Abstract
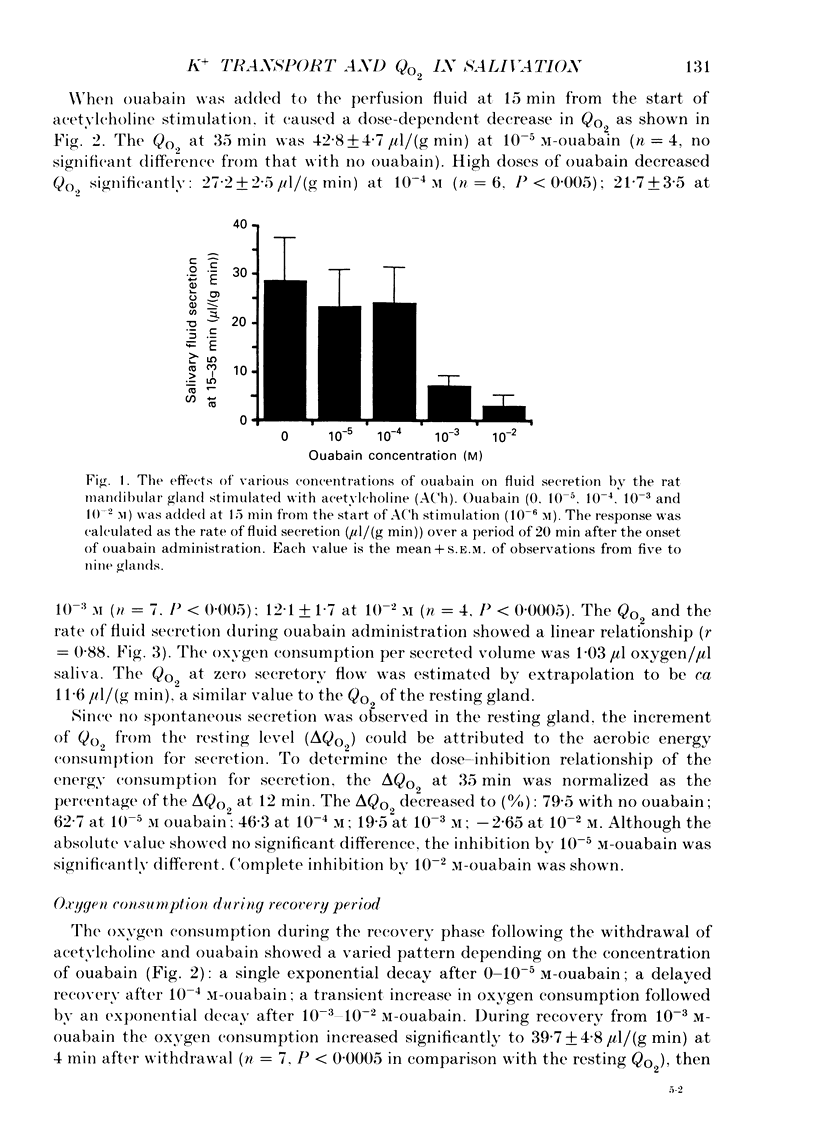
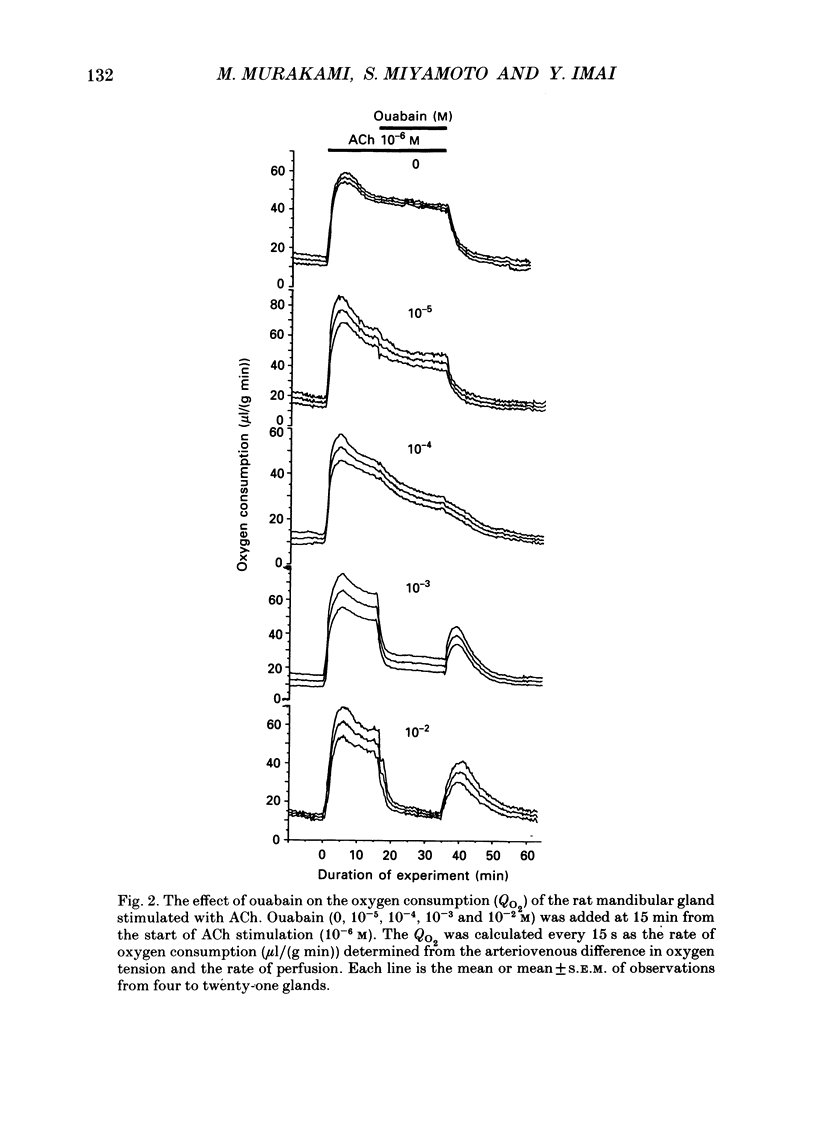
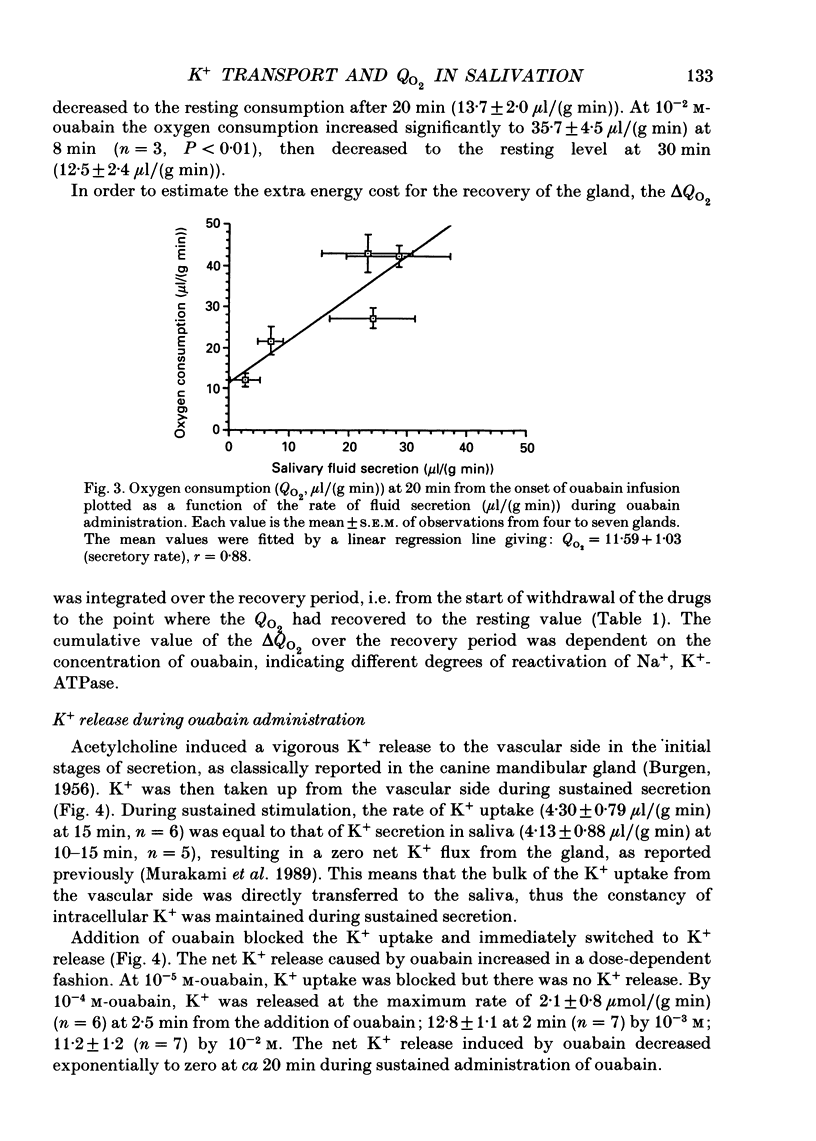
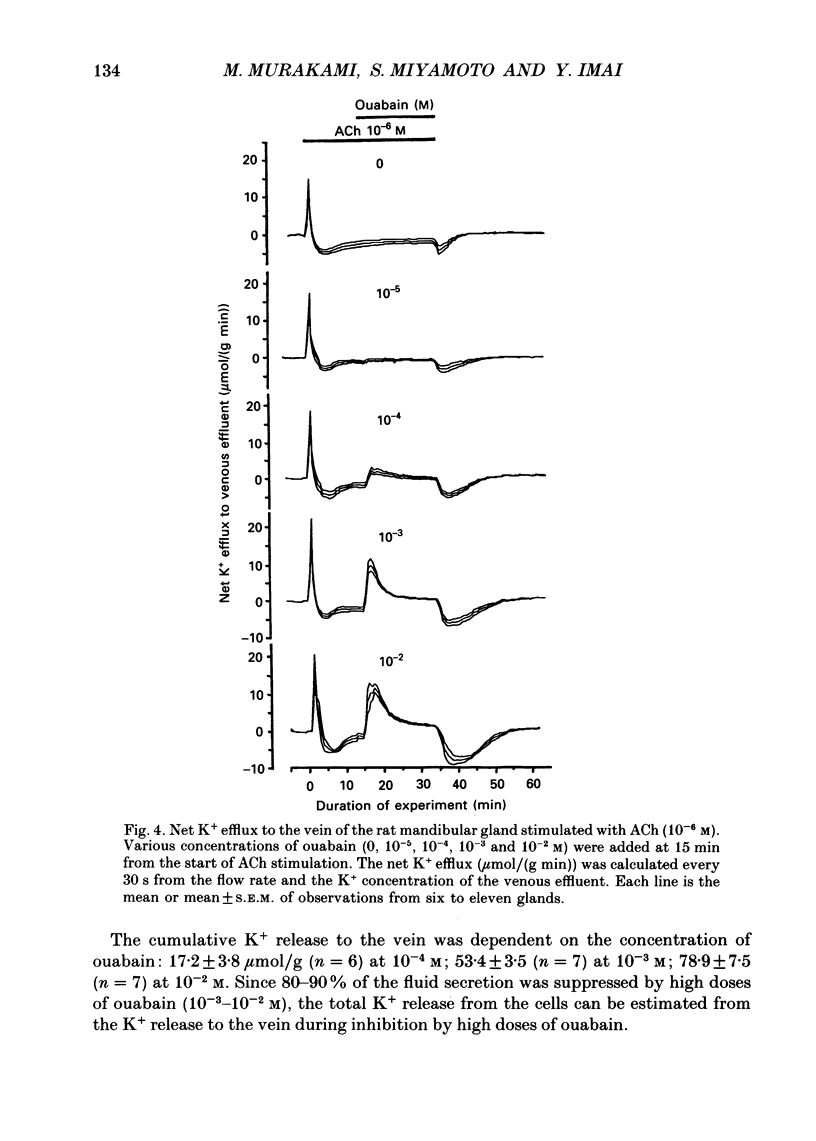
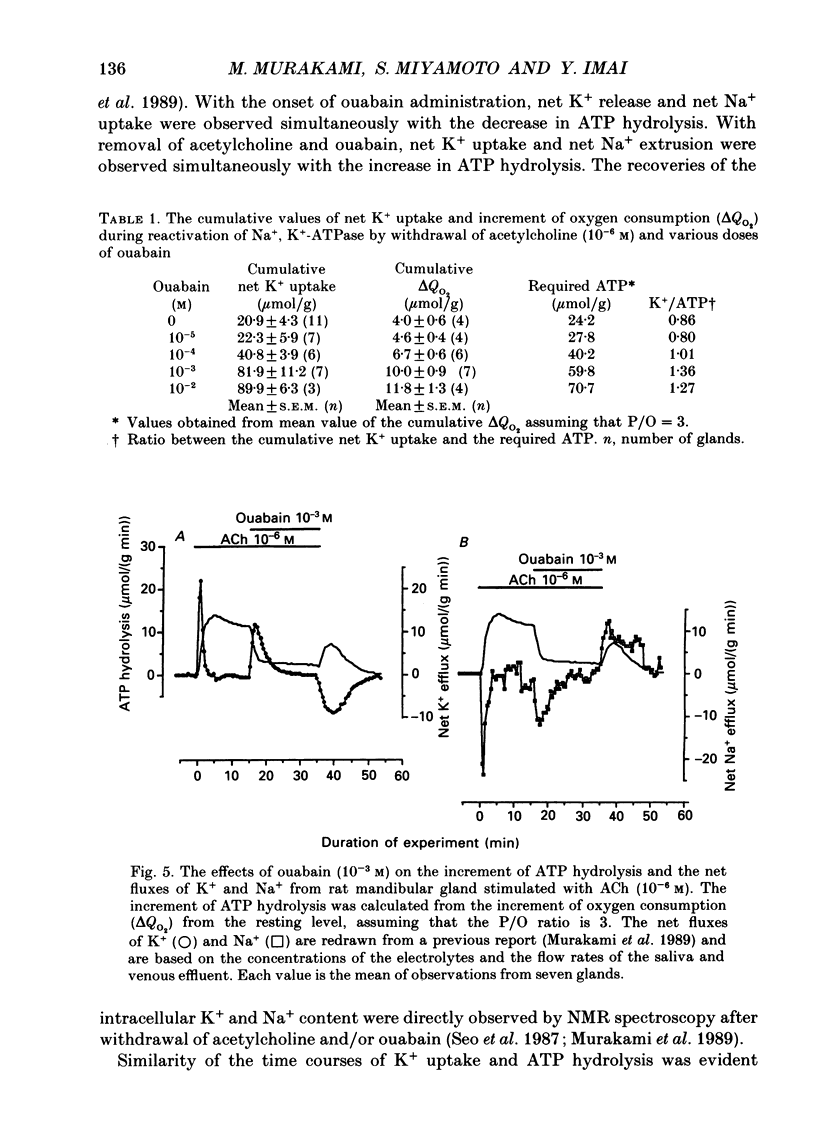
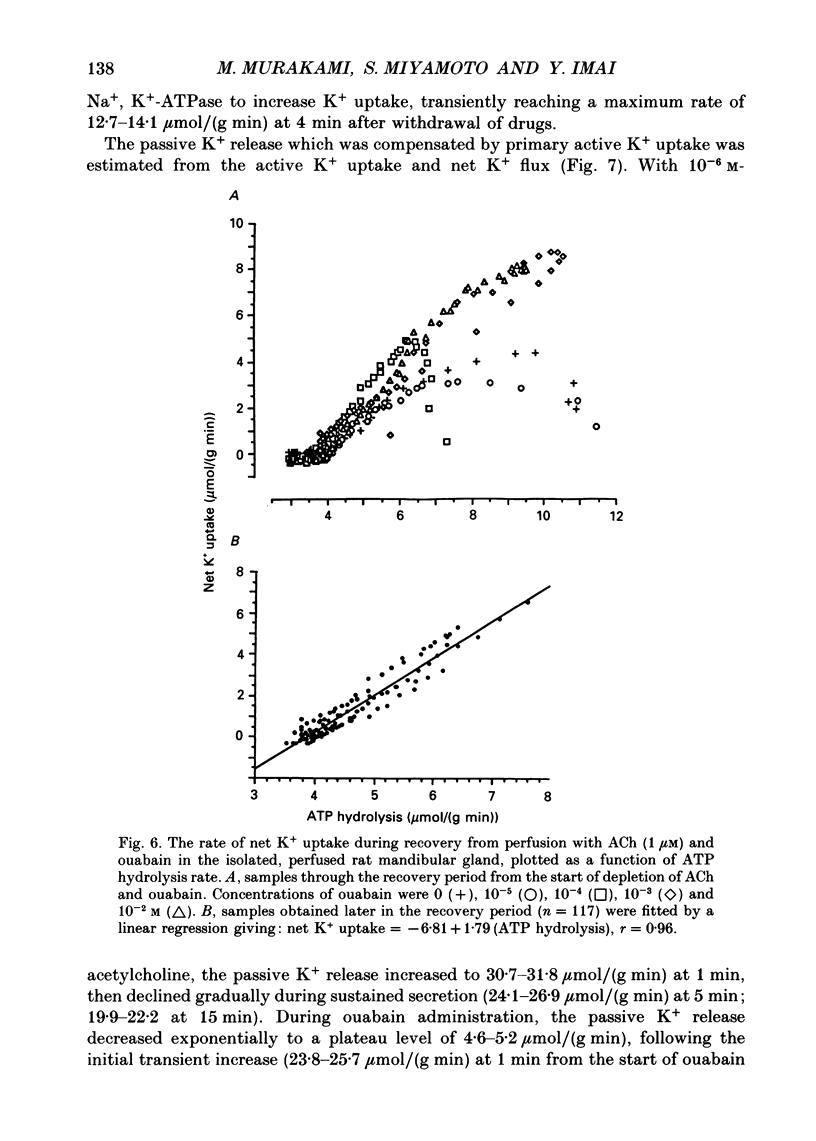
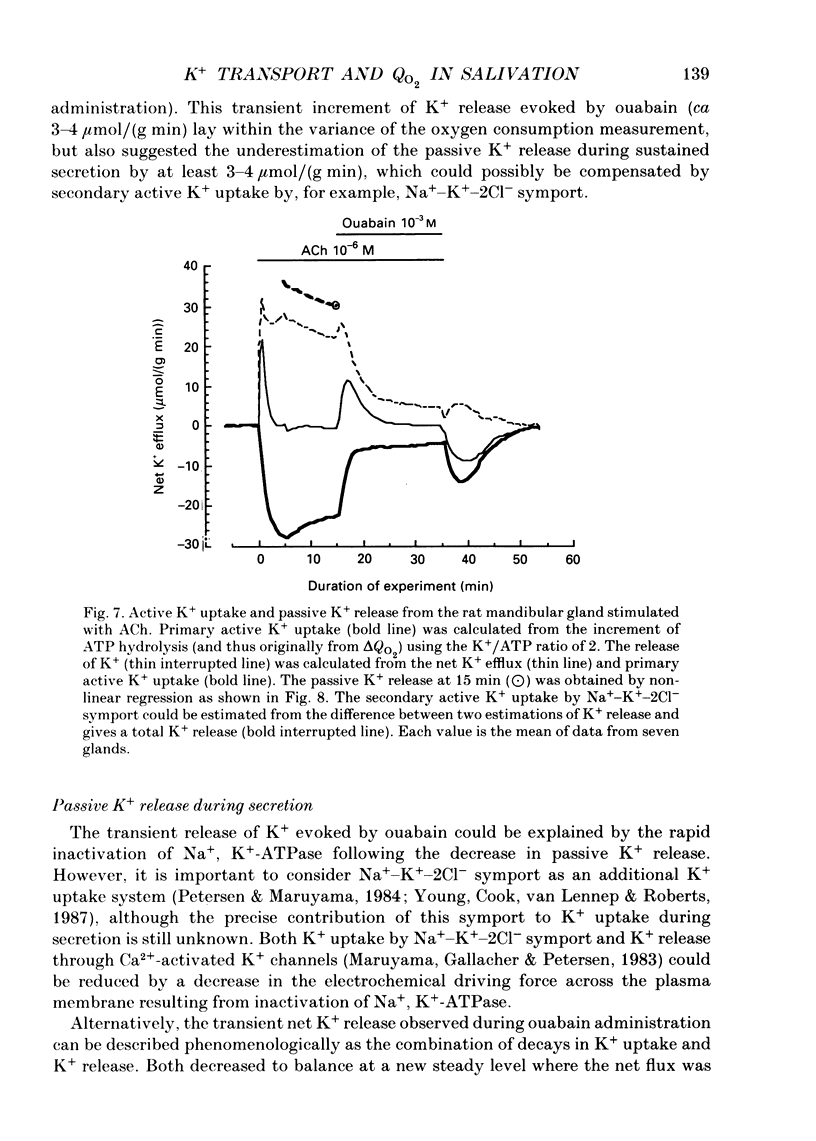
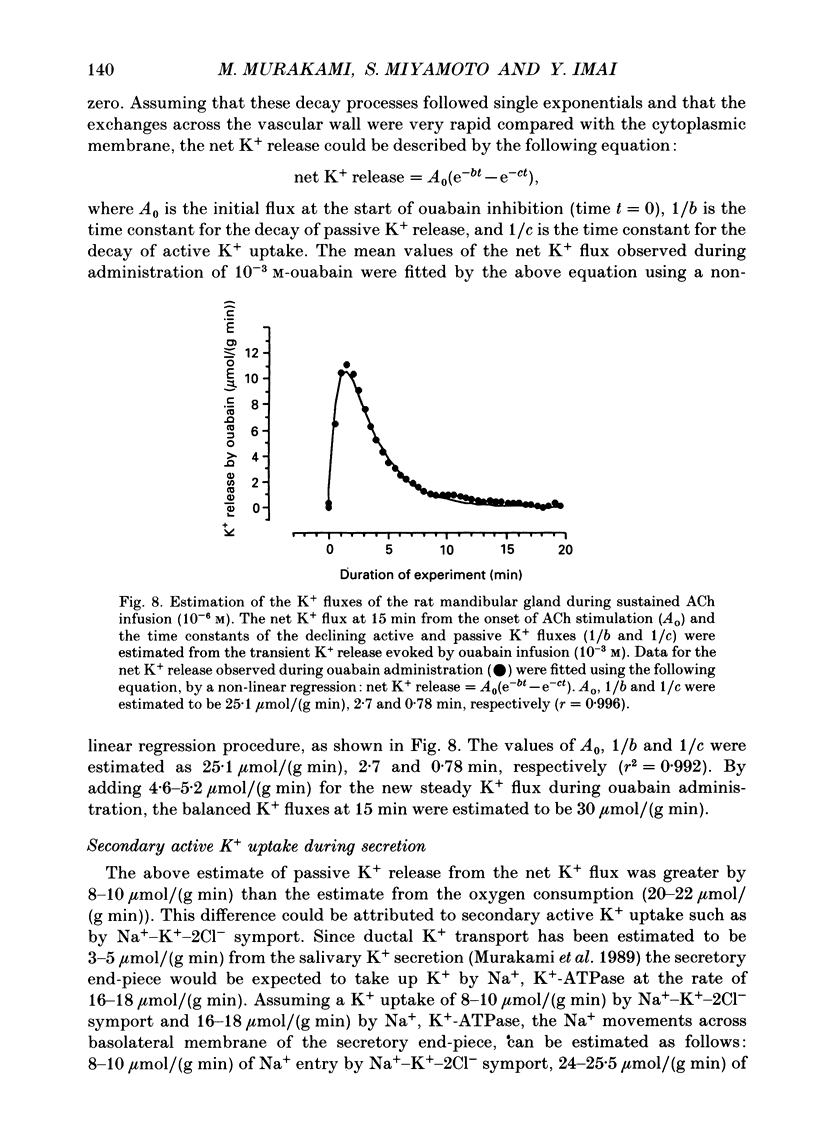
1. In order to determine the stoichiometry of K+ uptake and ATP consumption by Na+, K(+)-ATPase in the isolated, perfused mandibular gland of the rat, oxygen consumption and net K+ uptake from the vascular side were measured during the recovery period following K+ depletion by stimulation with acetylcholine in combination with ouabain. 2. Acetylcholine (10(-6) M) induced fluid secretion and an initial, transient release of K+ from the gland. Addition of ouabain suppressed salivary fluid secretion and caused an additional, dose-dependent, transient release of K+. 3. With acetylcholine (10(-6) M), the oxygen consumption of the gland increased to 62.4 +/- 4.2 microliters/(g min) from a resting value of 12.9 +/- 1.2 microliters/(g min). The increased oxygen consumption was suppressed by ouabain, in a dose-dependent fashion. 4. Withdrawal of acetylcholine and ouabain induced a net uptake of K+ and, simultaneously, an increase in oxygen consumption. The cumulative K+ uptake and the increment of oxygen consumption during the recovery period were dependent on the concentration of used ouabain. 5. The rates of K+ uptake and ATP hydrolysis were compared during recovery, assuming that six moles of ATP were hydrolysed for each mole of oxygen consumed. The data obtained during the later phase of the recovery lay on a single straight line with a slope of 1.8 for each of the various concentrations of ouabain, suggesting that the relationship between K+ uptake and energy consumption was linear. 6. Assuming the K+:ATP stoichiometry of the Na+, K(+)-ATPase to be 2:1, K+ would appear to be transported with ca 90% efficiency by Na+, K(+)-ATPase in the rat mandibular gland. Using 1.8-2.0 as a K+/ATP stoichiometry, the rate of primary active K+ uptake and the corresponding passive K+ efflux during secretion were estimated to be 20-22 mumol/(g min) from the oxygen consumption and the net K+ flux. 7. The passive K+ efflux was estimated, from the initial rate of K+ release caused by addition of 10(-3) M-ouabain, to be 30 mumol/g min). The discrepancy between the estimated active K+ uptake and passive K+ release (8-10 mumol/(g min] could be attributed to a secondary active K+ uptake process such as Na(+)-K(+)-2Cl-symport.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGEN A. S. The secretion of potassium in saliva. J Physiol. 1956 Apr 27;132(1):20–39. doi: 10.1113/jphysiol.1956.sp005500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Conigrave A. D., Favaloro E. J., Novak I., Thompson C. H., Young J. A. The role of buffer anions and protons in secretion by the rabbit mandibular salivary gland. J Physiol. 1982 Jan;322:273–286. doi: 10.1113/jphysiol.1982.sp014037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Conigrave A. D., Novak I., Young J. A. Electrolyte and protein secretion by the perfused rabbit mandibular gland stimulated with acetylcholine or catecholamines. J Physiol. 1980 Mar;300:467–487. doi: 10.1113/jphysiol.1980.sp013173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Hunter M., Novak I., Young J. A. The anionic basis of fluid secretion by the rabbit mandibular salivary gland. J Physiol. 1984 Apr;349:619–630. doi: 10.1113/jphysiol.1984.sp015177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton J., Martinez J. R., Martinez A. M., Young J. A. Fluid and electrolyte secretion from the isolated, perfused submandibular and sublingual glands of the rat. Arch Oral Biol. 1981;26(7):555–561. doi: 10.1016/0003-9969(81)90017-0. [DOI] [PubMed] [Google Scholar]
- Laugesen L. P., Nielsen J. O., Poulsen J. H. Partial dissociation between salivary secretion and active potassium transport in the perfused cat submandibular gland. Pflugers Arch. 1976 Jul 30;364(2):167–173. doi: 10.1007/BF00585186. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Gallacher D. V., Petersen O. H. Voltage and Ca2+-activated K+ channel in baso-lateral acinar cell membranes of mammalian salivary glands. Nature. 1983 Apr 28;302(5911):827–829. doi: 10.1038/302827a0. [DOI] [PubMed] [Google Scholar]
- Murakami M., Imai Y., Seo Y., Morimoto T., Shiga K., Watari H. Phosphorus nuclear magnetic resonance of perfused salivary gland. Biochim Biophys Acta. 1983 Feb 16;762(1):19–24. doi: 10.1016/0167-4889(83)90111-8. [DOI] [PubMed] [Google Scholar]
- Murakami M. Measurement of heat production in dog submandibular gland. Jpn J Physiol. 1979;29(4):491–507. doi: 10.2170/jjphysiol.29.491. [DOI] [PubMed] [Google Scholar]
- Murakami M., Seo Y., Nakahari T., Mori H., Imai Y., Watari H. Effects of Na+ depletion on fluid secretion and levels of phosphorus compounds as measured by 31P-NMR in perfused canine mandibular gland. Jpn J Physiol. 1984;34(4):587–597. doi: 10.2170/jjphysiol.34.587. [DOI] [PubMed] [Google Scholar]
- Murakami M., Seo Y., Watari H. Dissociation of fluid secretion and energy supply in rat mandibular gland by high dose of ACh. Am J Physiol. 1988 May;254(5 Pt 1):G781–G787. doi: 10.1152/ajpgi.1988.254.5.G781. [DOI] [PubMed] [Google Scholar]
- Murakami M., Seo Y., Watari H., Ueda H., Hashimoto T., Tagawa K. 31P NMR studies on the isolated perfused mandibular gland of the rat. Jpn J Physiol. 1987;37(3):411–423. doi: 10.2170/jjphysiol.37.411. [DOI] [PubMed] [Google Scholar]
- Murakami M., Suzuki E., Miyamoto S., Seo Y., Watari H. Direct measurement of K movement by 39K NMR in perfused rat mandibular salivary gland stimulated with acetylcholine. Pflugers Arch. 1989 Aug;414(4):385–392. doi: 10.1007/BF00585047. [DOI] [PubMed] [Google Scholar]
- Murakami M. [Heat production, blood flow, O2 uptake and CO2 output in the secretary process of the dog submandibular gland (author's transl)]. Nihon Seirigaku Zasshi. 1981 May;43(5):135–147. [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Some factors influencing stimulation-induced release of potassium from the cat submandibular gland to fluid perfused through the gland. J Physiol. 1970 Jun;208(2):431–447. doi: 10.1113/jphysiol.1970.sp009129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repke K., Est M., Portius H. J. Uber die Ursache der Speciesunterschiede in der Digitalisempfindlichkeit. Biochem Pharmacol. 1965 Dec;14(12):1785–1802. doi: 10.1016/0006-2952(65)90269-8. [DOI] [PubMed] [Google Scholar]
- Seo Y., Murakami M., Matsumoto T., Nishikawa H., Watari H. Direct measurement of Na influx by 23Na NMR during secretion with acetylcholine in perfused rat mandibular gland. Pflugers Arch. 1987 Aug;409(4-5):343–348. doi: 10.1007/BF00583787. [DOI] [PubMed] [Google Scholar]
- Smaje L. H., Poulsen J. H., Ussing H. H. Evidence from O2 uptake measurements for Na+ -K+ -2 Cl- co-transport in the rabbit submandibular gland. Pflugers Arch. 1986 May;406(5):492–496. doi: 10.1007/BF00583372. [DOI] [PubMed] [Google Scholar]
- Turner R. J., George J. N., Baum B. J. Evidence for a Na+/K+/Cl- cotransport system in basolateral membrane vesicles from the rabbit parotid. J Membr Biol. 1986;94(2):143–152. doi: 10.1007/BF01871194. [DOI] [PubMed] [Google Scholar]


