Abstract
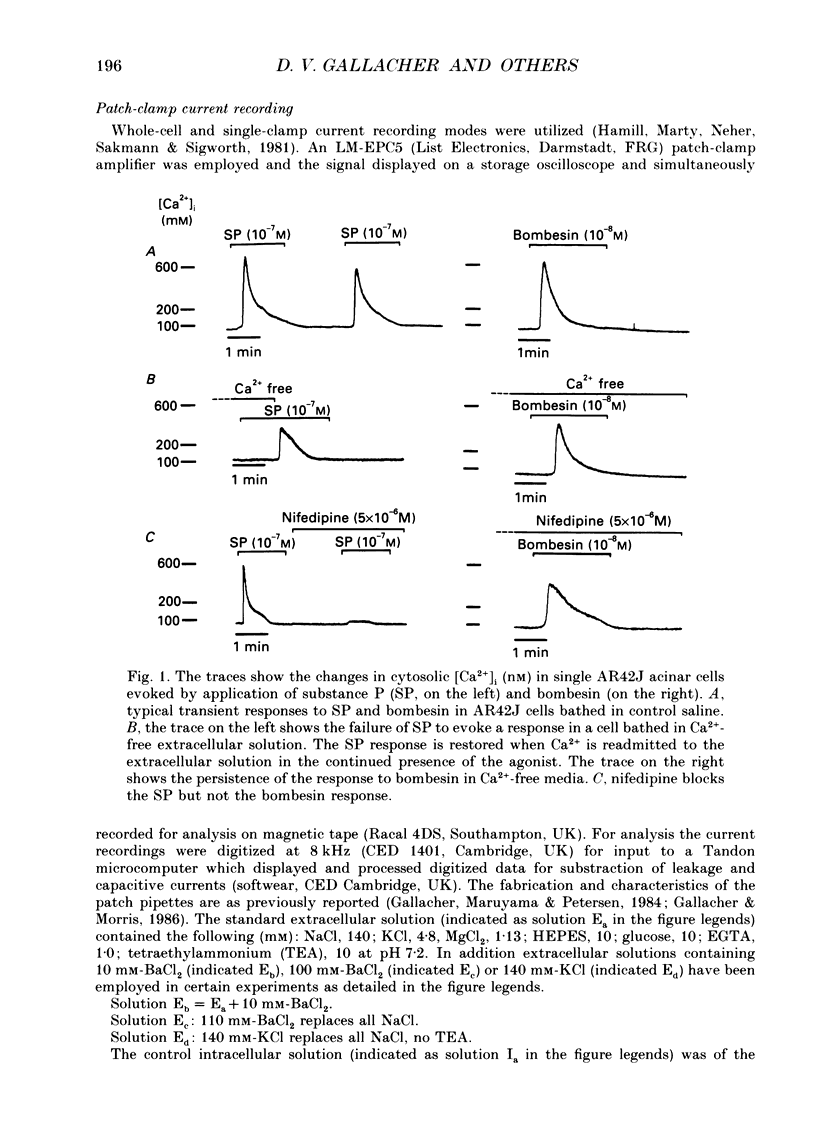
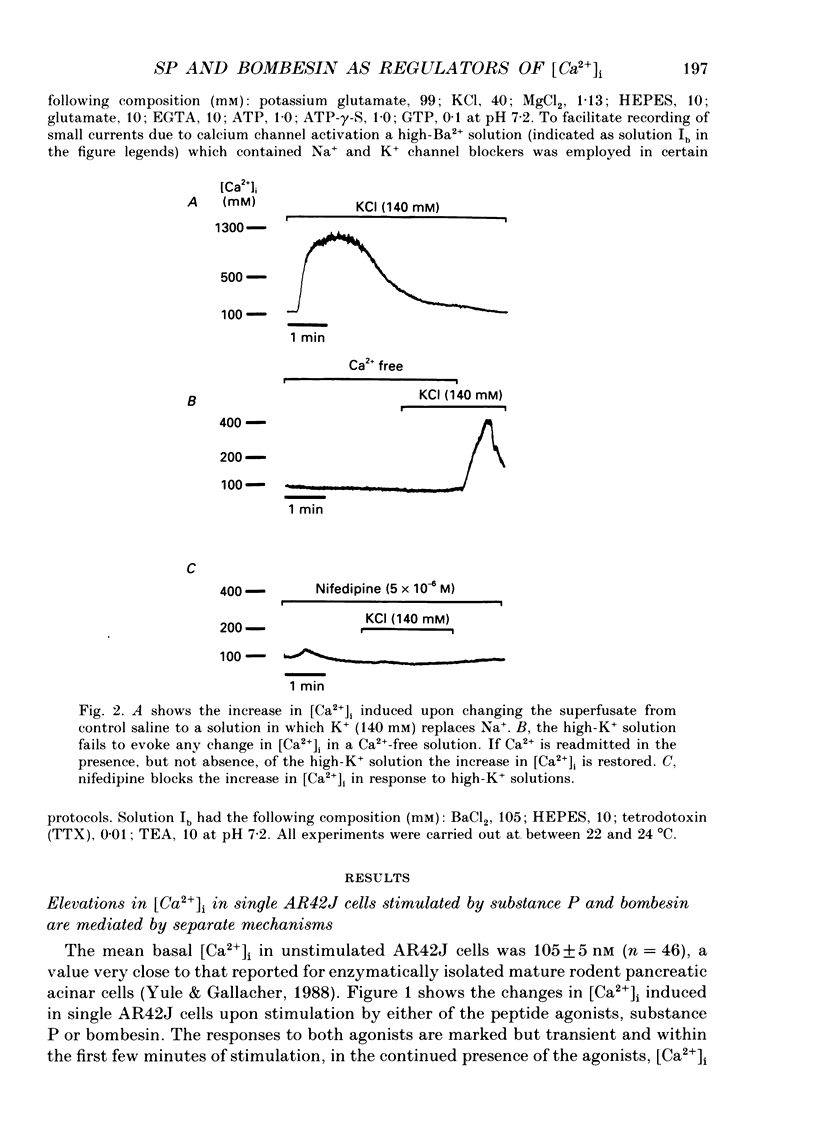
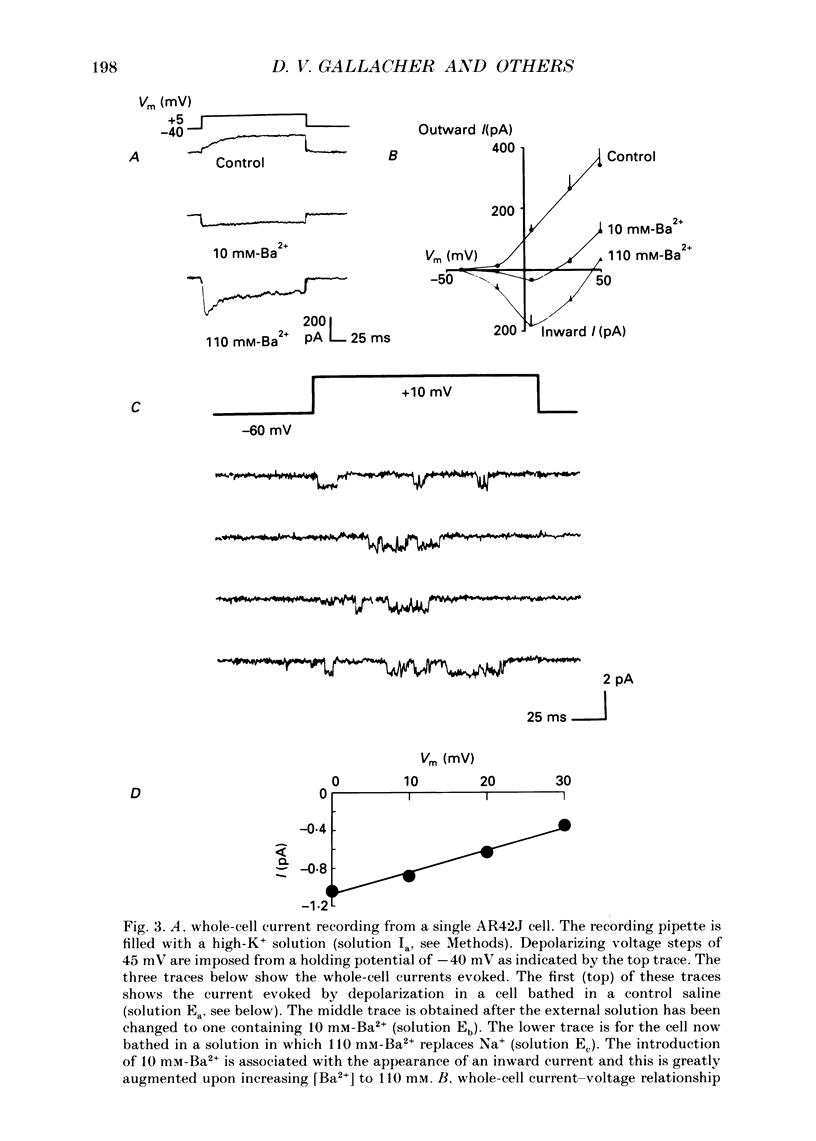
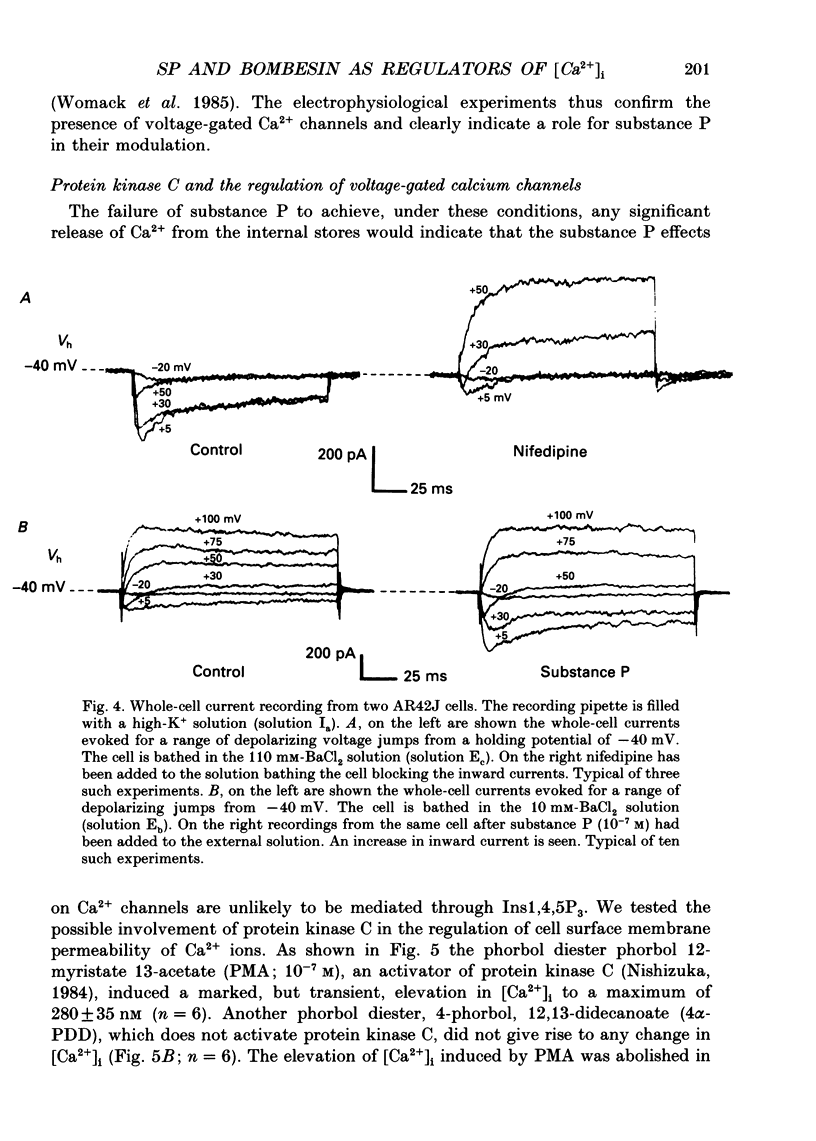
1. Dual-excitation microfluorometry (Fura-2 as indicator) was employed to monitor directly changes in the cytosolic calcium concentration [( Ca2+]i) in single cells. We investigated and compared the effects of stimulation of AR42J rat pancreatic acinar cells by two peptide agonists, substance P and bombesin. 2. Substance P (10(-7) M) and bombesin (10(-8) M) each gave rise to a marked, but transient, elevation in [Ca2+]i. The calcium signals evoked by the two peptides were qualitatively and quantitatively very similar. However, in the absence of extracellular Ca2+ the response to substance P, but not bombesin, was abolished. These results suggest that substance P induces calcium influx across the cell surface membrane but does not release calcium from internal stores. Bombesin in marked contrast releases calcium from intracellular stores in the absence of any detectable calcium influx. 3. Depolarization by high-K+ extracellular solutions evoked a marked, but transient, rise in [Ca2+]i. This elevation in [Ca2+]i was strictly dependent upon the presence of Ca2+ in extracellular media. 4. Nifedipine (5 x 10(-6) M), an antagonist of L-type voltage-dependent Ca2+ channels, blocked the elevations in [Ca2+]i induced by either substance P or high-K+ solutions, but not that evoked by application of bombesin. 5. Patch-clamp, single-channel current recordings from cell-attached patches of membrane confirmed the presence of voltage-dependent calcium channels in the surface membranes of AR42J cells. Whole-cell current recordings demonstrated voltage-dependent inward Ca2+ (Ba2+) currents which were increased in amplitude by substance P and blocked by nifedipine. 6. The protein kinase C (PKC) activators, the phorbol diester, phorbol 1,2-myristate 13-acetate (PMA, 10(-7) M), and cell-permeable diacylglycerol analogues, 1-oleoyl-2-acetyl-sn-glycerol (OAG, 2.5 x 10(-6) M) and sn-2-dioctanoyl glycerol (DiC8, 2.5 x 10(-6) M), mimicked the effect of substance P, but not bombesin, in elevating [Ca2+]i in a manner that was blocked by removal of extracellular Ca2+ or application of nifedipine. 7. The PKC inhibitor, polymyxin B (2.5 x 10(-6) M), applied 2 min prior to stimulation blocked the effects of substance P and PKC activators, but not bombesin, in elevating [Ca2+]i. 8. The calcium signals evoked by substance P and bombesin are achieved by activation of different molecular mechanisms. Substance P, the evidence suggests, activates PKC which in turn stimulates calcium influx by opening voltage-dependent Ca2+ channels in the cell surface membranes.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Changya L., Gallacher D. V., Irvine R. F., Petersen O. H. Inositol 1,3,4,5-tetrakisphosphate and inositol 1,4,5-trisphosphate act by different mechanisms when controlling Ca2+ in mouse lacrimal acinar cells. FEBS Lett. 1989 Jul 17;251(1-2):43–48. doi: 10.1016/0014-5793(89)81425-5. [DOI] [PubMed] [Google Scholar]
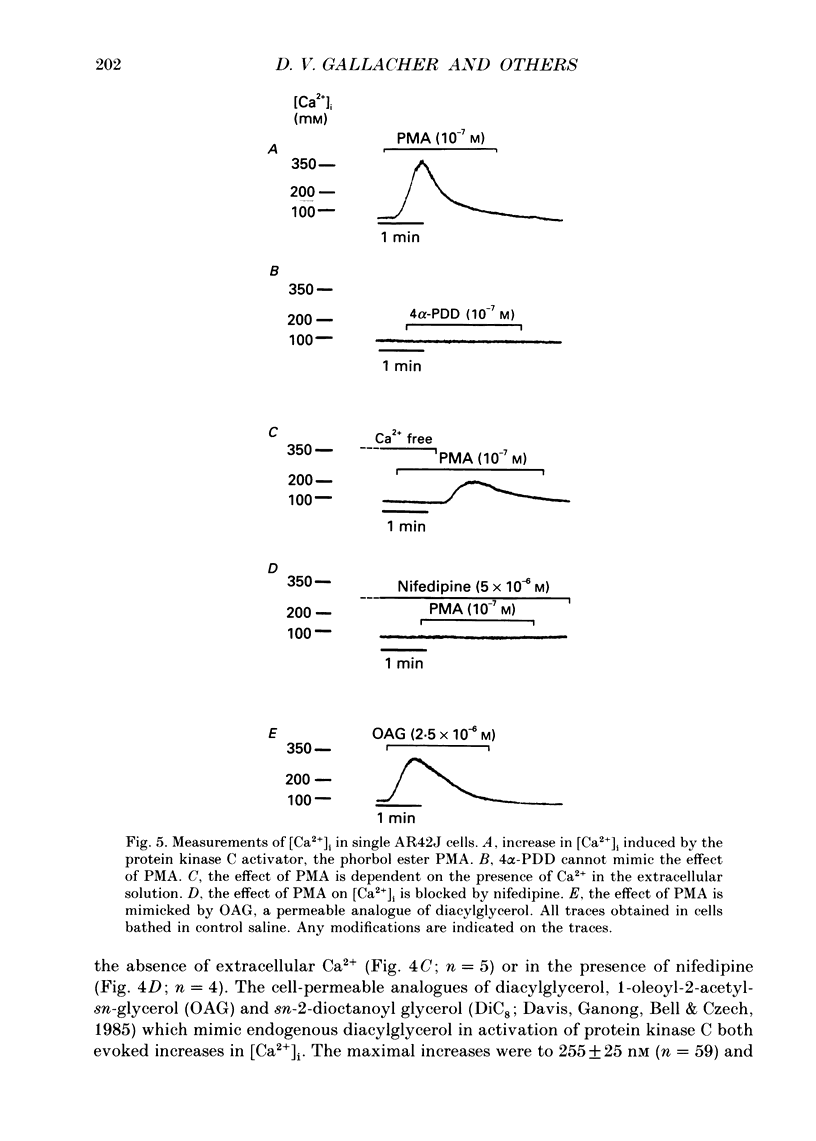
- Davis R. J., Ganong B. R., Bell R. M., Czech M. P. sn-1,2-Dioctanoylglycerol. A cell-permeable diacylglycerol that mimics phorbol diester action on the epidermal growth factor receptor and mitogenesis. J Biol Chem. 1985 Feb 10;260(3):1562–1566. [PubMed] [Google Scholar]
- Gallacher D. V., Maruyama Y., Petersen O. H. Patch-clamp study of rubidium and potassium conductances in single cation channels from mammalian exocrine acini. Pflugers Arch. 1984 Aug;401(4):361–367. doi: 10.1007/BF00584336. [DOI] [PubMed] [Google Scholar]
- Gallacher D. V., Morris A. P. A patch-clamp study of potassium currents in resting and acetylcholine-stimulated mouse submandibular acinar cells. J Physiol. 1986 Apr;373:379–395. doi: 10.1113/jphysiol.1986.sp016054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hanley M. R., Lee C. M., Michell R. H., Jones L. M. Similar effects of substance P and related peptides on salivation and on phosphatidylinositol turnover in rat salivary glands. Mol Pharmacol. 1980 Jul;18(1):78–83. [PubMed] [Google Scholar]
- Heslop J. P., Blakeley D. M., Brown K. D., Irvine R. F., Berridge M. J. Effects of bombesin and insulin on inositol (1,4,5)trisphosphate and inositol (1,3,4)trisphosphate formation in Swiss 3T3 cells. Cell. 1986 Dec 5;47(5):703–709. doi: 10.1016/0092-8674(86)90513-1. [DOI] [PubMed] [Google Scholar]
- Hill T. D., Dean N. M., Boynton A. L. Inositol 1,3,4,5-tetrakisphosphate induces Ca2+ sequestration in rat liver cells. Science. 1988 Nov 25;242(4882):1176–1178. doi: 10.1126/science.2847317. [DOI] [PubMed] [Google Scholar]
- Horstman D. A., Takemura H., Putney J. W., Jr Formation and metabolism of [3H]inositol phosphates in AR42J pancreatoma cells. Substance P-induced Ca2+ mobilization in the apparent absence of inositol 1,4,5-trisphosphate 3-kinase activity. J Biol Chem. 1988 Oct 25;263(30):15297–15303. [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Rampe D., Brown A. M. Effects of protein kinase C activators on cardiac Ca2+ channels. Nature. 1988 Sep 15;335(6187):249–251. doi: 10.1038/335249a0. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Tanguy J. Dependence of intracellular effects of GTP gamma S and inositoltrisphosphate on cell membrane potential and on external Ca ions. Pflugers Arch. 1987 Aug;409(4-5):499–506. doi: 10.1007/BF00583807. [DOI] [PubMed] [Google Scholar]
- Löffelholz K. Receptor regulation of choline phospholipid hydrolysis. A novel source of diacylglycerol and phosphatidic acid. Biochem Pharmacol. 1989 May 15;38(10):1543–1549. doi: 10.1016/0006-2952(89)90299-2. [DOI] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Kawasaki H., Imajoh S., Suzuki K., Inagaki M., Yokokura H., Sakoh T., Hidaka H. Tissue-specific expression of three distinct types of rabbit protein kinase C. Nature. 1987 Jan 8;325(7000):161–166. doi: 10.1038/325161a0. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Gallacher D. V. Electrophysiology of pancreatic and salivary acinar cells. Annu Rev Physiol. 1988;50:65–80. doi: 10.1146/annurev.ph.50.030188.000433. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Takemura H., Hughes A. R., Horstman D. A., Thastrup O. How do inositol phosphates regulate calcium signaling? FASEB J. 1989 Jun;3(8):1899–1905. doi: 10.1096/fasebj.3.8.2542110. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Stutchfield J., Jones P. M., Howell S. L. The effects of polymyxin B, a protein kinase C inhibitor, on insulin secretion from intact and permeabilized islets of Langerhans. Biochem Biophys Res Commun. 1986 May 14;136(3):1001–1006. doi: 10.1016/0006-291x(86)90432-8. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Malhotra R. K., Wakade T. D. Phorbol ester facilitates 45Ca accumulation and catecholamine secretion by nicotine and excess K+ but not by muscarine in rat adrenal medulla. Nature. 1986 Jun 12;321(6071):698–700. doi: 10.1038/321698a0. [DOI] [PubMed] [Google Scholar]
- Womack M. D., Hanley M. R., Jessell T. M. Functional substance P receptors on a rat pancreatic acinar cell line. J Neurosci. 1985 Dec;5(12):3370–3378. doi: 10.1523/JNEUROSCI.05-12-03370.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack M. D., MacDermott A. B., Jessell T. M. Sensory transmitters regulate intracellular calcium in dorsal horn neurons. Nature. 1988 Jul 28;334(6180):351–353. doi: 10.1038/334351a0. [DOI] [PubMed] [Google Scholar]
- Wooten M. W., Wrenn R. W. Phorbol ester induces intracellular translocation of phospholipid/Ca2+-dependent protein kinase and stimulates amylase secretion in isolated pancreatic acini. FEBS Lett. 1984 Jun 11;171(2):183–186. doi: 10.1016/0014-5793(84)80484-6. [DOI] [PubMed] [Google Scholar]
- Yule D. I., Gallacher D. V. Oscillations of cytosolic calcium in single pancreatic acinar cells stimulated by acetylcholine. FEBS Lett. 1988 Nov 7;239(2):358–362. doi: 10.1016/0014-5793(88)80951-7. [DOI] [PubMed] [Google Scholar]



