Abstract
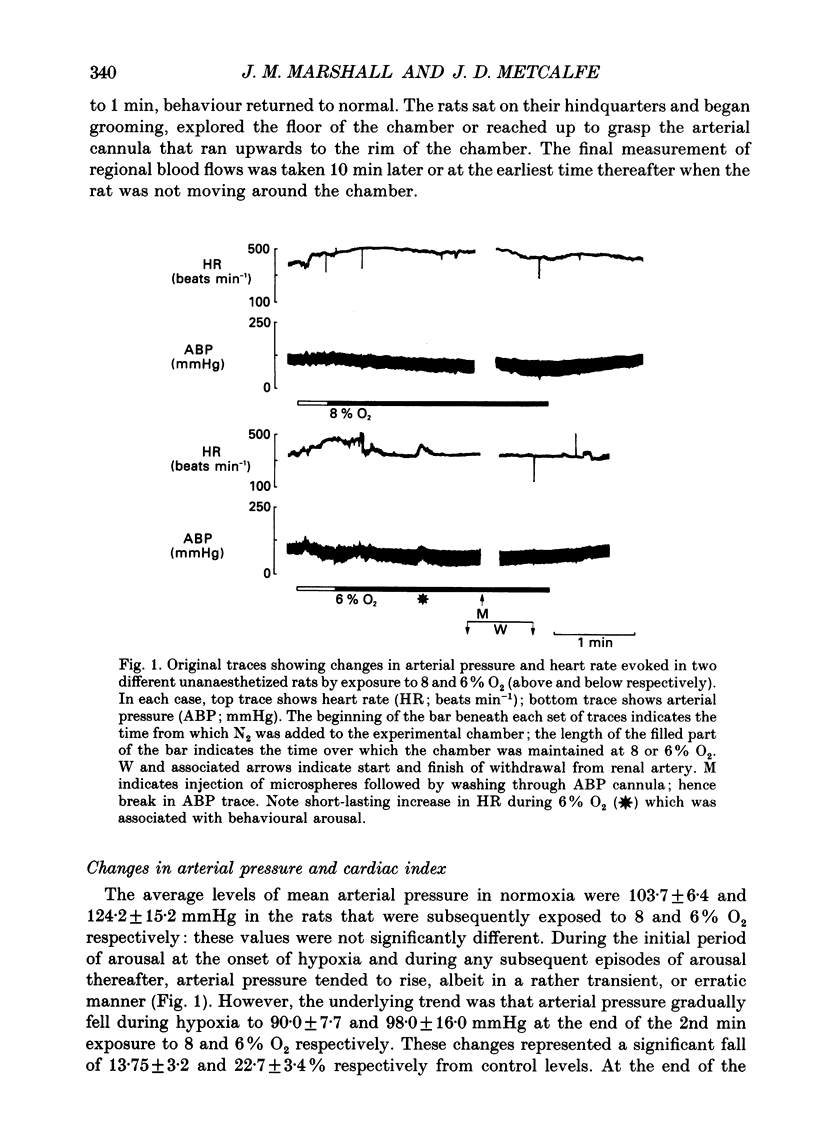
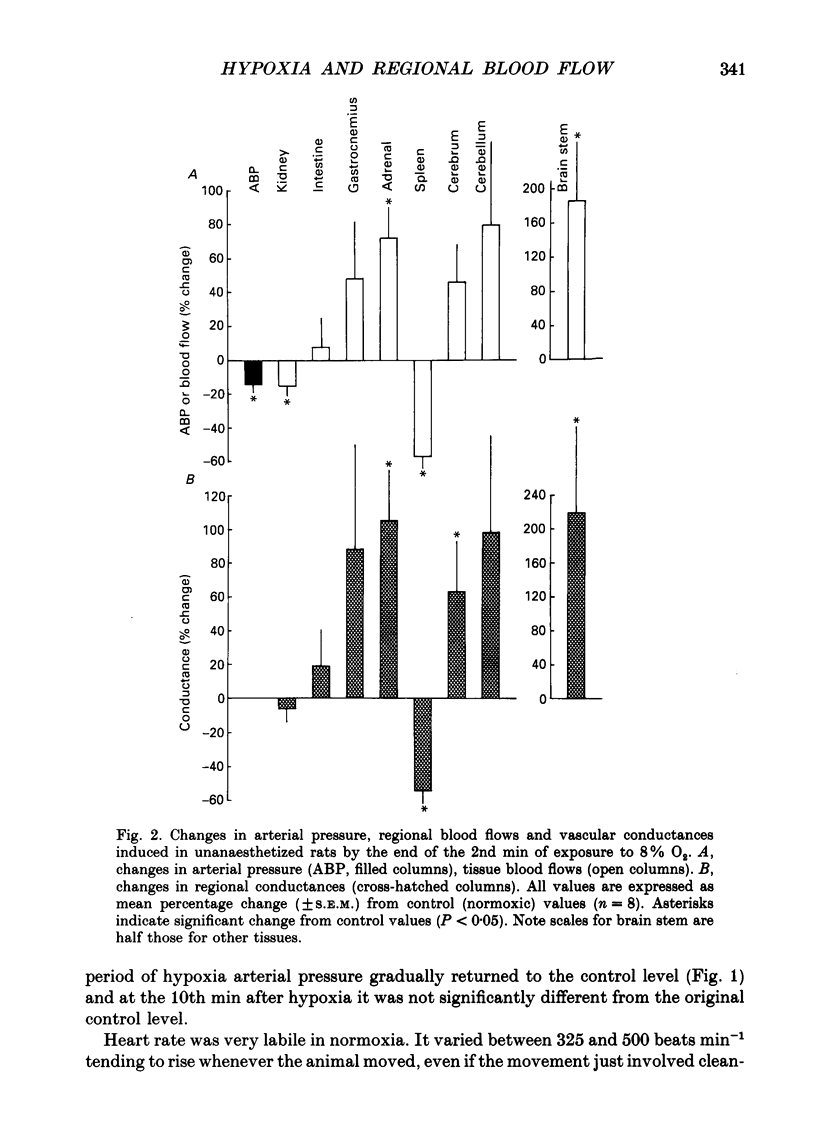
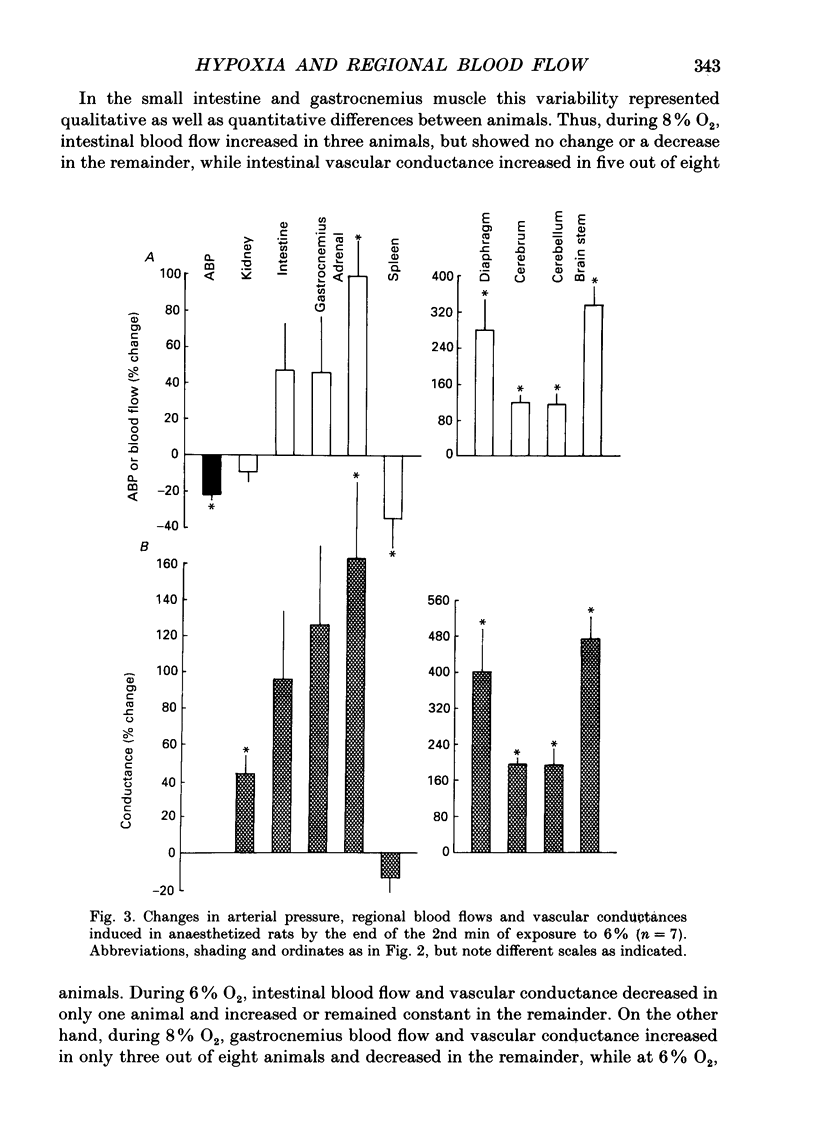
1. Studies were made in unanaesthetized rats of cardiovascular responses induced during 3 min periods of systemic hypoxia (inspirate 8 or 6% O2). Arterial pressure and heart rate were recorded continuously; cardiac index and regional blood flows were measured in normoxia and at the 2nd min of hypoxia by injection of radiolabelled microspheres. Comparisons are made with changes recorded in Saffan-anaesthetized rats during 8% O2 using microspheres and in previous studies using electromagnetic transducers on renal, mesenteric and femoral arteries (Marshall & Metcalfe, 1988a). 2. In unanaesthetized rats, the initial 1-1.5 min of hypoxia evoked behavioural arousal associated with a short-lasting rise in arterial pressure and heart rate. This agrees with our previous proposal that hypoxia activates the brain stem defence areas by stimulating peripheral chemoreceptors. 3. In unanaesthetized rats, these changes were superimposed upon a gradual fall in arterial pressure and tachycardia, the responses being greater during 6 than 8% O2 (cf. Saffan-anaesthetized rats). Further, in all rats at the 2nd min of hypoxia, cardiac index and vascular conductance of most body tissues was increased. It is concluded that the fall in arterial pressure is due to peripheral vasodilatation. 4. In the unanaesthetized rats, the tendency for vascular conductance in kidney, intestine and gastrocnemius muscle to increase (more during 6 than 8% O2) allowed increases in blood flow in the last two regions. These changes accord with those recorded under Saffan anaesthesia. 5. In both unanaesthetized and anaesthetized rats, hypoxia induced pronounced increases in vascular conductance of diaphragm, adrenal gland, cerebral hemispheres, cerebellum and brain stem, the resultant increases in blood flow being larger in the unanaesthetized rats. 6. It is proposed that in unanaesthetized, as in anaesthetized, rats the regional dilator responses predominantly reflect the local dilator effects of tissue hypoxia. Possible dilator factors are considered.
Full text
PDF







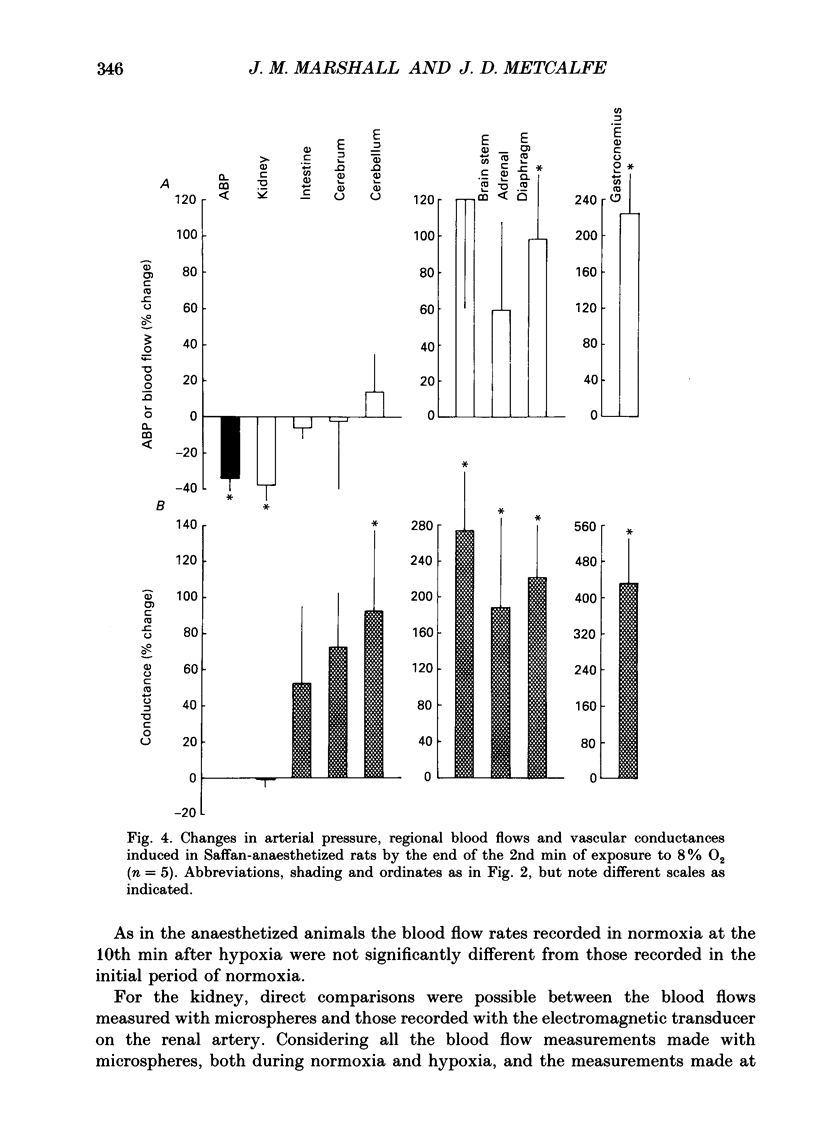







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAMS V. C., HILTON S. M., ZBROZYNA A. Active muscle vasodilatation produced by stimulation of the brain stem: its significance in the defence reaction. J Physiol. 1960 Dec;154:491–513. doi: 10.1113/jphysiol.1960.sp006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. B., Laughlin M. H. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol. 1983 Nov;344:189–208. doi: 10.1113/jphysiol.1983.sp014933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne R. M., Knabb R. M., Ely S. W., Rubio R. Adenosine in the local regulation of blood flow: a brief overview. Fed Proc. 1983 Dec;42(15):3136–3142. [PubMed] [Google Scholar]
- Berns A. S., Anderson R. J., McDonald K. M., Arnold P. E. Effect of hypercapnic acidosis on renal water excretion in the dog. Kidney Int. 1979 Feb;15(2):116–125. doi: 10.1038/ki.1979.17. [DOI] [PubMed] [Google Scholar]
- Heymann M. A., Payne B. D., Hoffman J. I., Rudolph A. M. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977 Jul-Aug;20(1):55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Hilton S. M., Hudlická O., Marshall J. M. Possible mediators of functional hyperaemia in skeletal muscle. J Physiol. 1978 Sep;282:131–147. doi: 10.1113/jphysiol.1978.sp012453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M. A., Blantz R. C., Rector F. C., Jr, Seldin D. W. Measurement of intrarenal blood flow. I. Analysis of microsphere method. Am J Physiol. 1971 Jun;220(6):1903–1913. doi: 10.1152/ajplegacy.1971.220.6.1903. [DOI] [PubMed] [Google Scholar]
- Koehler R. C., McDonald B. W., Krasney J. A. Influence of CO2 on cardiovascular response to hypoxia in conscious dogs. Am J Physiol. 1980 Oct;239(4):H545–H558. doi: 10.1152/ajpheart.1980.239.4.H545. [DOI] [PubMed] [Google Scholar]
- Laughlin M. H., Armstrong R. B., White J., Rouk K. A method for using microspheres to measure muscle blood flow in exercising rats. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jun;52(6):1629–1635. doi: 10.1152/jappl.1982.52.6.1629. [DOI] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Analysis of factors that contribute to cardiovascular changes induced in the cat by graded levels of systemic hypoxia. J Physiol. 1989 May;412:429–448. doi: 10.1113/jphysiol.1989.sp017625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Analysis of the cardiovascular changes induced in the rat by graded levels of systemic hypoxia. J Physiol. 1988 Dec;407:385–403. doi: 10.1113/jphysiol.1988.sp017422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Cardiovascular changes associated with augmented breaths in normoxia and hypoxia in the rat. J Physiol. 1988 Jun;400:15–27. doi: 10.1113/jphysiol.1988.sp017107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Influences on the cardiovascular response to graded levels of systemic hypoxia of the accompanying hypocapnia in the rat. J Physiol. 1989 Mar;410:381–394. doi: 10.1113/jphysiol.1989.sp017539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahapill P. A., Schlichter L. C. Modulation of potassium channels in human T lymphocytes: effects of temperature. J Physiol. 1990 Mar;422:103–126. doi: 10.1113/jphysiol.1990.sp017975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose C. E., Jr, Althaus J. A., Kaiser D. L., Miller E. D., Carey R. M. Acute hypoxemia and hypercapnia: increase in plasma catecholamines in conscious dogs. Am J Physiol. 1983 Dec;245(6):H924–H929. doi: 10.1152/ajpheart.1983.245.6.H924. [DOI] [PubMed] [Google Scholar]
- Rowell L. B., Blackmon J. R. Lack of sympathetic vasoconstriction in hypoxemic humans at rest. Am J Physiol. 1986 Sep;251(3 Pt 2):H562–H570. doi: 10.1152/ajpheart.1986.251.3.H562. [DOI] [PubMed] [Google Scholar]
- Stanek K. A., Coleman T. G., Smith T. L., Murphy W. R. Two hemodynamic problems commonly associated with the microsphere technique for measuring regional blood flow in rats. J Pharmacol Methods. 1985 Apr;13(2):117–124. doi: 10.1016/0160-5402(85)90055-5. [DOI] [PubMed] [Google Scholar]
- TAKACS L., KALLAY K., VAJDA V. The effect of acute arterial hypoxia on the organ blood flow in rats. Acta Physiol Acad Sci Hung. 1962;21:87–91. [PubMed] [Google Scholar]
- Timms R. J. A study of the amygdaloid defence reaction showing the value of Althesin anaesthesia in studies of the functions of the fore-brain in cats. Pflugers Arch. 1981 Jul;391(1):49–56. doi: 10.1007/BF00580694. [DOI] [PubMed] [Google Scholar]
- Timms R. J. Central blocking action of ketamine anaesthesia on the visceral alerting and chemoreceptor reflex responses in the cat. Pflugers Arch. 1982 Jul;394(1):12–15. doi: 10.1007/BF01108301. [DOI] [PubMed] [Google Scholar]
- Uther J. B., Hunyor S. N., Shaw J., Korner P. I. Bulbar and suprabulbar control of the cardiovascular autonomic effects during arterial hypoxia in the rabbit. Circ Res. 1970 Apr;26(4):491–506. doi: 10.1161/01.res.26.4.491. [DOI] [PubMed] [Google Scholar]
- Walker B. R. Role of vasopressin in the cardiovascular response to hypoxia in the conscious rat. Am J Physiol. 1986 Dec;251(6 Pt 2):H1316–H1323. doi: 10.1152/ajpheart.1986.251.6.H1316. [DOI] [PubMed] [Google Scholar]
- Winn H. R., Rubio R., Berne R. M. Brain adenosine concentration during hypoxia in rats. Am J Physiol. 1981 Aug;241(2):H235–H242. doi: 10.1152/ajpheart.1981.241.2.H235. [DOI] [PubMed] [Google Scholar]
- Yardley C. P., Hilton S. M. The hypothalamic and brainstem areas from which the cardiovascular and behavioural components of the defence reaction are elicited in the rat. J Auton Nerv Syst. 1986 Mar;15(3):227–244. doi: 10.1016/0165-1838(86)90066-4. [DOI] [PubMed] [Google Scholar]


