Abstract
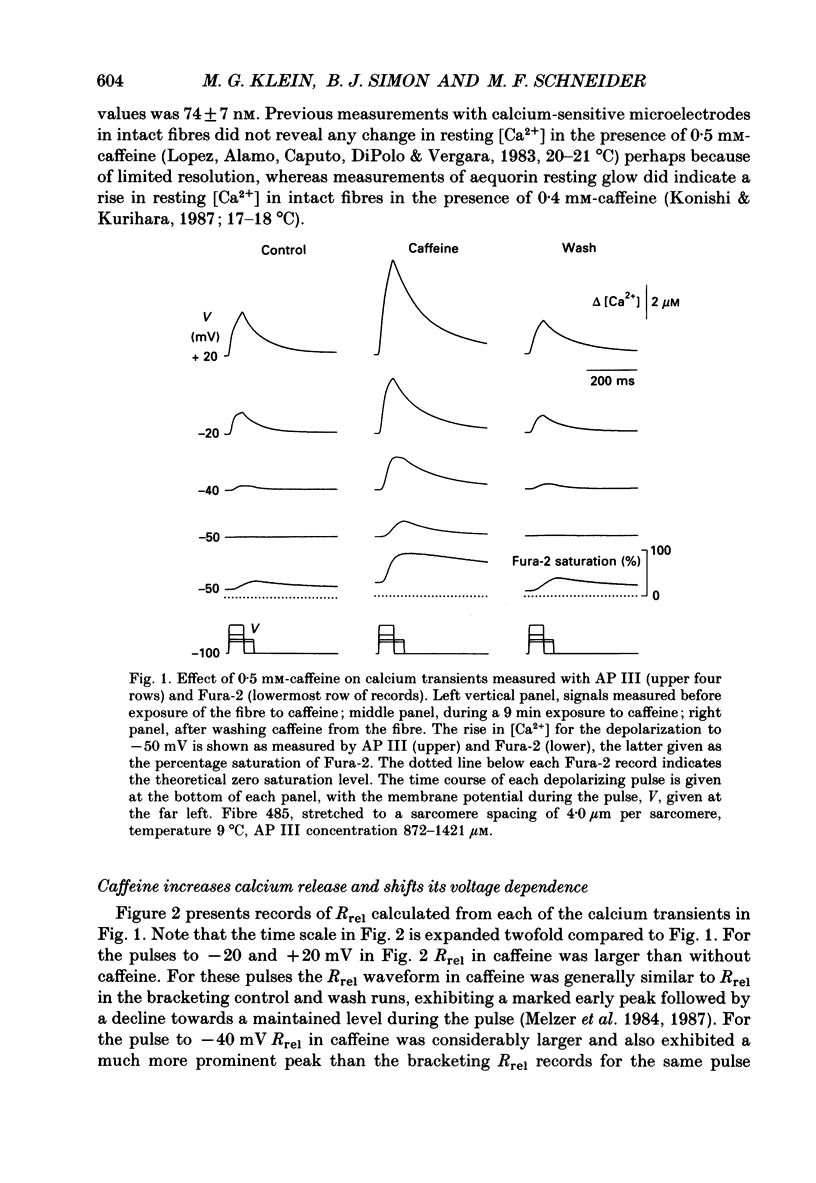
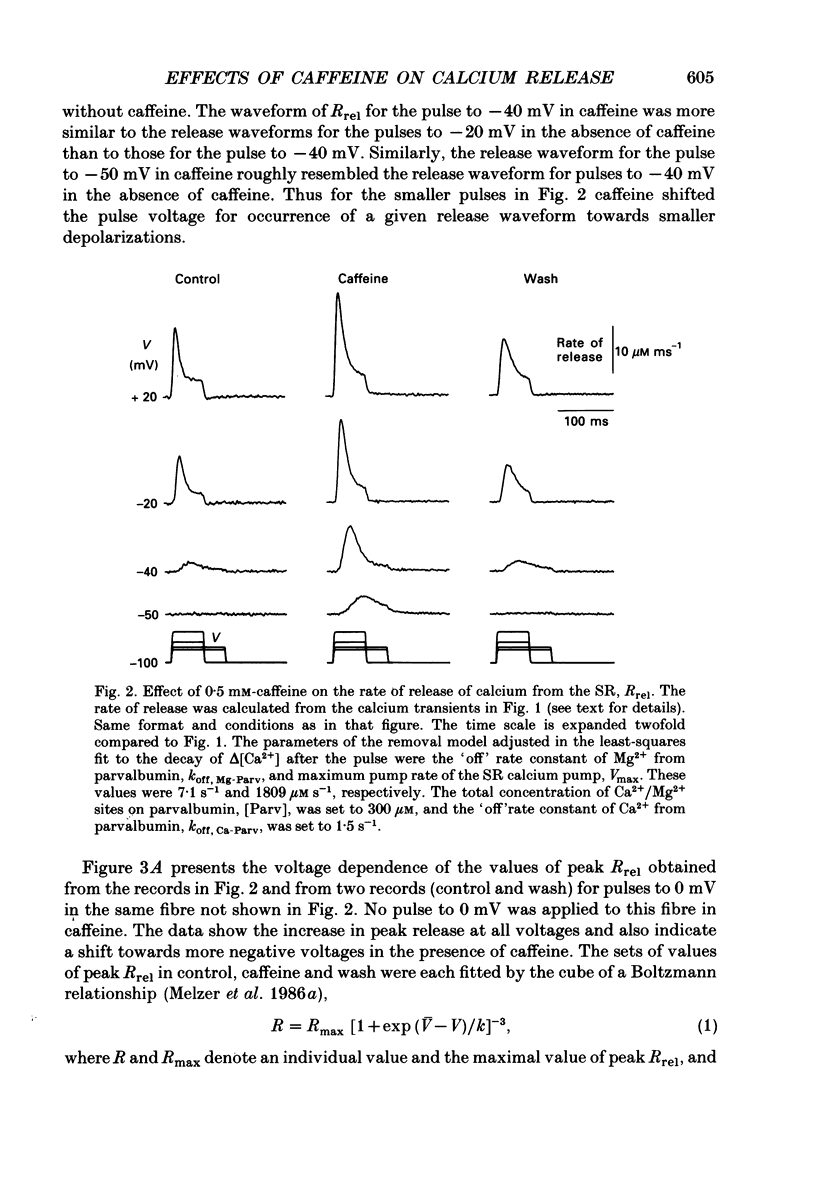
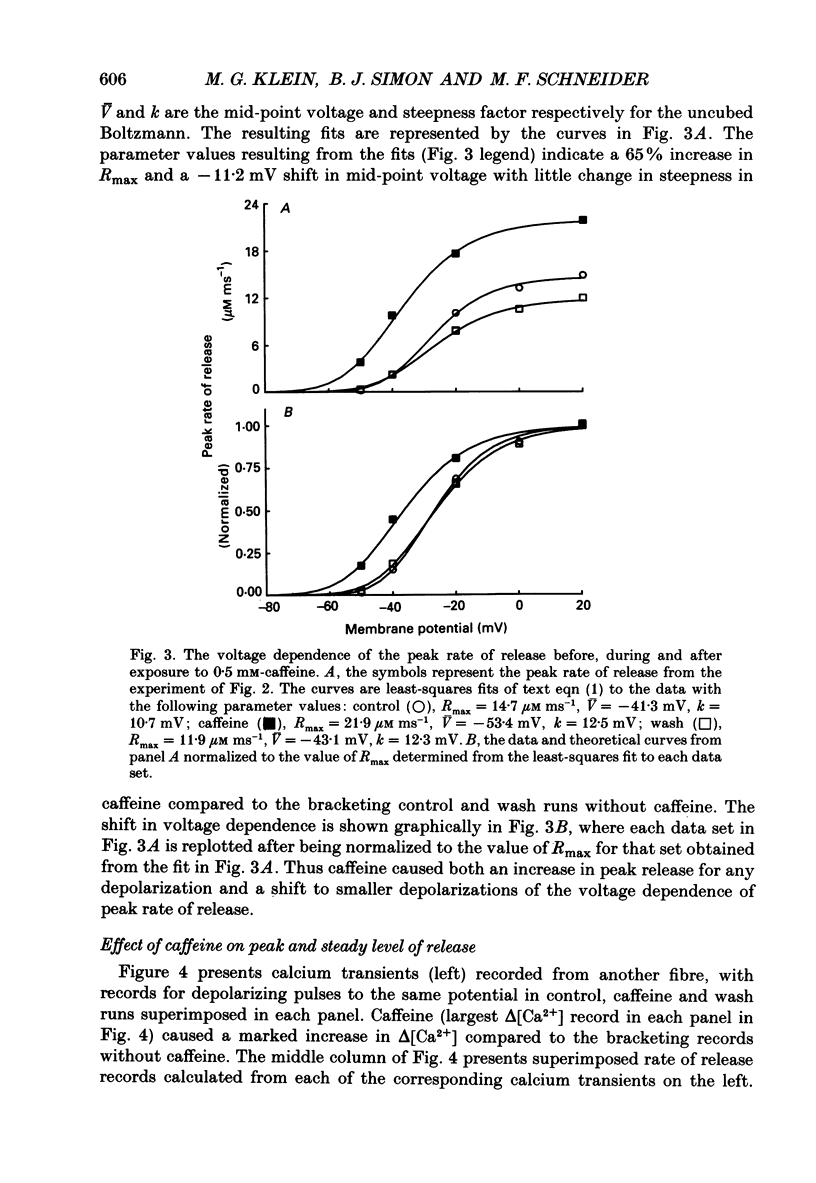
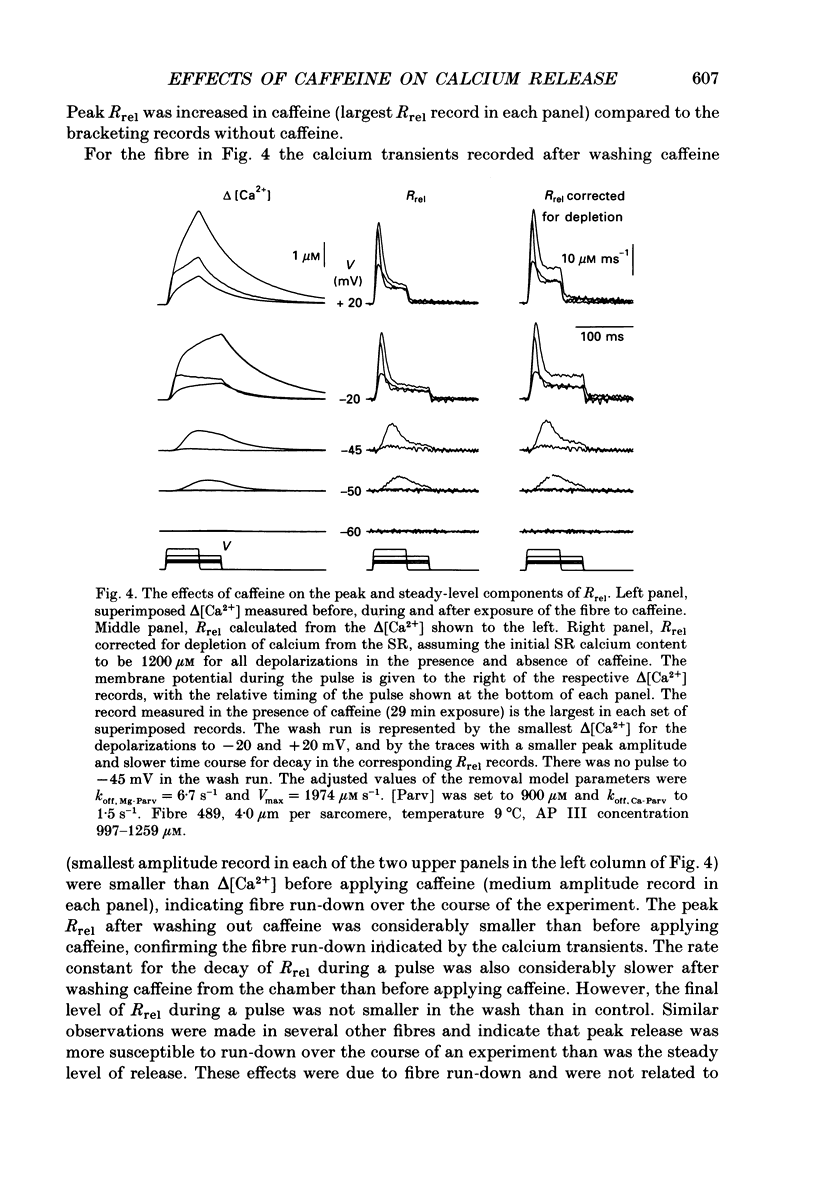
1. Resting myoplasmic [Ca2+] and [Ca2+] transients (delta [Ca2+]) were monitored using Fura-2 fluorescence and Antipyrylazo III absorbance signals from voltage-clamped segments of cut frog skeletal muscle fibres in the presence and absence of 0.5 mM-caffeine. The rate of release (Rrel) of calcium from the sarcoplasmic reticulum was calculated from delta [Ca2+]. 2. delta [Ca2+] and Rrel were increased in caffeine for all pulses. The decline of delta [Ca2+] was slower after a given pulse in caffeine than without caffeine. Resting [Ca2+] was slightly elevated in caffeine. 3. The voltage dependence of the peak value of Rrel and of the steady level of Rrel at the end of a 60-120 ms pulse were both shifted towards more negative voltages in caffeine. For relatively small pulses the voltage at which a given release waveform was observed was also shifted to more negative voltages. 4. Intramembrane charge movements measured in the same fibres in which the above changes in Rrel were observed showed no significant changes in caffeine. 5. In caffeine calcium release continued for many milliseconds after the end of a short (10 ms) pulse. Continued release after a pulse was not observed without caffeine and was probably due to positive feedback of elevated [Ca2+] on calcium release resulting from calcium-induced calcium release in caffeine. 6. Intramembrane charge movements after short pulses showed no change in caffeine that could account for the continued calcium release after the pulse. 7. Continued release after short pulses in caffeine decreased as the pulse duration was increased and was absent for pulses of 60 ms or longer. Rrel also inactivated during such pulses. 8. Relatively large and long conditioning pulses in caffeine suppressed both the peak Rrel and the continued release after short pulses. Peak release and continued release after short pulses recovered in parallel with increasing recovery time following suppression by a conditioning pulse in caffeine. 9. These results indicate that in the presence of caffeine, charge movement and calcium-induced calcium release both contribute significantly to the activation of sarcoplasmic reticulum calcium release during fibre depolarization. Release activated by either mechanism appears to be inactivated by calcium-dependent inactivation. A significant contribution of calcium-induced calcium release during depolarization in the absence of caffeine is not ruled out by present observations.
Full text
PDF







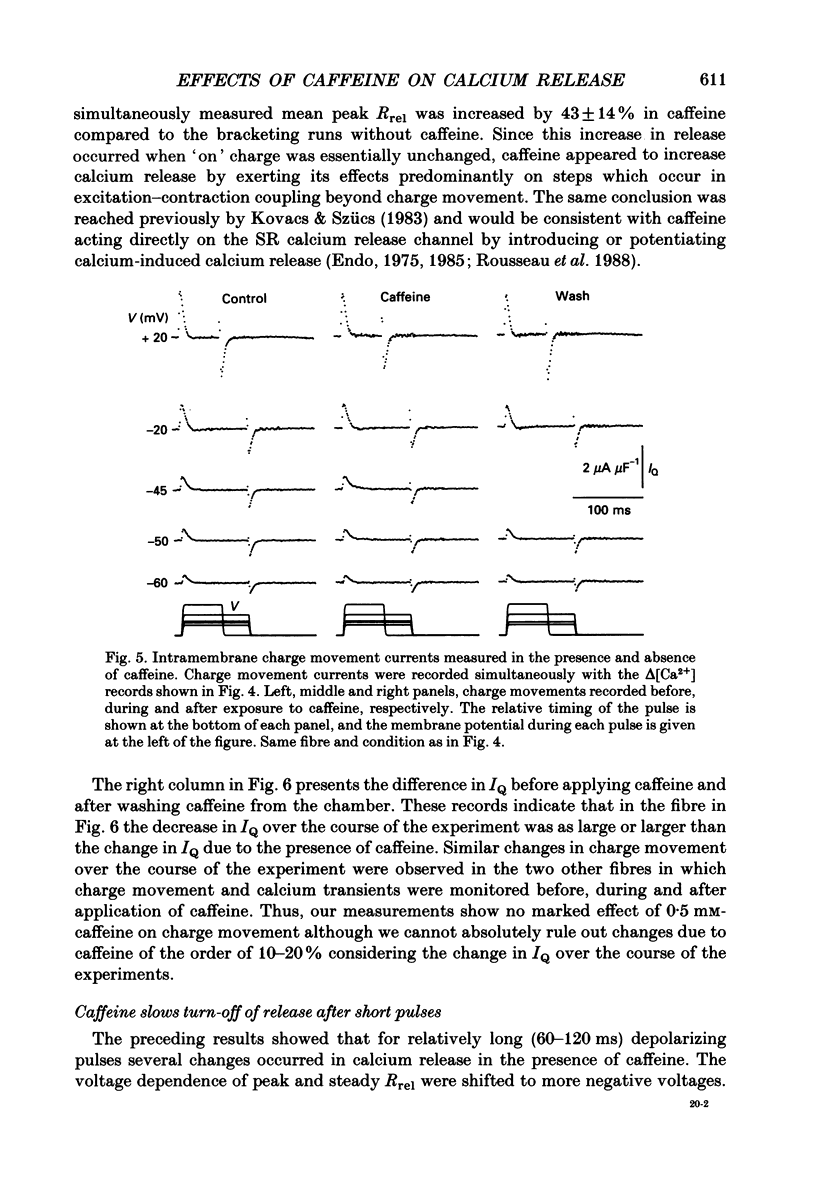
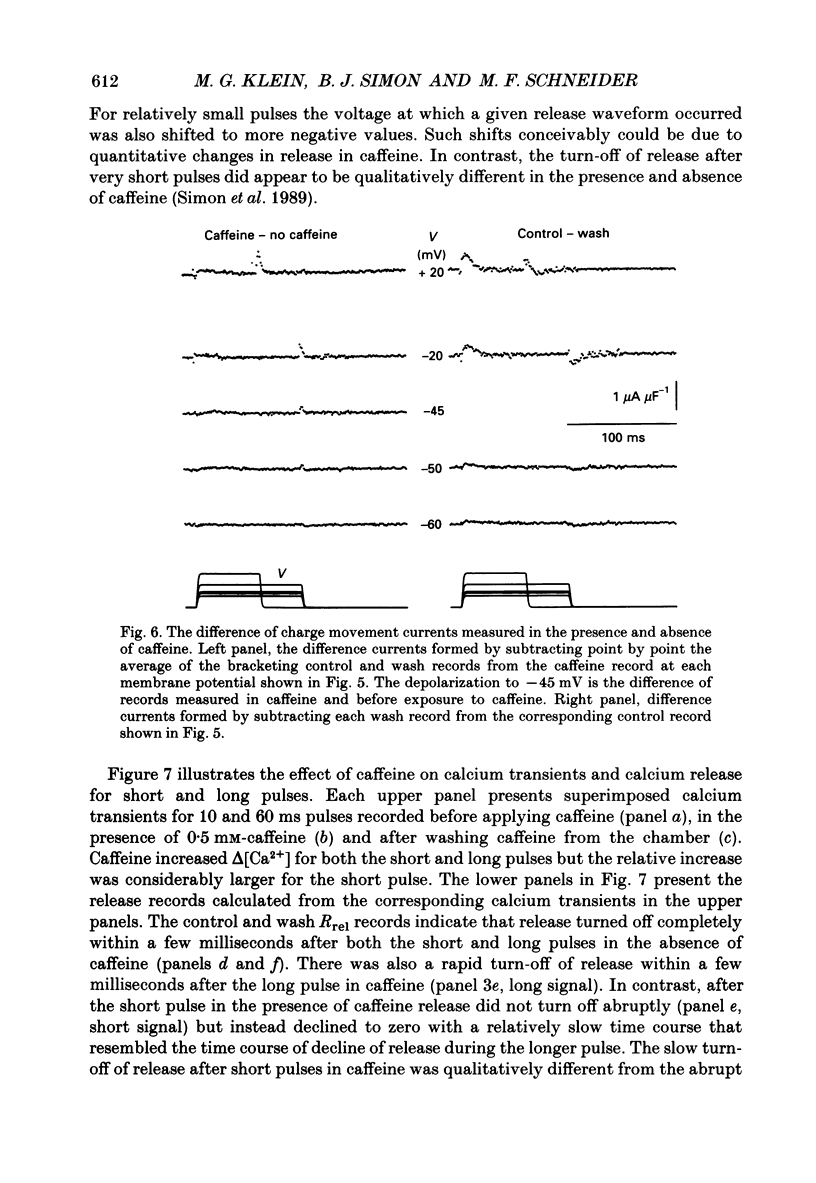
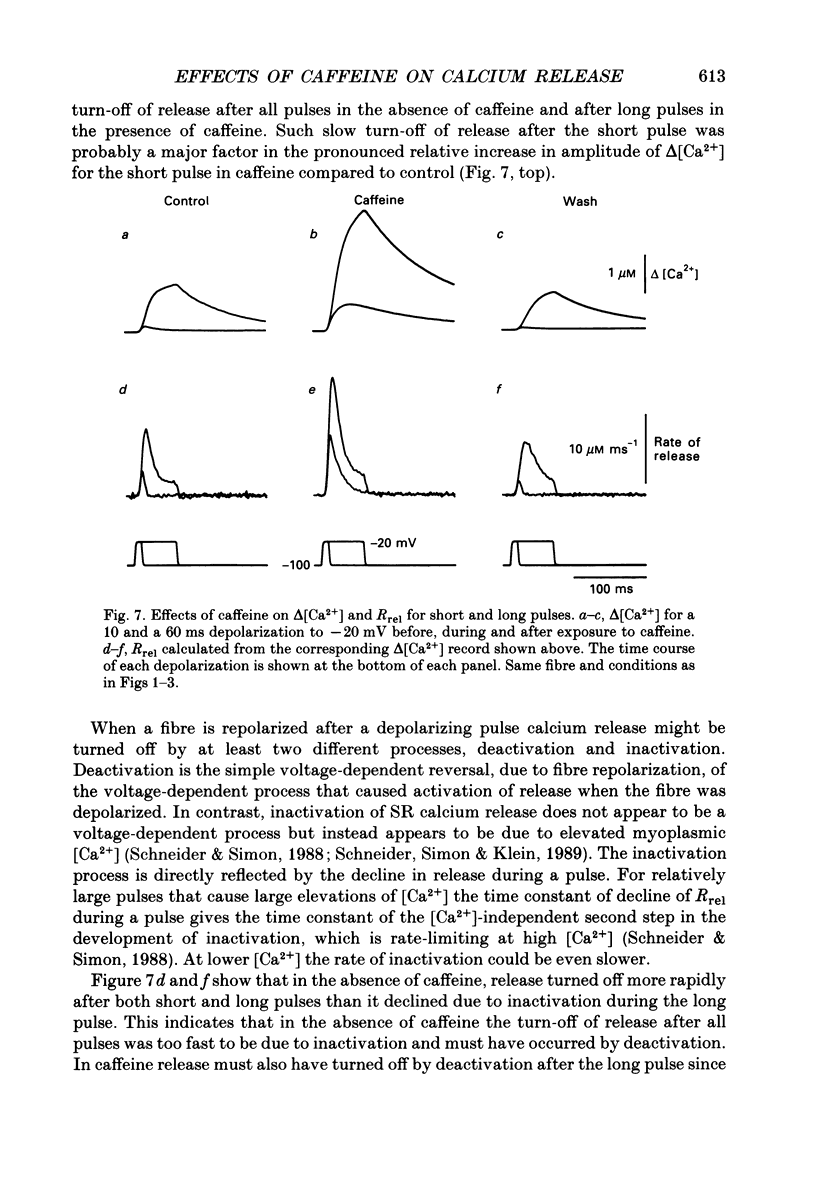

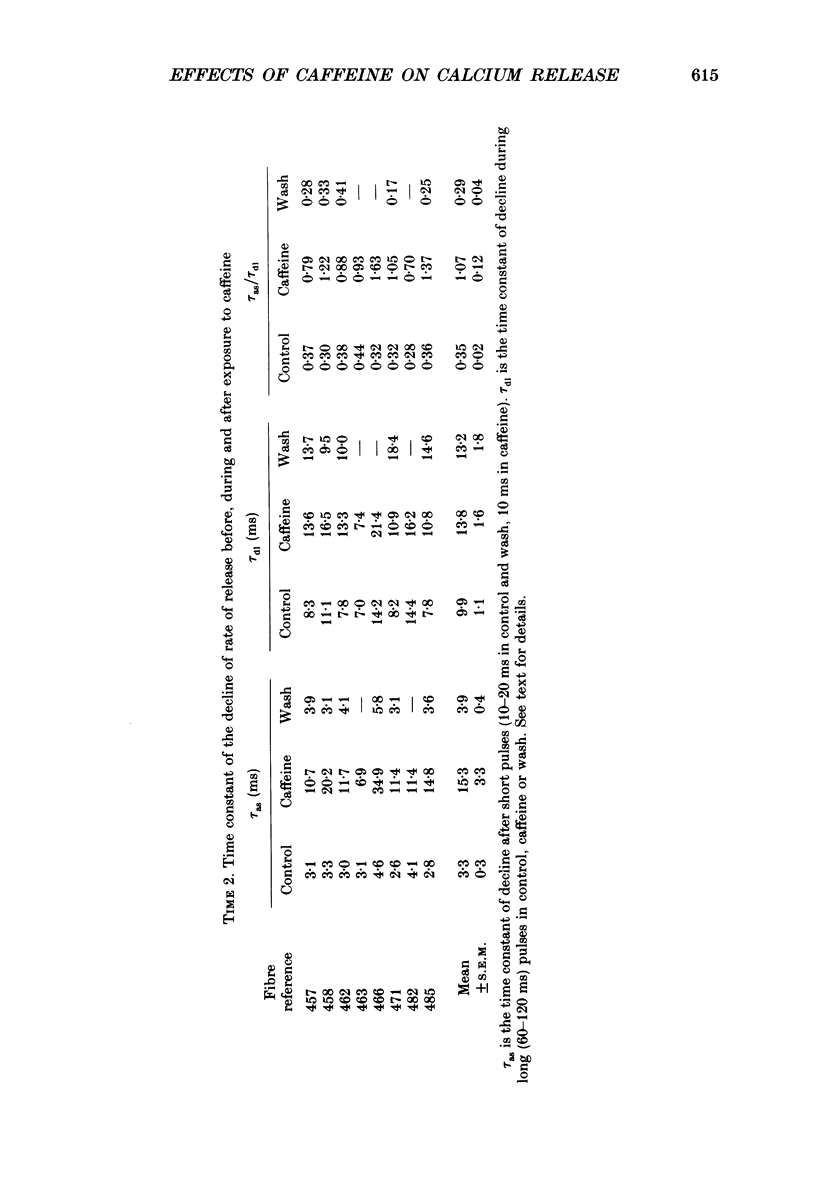
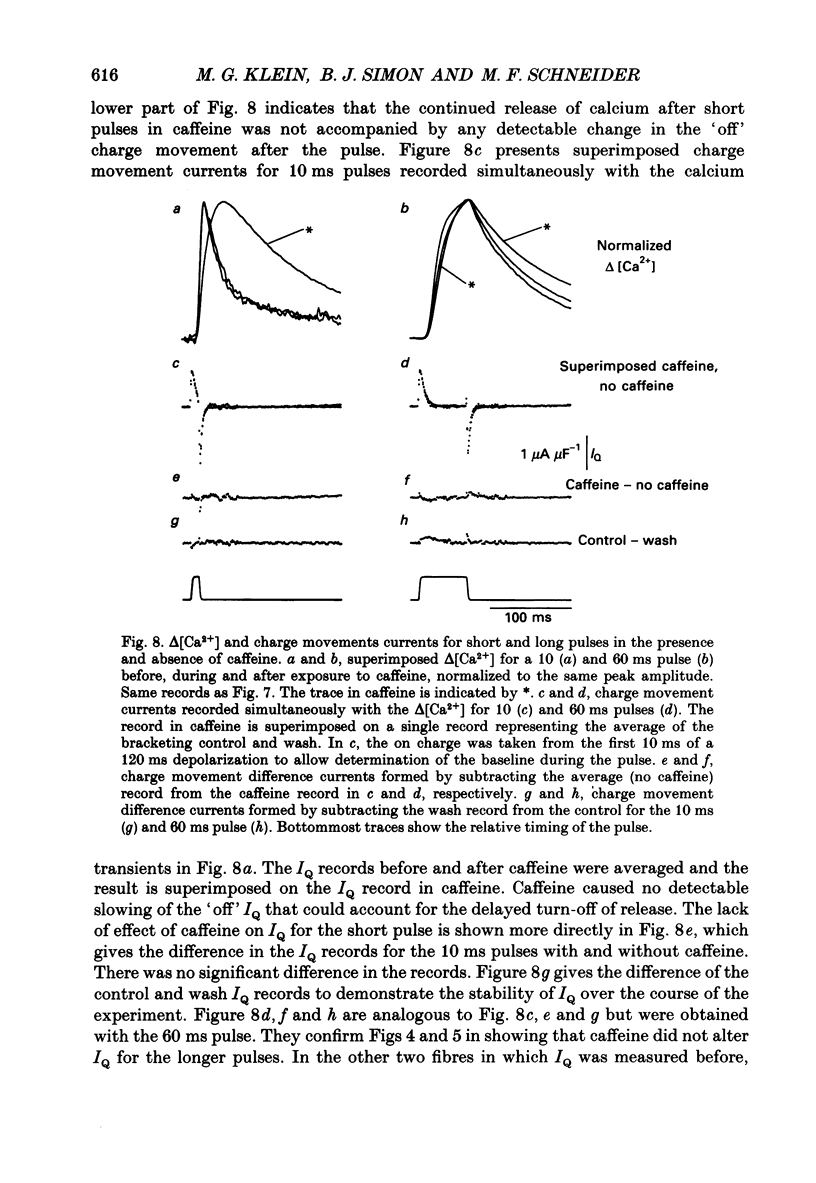

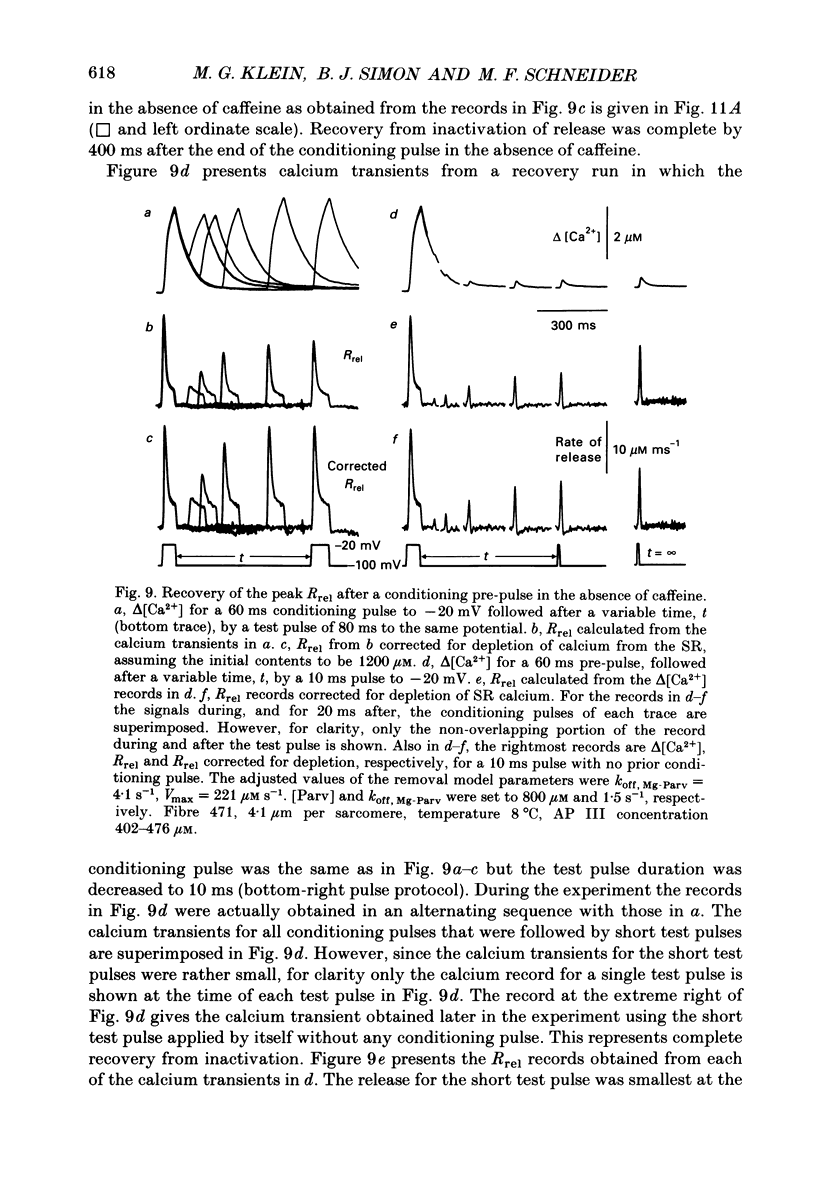
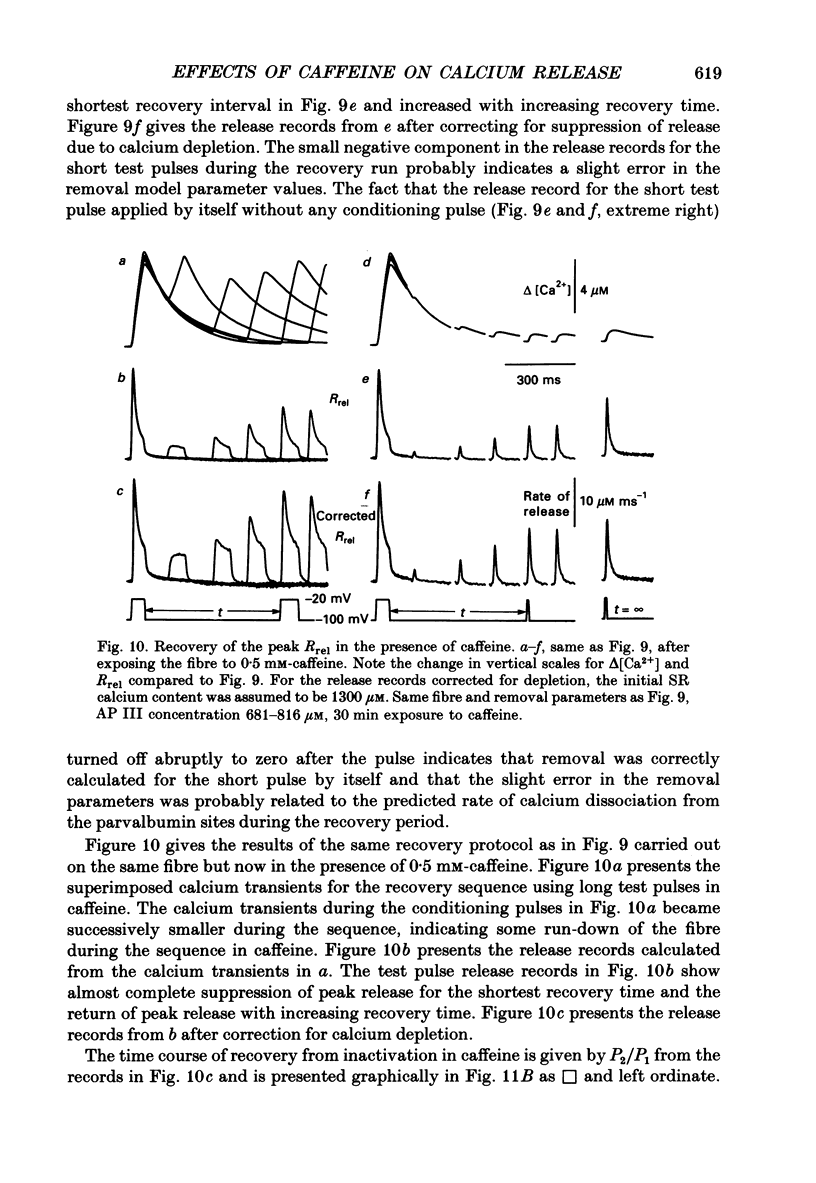
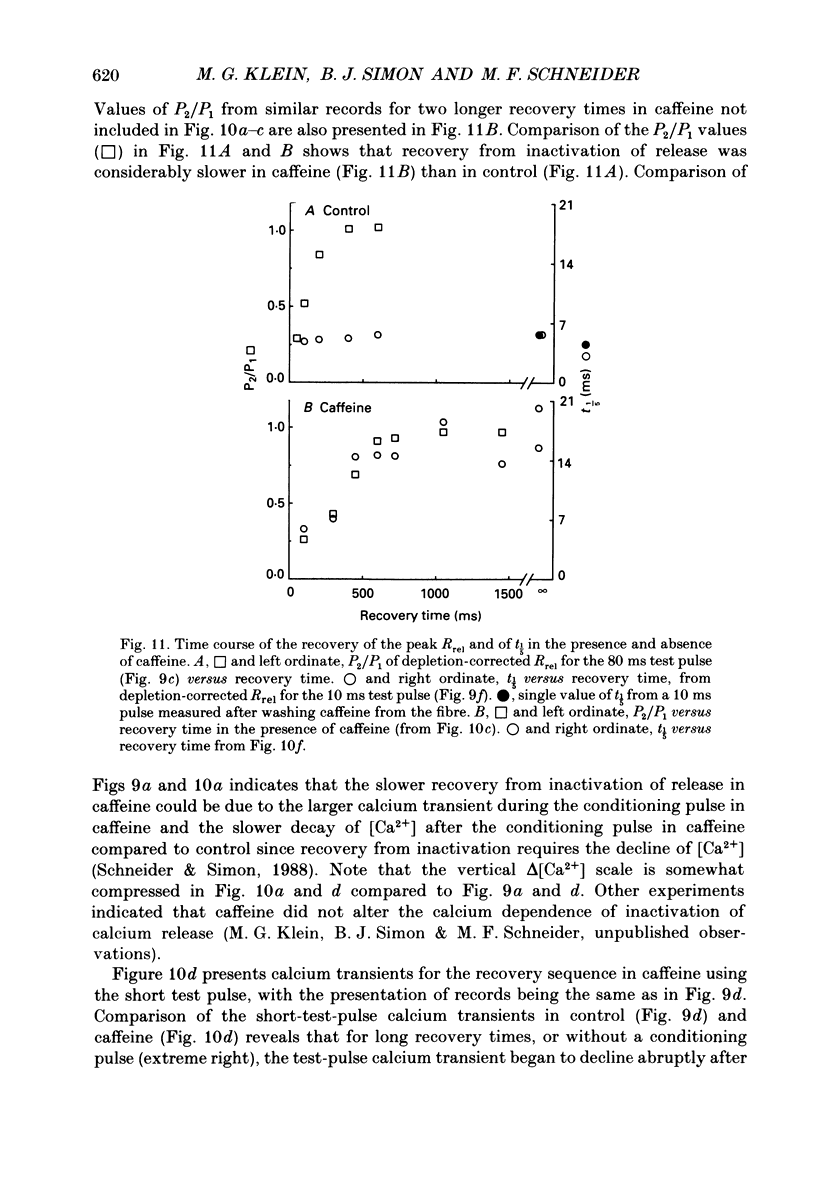






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batra S. The effects of drugs on calcium uptake and calcium release by mitochondria and sarcoplasmic reticulum of frog skeletal muscle. Biochem Pharmacol. 1974 Jan 1;23(1):89–101. doi: 10.1016/0006-2952(74)90316-5. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983 Nov;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Ríos E., Stéfani E. Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres. J Physiol. 1988 Apr;398:441–473. doi: 10.1113/jphysiol.1988.sp017052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Allen D. G. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J. 1984 May;45(5):913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay M., Ribalet B., Vergara J. Caffeine potentiation of calcium release in frog skeletal muscle fibres. J Physiol. 1986 Jun;375:535–559. doi: 10.1113/jphysiol.1986.sp016132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Determining the rate of calcium release from the sarcoplasmic reticulum in muscle fibers. Biophys J. 1987 Jun;51(6):1005–1007. doi: 10.1016/S0006-3495(87)83430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G. B. The current view of the source of trigger calcium in excitation-contraction coupling in vertebrate skeletal muscle. Biochem Pharmacol. 1980 Sep 15;29(18):2399–2406. doi: 10.1016/0006-2952(80)90341-x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Kim D. H., Ohnishi S. T., Ikemoto N. Kinetic studies of calcium release from sarcoplasmic reticulum in vitro. J Biol Chem. 1983 Aug 25;258(16):9662–9668. [PubMed] [Google Scholar]
- Klein M. G., Simon B. J., Szucs G., Schneider M. F. Simultaneous recording of calcium transients in skeletal muscle using high- and low-affinity calcium indicators. Biophys J. 1988 Jun;53(6):971–988. doi: 10.1016/S0006-3495(88)83178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Kurihara S. Effects of caffeine on intracellular calcium concentrations in frog skeletal muscle fibres. J Physiol. 1987 Feb;383:269–283. doi: 10.1113/jphysiol.1987.sp016408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs L., Rios E., Schneider M. F. Measurement and modification of free calcium transients in frog skeletal muscle fibres by a metallochromic indicator dye. J Physiol. 1983 Oct;343:161–196. doi: 10.1113/jphysiol.1983.sp014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Schneider M. F. Contractile activation by voltage clamp depolarization of cut skeletal muscle fibres. J Physiol. 1978 Apr;277:483–506. doi: 10.1113/jphysiol.1978.sp012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Szücs G. Effect of caffeine on intramembrane charge movement and calcium transients in cut skeletal muscle fibres of the frog. J Physiol. 1983 Aug;341:559–578. doi: 10.1113/jphysiol.1983.sp014824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López J. R., Alamo L., Caputo C., DiPolo R., Vergara S. Determination of ionic calcium in frog skeletal muscle fibers. Biophys J. 1983 Jul;43(1):1–4. doi: 10.1016/S0006-3495(83)84316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G., Darling E., Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986 Jan 14;25(1):236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Melzer W., Rios E., Schneider M. F. A general procedure for determining the rate of calcium release from the sarcoplasmic reticulum in skeletal muscle fibers. Biophys J. 1987 Jun;51(6):849–863. doi: 10.1016/S0006-3495(87)83413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Rios E., Schneider M. F. Time course of calcium release and removal in skeletal muscle fibers. Biophys J. 1984 Mar;45(3):637–641. doi: 10.1016/S0006-3495(84)84203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Ríos E., Schneider M. F. The removal of myoplasmic free calcium following calcium release in frog skeletal muscle. J Physiol. 1986 Mar;372:261–292. doi: 10.1113/jphysiol.1986.sp016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Schneider M. F., Simon B. J., Szucs G. Intramembrane charge movement and calcium release in frog skeletal muscle. J Physiol. 1986 Apr;373:481–511. doi: 10.1113/jphysiol.1986.sp016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H., Racker E. Mechanism of calcium release from skeletal sarcoplasmic reticulum. J Membr Biol. 1982;66(3):193–201. doi: 10.1007/BF01868494. [DOI] [PubMed] [Google Scholar]
- Ogawa Y. Some properties of fragmented frog sarcoplasmic reticulum with particular reference to its response to caffeine. J Biochem. 1970 May;67(5):667–683. doi: 10.1093/oxfordjournals.jbchem.a129295. [DOI] [PubMed] [Google Scholar]
- Palade P. Drug-induced Ca2+ release from isolated sarcoplasmic reticulum. I. Use of pyrophosphate to study caffeine-induced Ca2+ release. J Biol Chem. 1987 May 5;262(13):6135–6141. [PubMed] [Google Scholar]
- Rousseau E., Ladine J., Liu Q. Y., Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988 Nov 15;267(1):75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Rios E., Melzer W. Use of a metallochromic indicator to study intracellular calcium movements in skeletal muscle. Cell Calcium. 1985 Apr;6(1-2):109–118. doi: 10.1016/0143-4160(85)90038-7. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Simon B. J. Inactivation of calcium release from the sarcoplasmic reticulum in frog skeletal muscle. J Physiol. 1988 Nov;405:727–745. doi: 10.1113/jphysiol.1988.sp017358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Simon B. J., Szucs G. Depletion of calcium from the sarcoplasmic reticulum during calcium release in frog skeletal muscle. J Physiol. 1987 Nov;392:167–192. doi: 10.1113/jphysiol.1987.sp016775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon B. J., Klein M. G., Schneider M. F. Caffeine slows turn-off of calcium release in voltage clamped skeletal muscle fibers. Biophys J. 1989 Apr;55(4):793–797. doi: 10.1016/S0006-3495(89)82878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


