Abstract
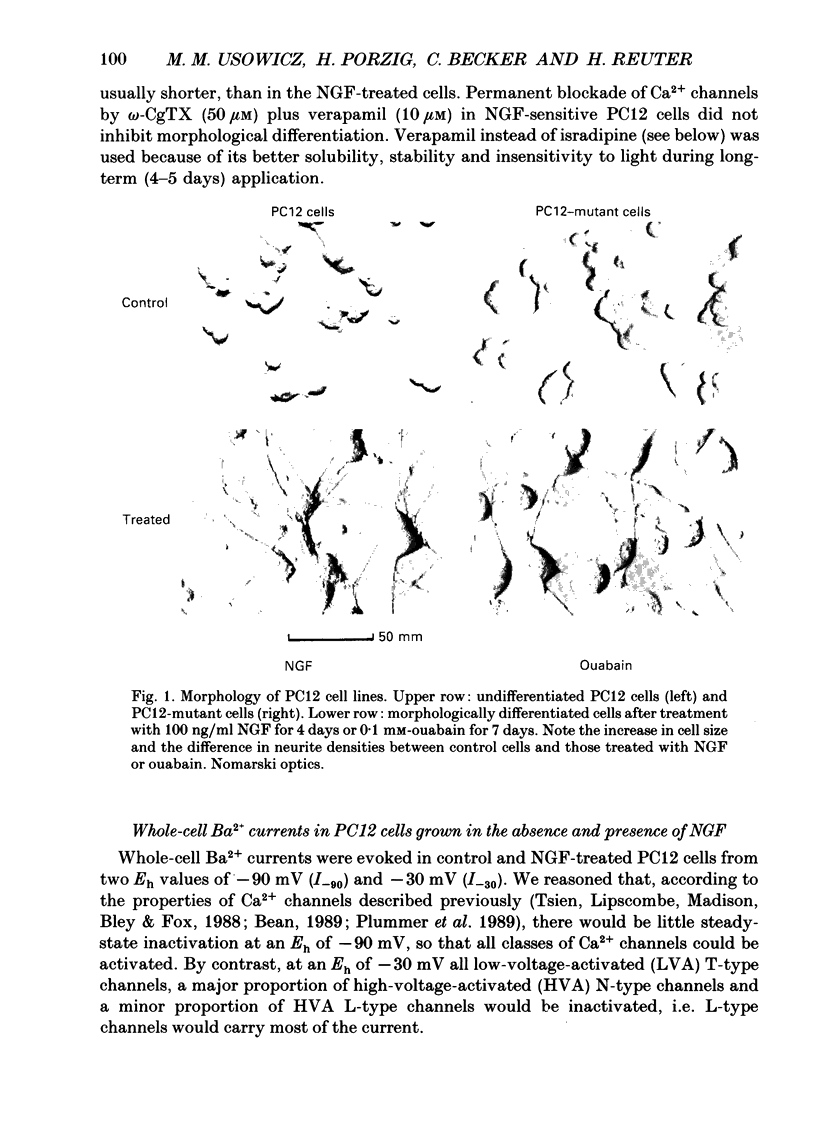
1. Two clones of rat phaeochromocytoma PC12 cells have been used to study the expression of Ca2+ channels and their possible involvement in neuronal differentiation. One clone differentiated morphologically when exposed to nerve growth factor (NGF) for 4 days (PC12 cells), while the other clone was insensitive to NGF, but differentiated morphologically in the presence of ouabain (0.1 mM) for 7 days (PC12-mutant cells). 2. Whole-cell Ba2+ currents through Ca2+ channels were measured in PC12 cells at a test potential (Et) of +10 mV, from two holding potentials (Eh) of -90 and -30 mV (I-90 and I-30). NGF-induced differentiation increased I-90 by 248% and I-30 by 133%. The cells that differentiate in the presence of ouabain had only small, if any, Ba2+ currents that did not appear to change during morphological differentiation or after the addition of NGF. 3. Barium currents in PC12 cells could be separated into two components by selective antagonists. The component of I-90 that could be inhibited by omega-conotoxin GVIA (omega-CgTX) in NGF-differentiated cells was 458 +/- 84 pA (mean +/- S.E.M.), compared with 79 +/- 44 pA in native cells. I-30 was reduced by 50 +/- 17 pA in NGF-treated cells and was virtually insensitive to the toxin in native cells. By contrast, the dihydropyridine (DHP) isradipine reduced I-30 in NGF-treated cells by 30 +/- 8 pA and in native cells by 20 +/- 3 pA. 4. Radioligand binding studies with 125I-omega-CgTX in PC12 cell membrane fragments and in PC12 cells showed a 2- to 3-fold increase in maximal binding capacity after NGF exposure, while mutant cells showed no such change in binding capacity after treatment with NGF or ouabain. Staurosporine inhibited the effect of NGF on 125I-omega-CgTX binding. [3H](+)-isradipine binding capacity was increased 1.8-fold by NGF in depolarized PC12 cells while no change was observed in mutant cells after NGF or ouabain. There was no interaction between omega-CgTX and DHP binding sites. 5. Both the electrophysiological and the binding data indicate a preferential expression of omega-CgTX-sensitive Ca2+ channels (N type) over isradipine-sensitive channels (L type) in PC12 cells treated with NGF. By contrast, ouabain-induced differentiation of a mutant PC12 cell line, that lacks functional NFG receptors, was not associated with the expression of Ca2+ channels.
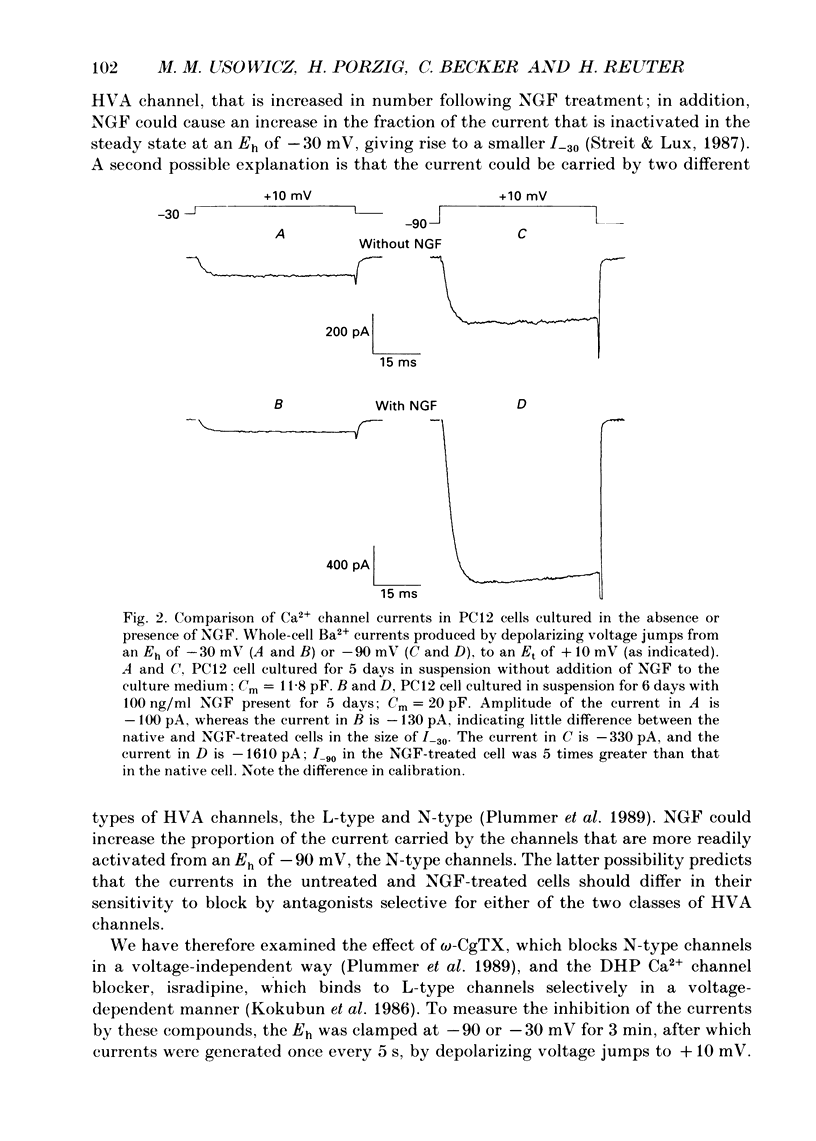
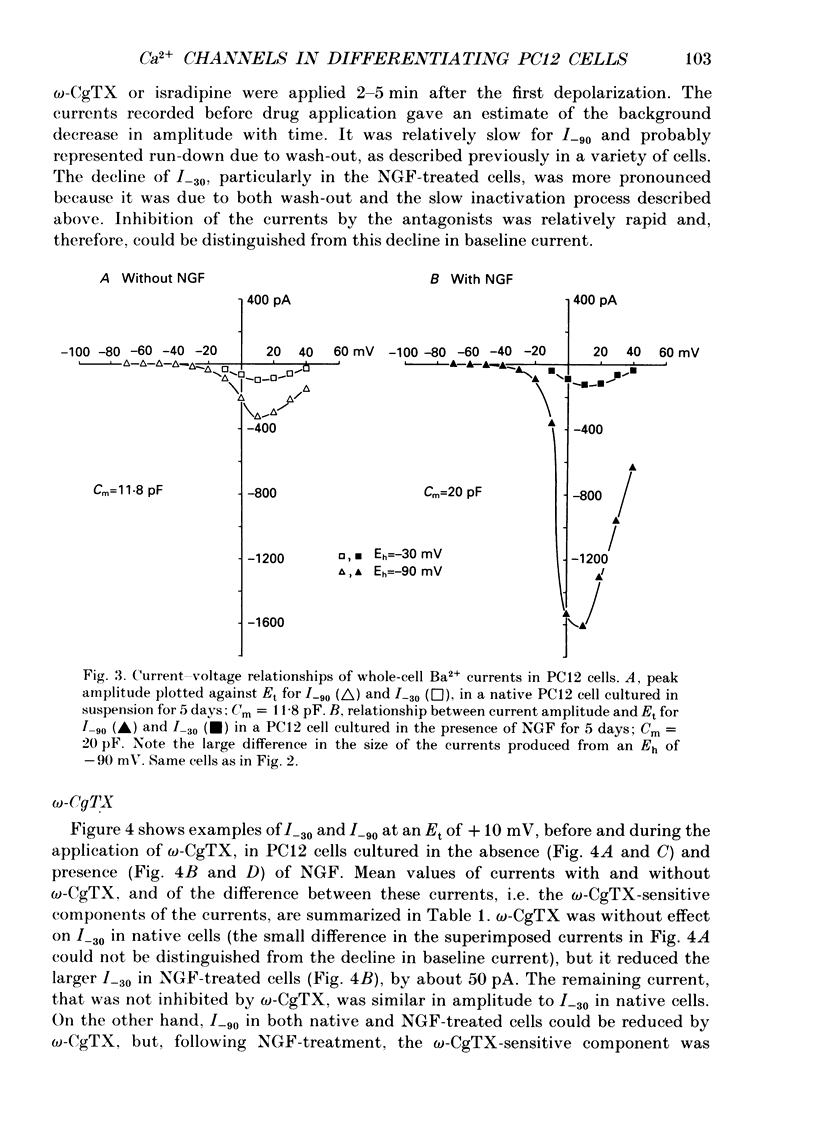
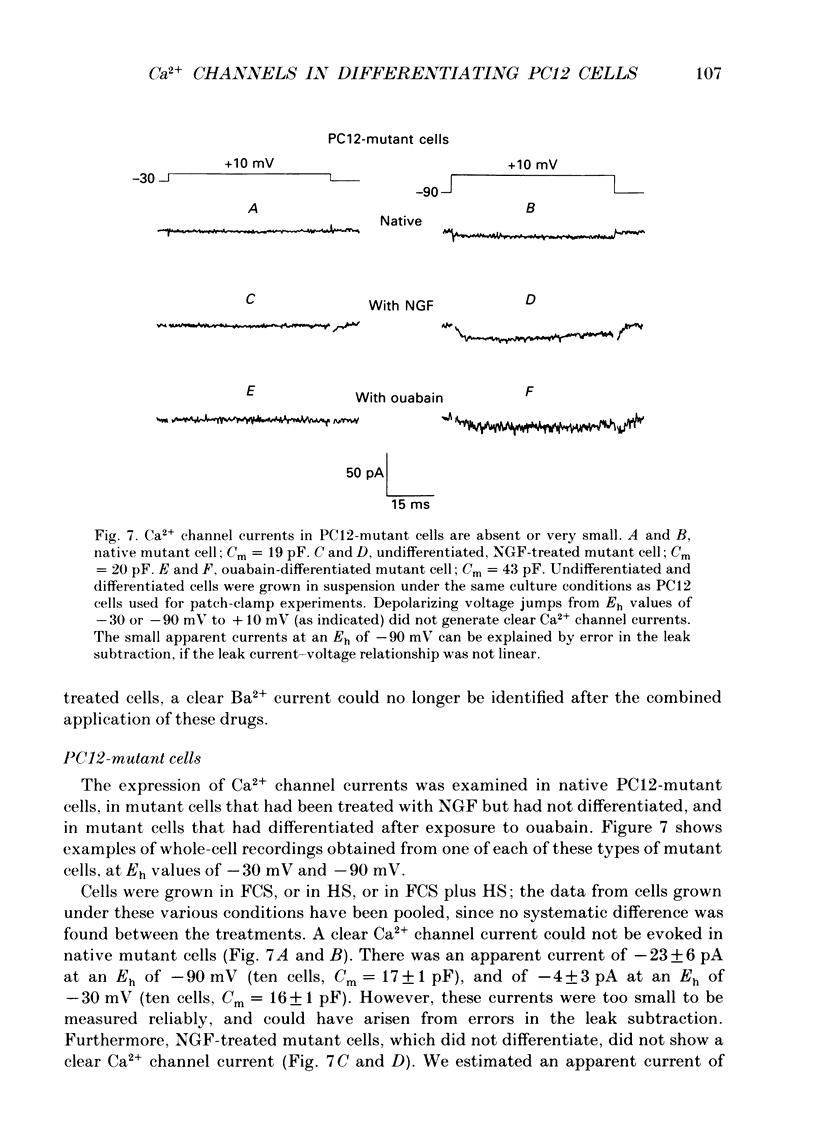
Full text
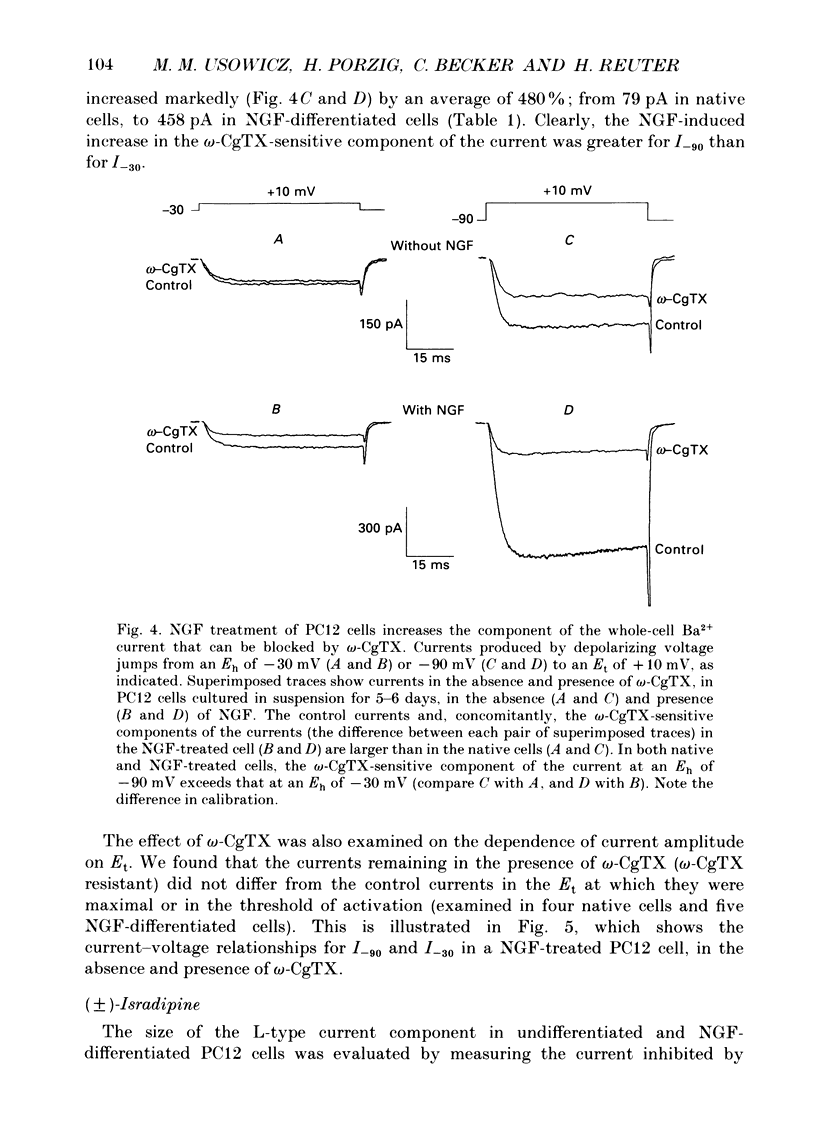
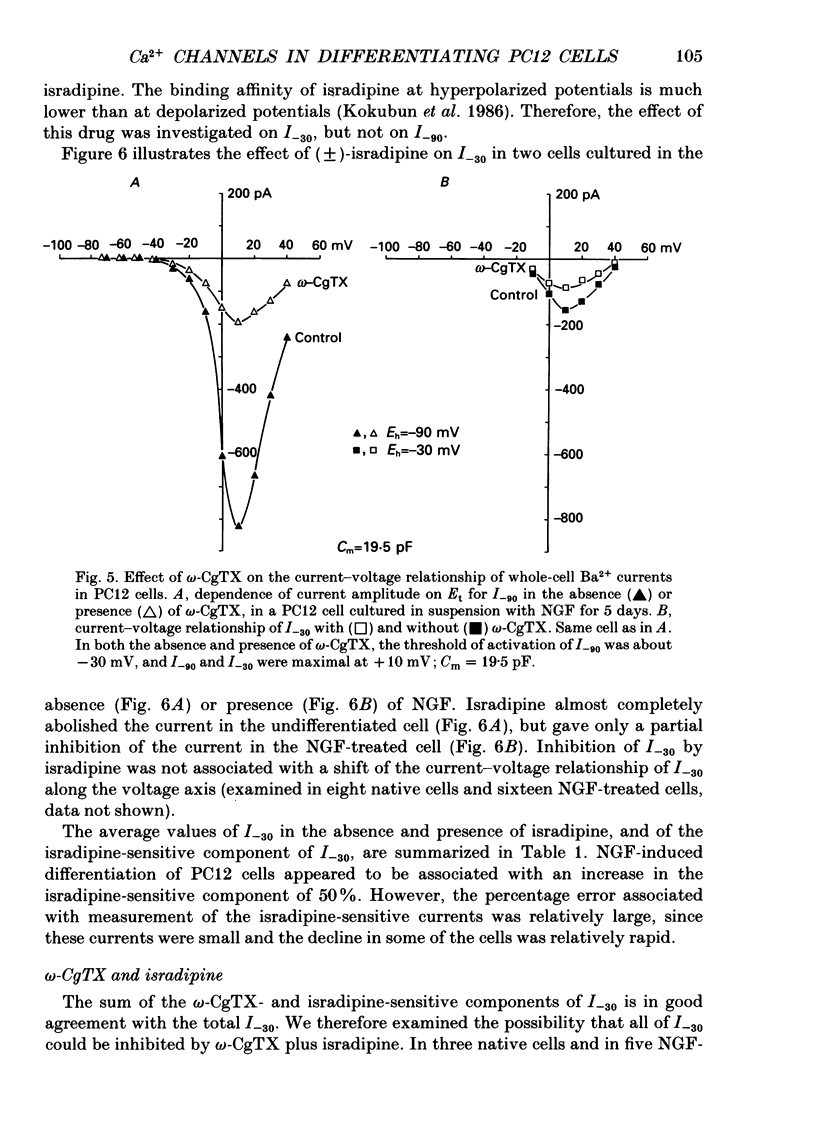
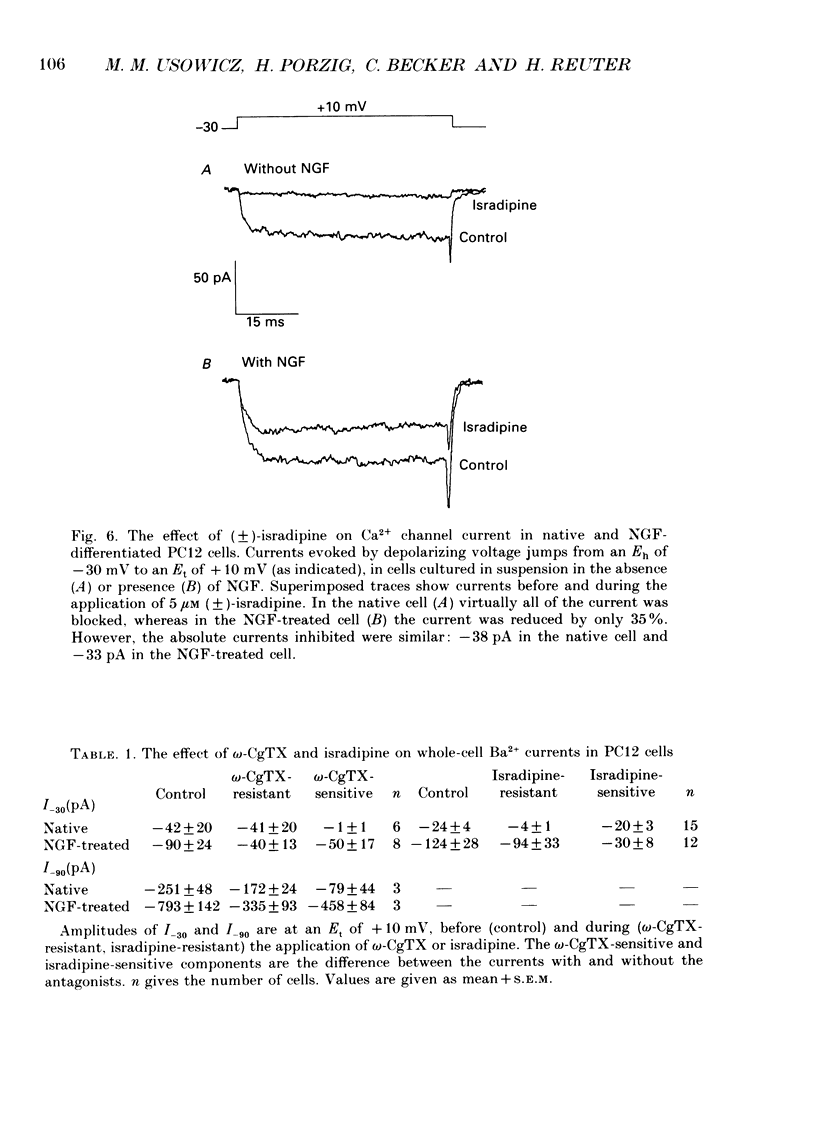
PDF





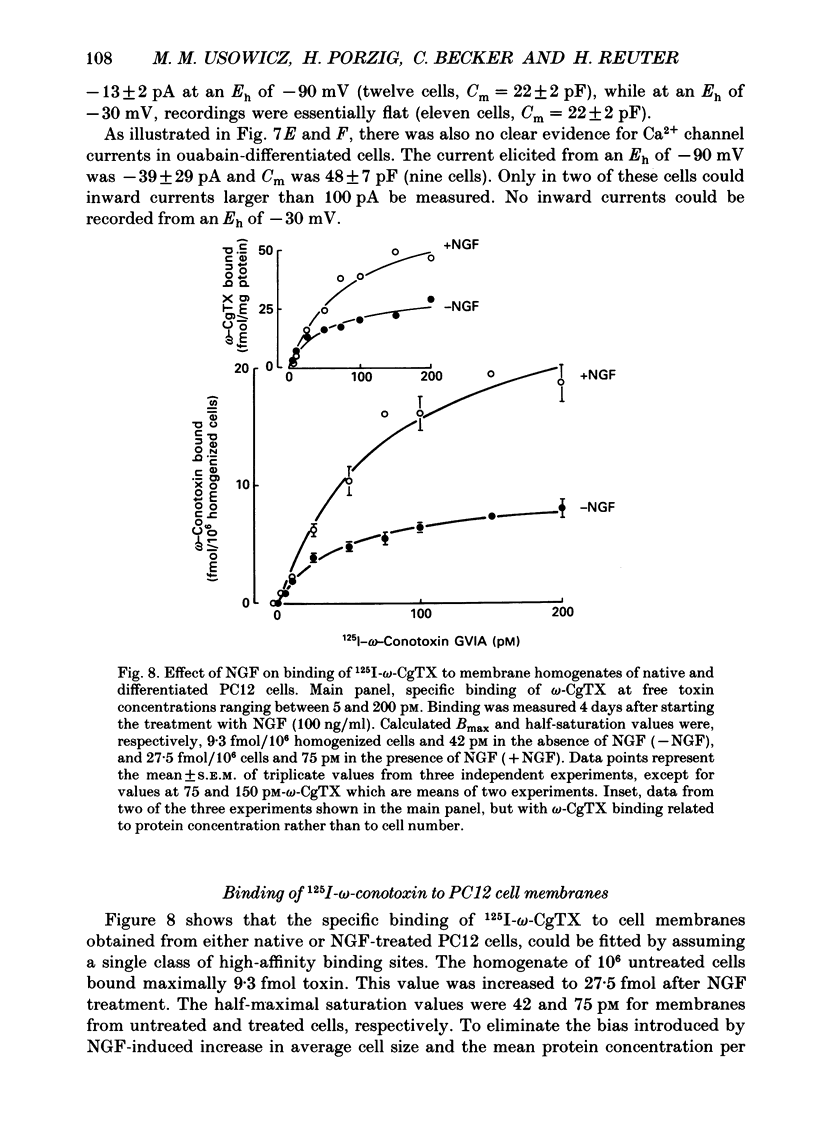

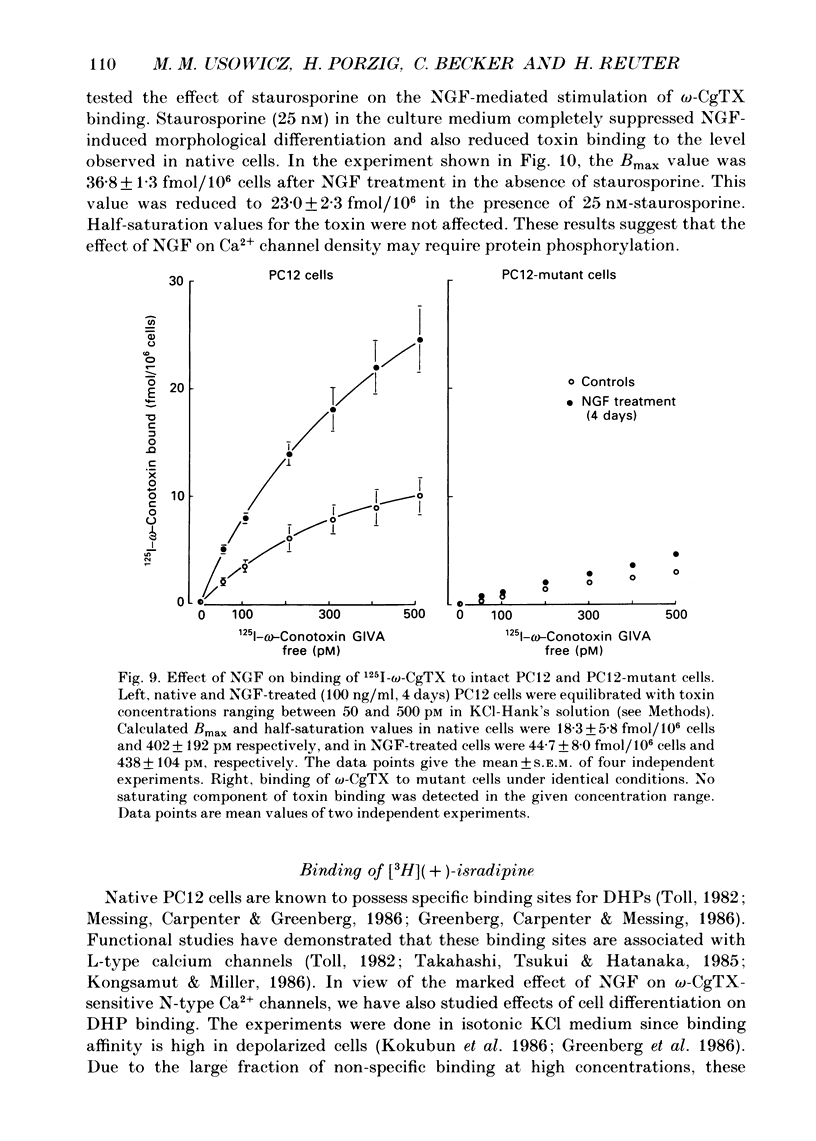
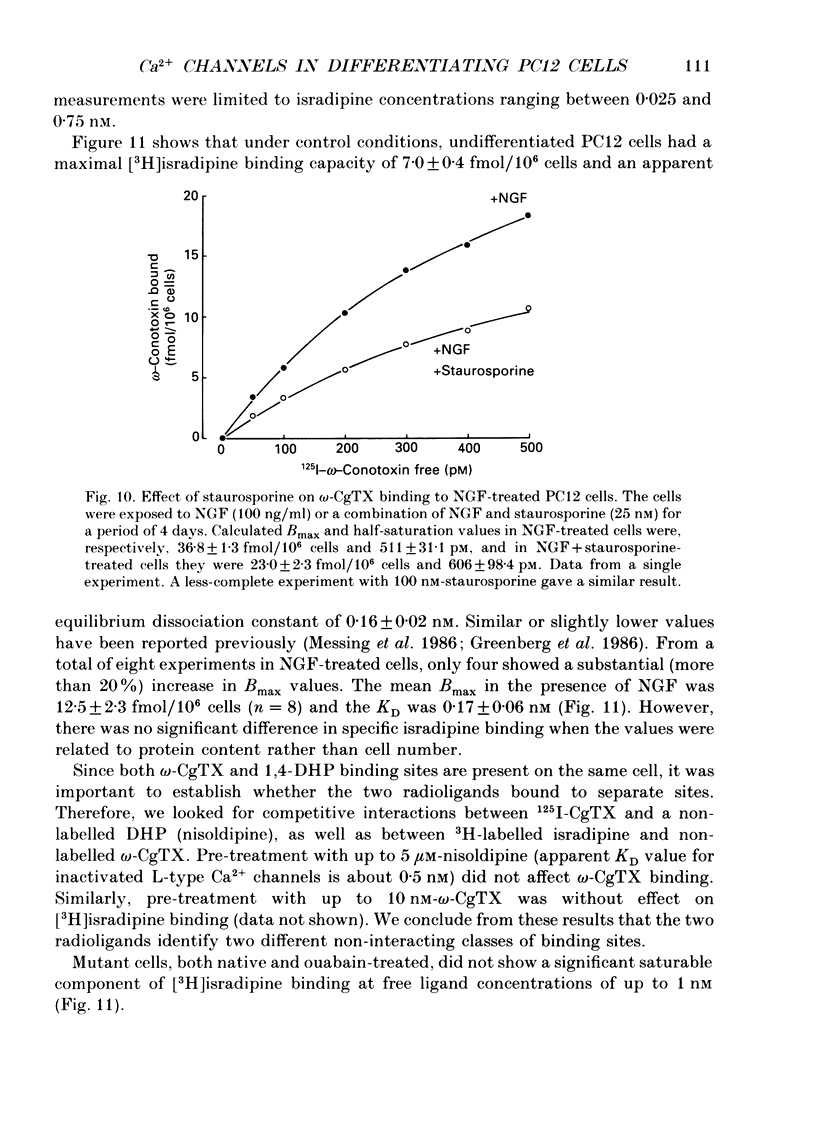
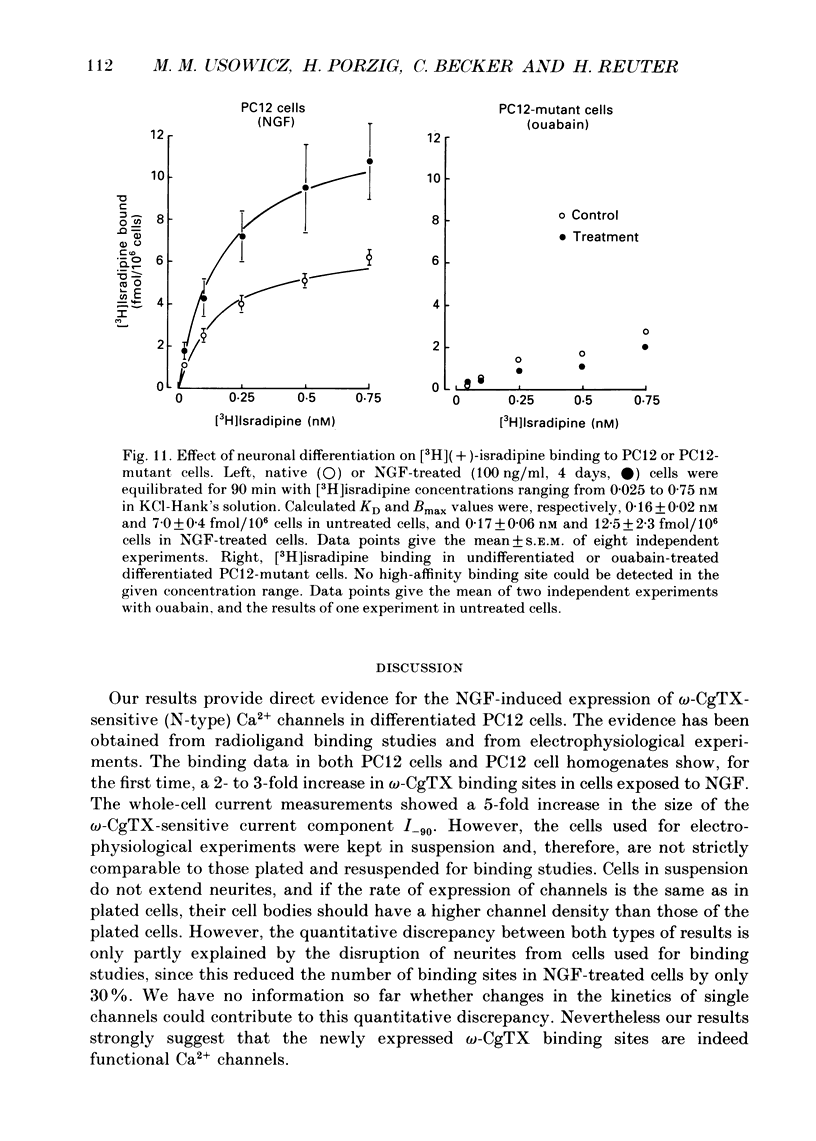




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Koyano K., Saisu H., Nishiuchi Y., Sakakibara S. Binding of omega-conotoxin to receptor sites associated with the voltage-sensitive calcium channel. Neurosci Lett. 1986 Nov 11;71(2):203–208. doi: 10.1016/0304-3940(86)90559-8. [DOI] [PubMed] [Google Scholar]
- Anglister L., Farber I. C., Shahar A., Grinvald A. Localization of voltage-sensitive calcium channels along developing neurites: their possible role in regulating neurite elongation. Dev Biol. 1982 Dec;94(2):351–365. doi: 10.1016/0012-1606(82)90353-0. [DOI] [PubMed] [Google Scholar]
- Bernstein A., Hunt D. M., Crichley V., Mak T. W. Induction by ouabain of hemoglobin synthesis in cultured Friend erythroleukemic cells. Cell. 1976 Nov;9(3):375–381. doi: 10.1016/0092-8674(76)90082-9. [DOI] [PubMed] [Google Scholar]
- Garber S. S., Hoshi T., Aldrich R. W. Regulation of ionic currents in pheochromocytoma cells by nerve growth factor and dexamethasone. J Neurosci. 1989 Nov;9(11):3976–3987. doi: 10.1523/JNEUROSCI.09-11-03976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. L., King V. F., Siegl P. K., Reuben J. P., Kaczorowski G. J. Binding of Ca2+ entry blockers to cardiac sarcolemmal membrane vesicles. Characterization of diltiazem-binding sites and their interaction with dihydropyridine and aralkylamine receptors. J Biol Chem. 1986 Jun 25;261(18):8146–8157. [PubMed] [Google Scholar]
- Greenberg D. A., Carpenter C. L., Messing R. O. Depolarization-dependent binding of the calcium channel antagonist, (+)-[3H]PN200-110, to intact cultured PC12 cells. J Pharmacol Exp Ther. 1986 Sep;238(3):1021–1027. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. II. Inactivation. J Gen Physiol. 1989 Jul;94(1):169–182. doi: 10.1085/jgp.94.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater S. B., Mattson M. P., Cohan C., Connor J. Calcium regulation of the neuronal growth cone. Trends Neurosci. 1988 Jul;11(7):315–321. doi: 10.1016/0166-2236(88)90094-x. [DOI] [PubMed] [Google Scholar]
- Knaus H. G., Striessnig J., Koza A., Glossmann H. Neurotoxic aminoglycoside antibiotics are potent inhibitors of [125I]-Omega-Conotoxin GVIA binding to guinea-pig cerebral cortex membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987 Nov;336(5):583–586. doi: 10.1007/BF00169318. [DOI] [PubMed] [Google Scholar]
- Koike T., Takashima A. Clonal variability of PC12 pheochromocytoma cells with respect to catecholamine biosynthesis. J Neurochem. 1984 May;42(5):1472–1475. doi: 10.1111/j.1471-4159.1984.tb02812.x. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Contreras M. L., Matsuda Y., Hama T., Lazarovici P., Guroff G. K-252a: a specific inhibitor of the action of nerve growth factor on PC 12 cells. J Neurosci. 1988 Feb;8(2):715–721. doi: 10.1523/JNEUROSCI.08-02-00715.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubun S., Prod'hom B., Becker C., Porzig H., Reuter H. Studies on Ca channels in intact cardiac cells: voltage-dependent effects and cooperative interactions of dihydropyridine enantiomers. Mol Pharmacol. 1986 Dec;30(6):571–584. [PubMed] [Google Scholar]
- Kongsamut S., Miller R. J. Nerve growth factor modulates the drug sensitivity of neurotransmitter release from PC-12 cells. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2243–2247. doi: 10.1073/pnas.83.7.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi A., Biocca S., Cattaneo A., Calissano P. The mode of action of nerve growth factor in PC12 cells. Mol Neurobiol. 1988 Fall;2(3):201–226. doi: 10.1007/BF02935346. [DOI] [PubMed] [Google Scholar]
- Mandel G., Cooperman S. S., Maue R. A., Goodman R. H., Brehm P. Selective induction of brain type II Na+ channels by nerve growth factor. Proc Natl Acad Sci U S A. 1988 Feb;85(3):924–928. doi: 10.1073/pnas.85.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Moutot N., Marqueze B., Azais F., Seagar M., Couraud F. Properties of the calcium channel associated omega-conotoxin receptor in rat brain. Ann N Y Acad Sci. 1989;560:53–55. doi: 10.1111/j.1749-6632.1989.tb24078.x. [DOI] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing R. O., Carpenter C. L., Greenberg D. A. Inhibition of calcium flux and calcium channel antagonist binding in the PC12 neural cell line by phorbol esters and protein kinase C. Biochem Biophys Res Commun. 1986 May 14;136(3):1049–1056. doi: 10.1016/0006-291x(86)90439-0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Wagner J. A., Snyder S. H., Thayer S. A., Olivera B. M., Miller R. J. Brain voltage-sensitive calcium channel subtypes differentiated by omega-conotoxin fraction GVIA. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8804–8807. doi: 10.1073/pnas.83.22.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B., Kirschenbaum B., Rukenstein A., Greene L. A. Nerve growth factor increases the number of functional Na channels and induces TTX-resistant Na channels in PC12 pheochromocytoma cells. J Neurosci. 1987 Jun;7(6):1613–1625. doi: 10.1523/JNEUROSCI.07-06-01613.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Whitlock C., Stallcup W. Alterations in the surface properties of cells responsive to nerve growth factor. Nature. 1978 Jun 29;273(5665):718–723. doi: 10.1038/273718a0. [DOI] [PubMed] [Google Scholar]
- Seifter J. L., Aronson P. S. Properties and physiologic roles of the plasma membrane sodium-hydrogen exchanger. J Clin Invest. 1986 Oct;78(4):859–864. doi: 10.1172/JCI112671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher E., Pandiella A., Clementi F. Omega-conotoxin binding and effects on calcium channel function in human neuroblastoma and rat pheochromocytoma cell lines. FEBS Lett. 1988 Aug 1;235(1-2):178–182. doi: 10.1016/0014-5793(88)81258-4. [DOI] [PubMed] [Google Scholar]
- Streit J., Lux H. D. Distribution of calcium currents in sprouting PC12 cells. J Neurosci. 1989 Dec;9(12):4190–4199. doi: 10.1523/JNEUROSCI.09-12-04190.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit J., Lux H. D. Voltage dependent calcium currents in PC12 growth cones and cells during NGF-induced cell growth. Pflugers Arch. 1987 May;408(6):634–641. doi: 10.1007/BF00581167. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Tsukui H., Hatanaka H. Neuronal differentiation of Ca2+ channel by nerve growth factor. Brain Res. 1985 Aug 26;341(2):381–384. doi: 10.1016/0006-8993(85)91079-0. [DOI] [PubMed] [Google Scholar]
- Toll L. Calcium antagonists High-affinity binding and inhibition of calcium transport in a clonal cell line. J Biol Chem. 1982 Nov 25;257(22):13189–13192. [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Wagner J. A., Snowman A. M., Biswas A., Olivera B. M., Snyder S. H. Omega-conotoxin GVIA binding to a high-affinity receptor in brain: characterization, calcium sensitivity, and solubilization. J Neurosci. 1988 Sep;8(9):3354–3359. doi: 10.1523/JNEUROSCI.08-09-03354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



