DOI] [PubMed] [Google Scholar]
- Jänig W., Morrison J. F. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res. 1986;67:87–114. doi: 10.1016/s0079-6123(08)62758-2. [DOI] [PubMed] [Google Scholar]
- Klevmark B. Motility of the urinary bladder in cats during filling at physiological rates. I. Intravesical pressure patterns studied by a new method of cystometry. Acta Physiol Scand. 1974 Mar;90(3):565–577. doi: 10.1111/j.1748-1716.1974.tb05621.x. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M., McMahon S. B. Plasma extravasation in the rat urinary bladder following mechanical, electrical and chemical stimuli: evidence for a new population of chemosensitive primary sensory afferents. Neurosci Lett. 1986 Dec 23;72(3):352–356. doi: 10.1016/0304-3940(86)90540-9. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Brodin E., Hua X., Saria A. Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand. 1984 Feb;120(2):217–227. doi: 10.1111/j.1748-1716.1984.tb00127.x. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Abel C. A model for the study of visceral pain states: chronic inflammation of the chronic decerebrate rat urinary bladder by irritant chemicals. Pain. 1987 Jan;28(1):109–127. doi: 10.1016/0304-3959(87)91065-7. [DOI] [PubMed] [Google Scholar]
- McMahon S. B. Neuronal and behavioural consequences of chemical inflammation of rat urinary bladder. Agents Actions. 1988 Dec;25(3-4):231–233. doi: 10.1007/BF01965020. [DOI] [PubMed] [Google Scholar]
- McMahon S. B. Sensory-motor integration in urinary bladder function. Prog Brain Res. 1986;67:245–253. doi: 10.1016/s0079-6123(08)62766-1. [DOI] [PubMed] [Google Scholar]
- Schaible H. G., Schmidt R. F. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol. 1988 Dec;60(6):2180–2195. doi: 10.1152/jn.1988.60.6.2180. [DOI] [PubMed] [Google Scholar]
- Simone D. A., Baumann T. K., LaMotte R. H. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989 Jul;38(1):99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- de Groat W. C. Neuropeptides in pelvic afferent pathways. Experientia. 1987 Jul 15;43(7):801–813. doi: 10.1007/BF01945358. [DOI] [PubMed] [Google Scholar]


