Abstract
1. An in vitro slice preparation of rat prefrontal cortex was used to analyse the responses of layer V pyramidal cells to electrical stimulation of layer II. We also studied the long-lasting modifications of synaptic efficacy following high-frequency stimulation of the same region. 2. Stable intracellular recordings were obtained from forty-three regular spiking pyramidal cells. The input resistance was 56 +/- 18 M omega (mean +/- S.D.) at a resting membrane potential of -71 +/- 4 mV. 3. At rest, a single stimulus of increasing strength evoked a monophasic, purely depolarizing postsynaptic potential (PSP) of increasing amplitude. In neurons recorded with potassium acetate-filled micropipettes, membrane depolarization disclosed an excitatory-inhibitory (EPSP-IPSP) sequence (onset latency of the EPSP, 3.6 +/- 0.6 ms). 4. Superfusion with the non-N-methyl-D-aspartate (NMDA) receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) reduced the EPSP and suppressed the IPSP. The small EPSP which remained was blocked by the NMDA receptor antagonist, D,L-2-amino-5-phosphonovalerate (APV). 5. In five cells, administration of 0.5 mumol l-1 bicuculline revealed a postsynaptic NMDA component in the evoked response as evidenced by its anomalous voltage dependence in the presence of Mg2+ and its sensitivity to APV. In these cells the latency of the APV-sensitive EPSP was the same as that of the APV-insensitive EPSP. 6. In six cells superfused with a high-Mg2+, low-Ca2+ artificial cerebrospinal fluid (ACSF) a small monosynaptic EPSP remained which had the same latency as the PSP recorded in control ACSF. 7. Patterned high-frequency stimulation (50-100 Hz) was applied to the afferents of twenty-eight neurons (twenty-three of them were recorded in the presence of bicuculline). During the train the membrane potential depolarized some 20 mV and each stimulus evoked a small PSP. The tetanic stimulation was followed by a short-term enhancement of the PSP amplitude and a slight increase in membrane input resistance. 8. Out of the twenty-eight cells, twenty-four showed long-lasting (over 30 min) modifications of the PSP. Long-term depression (LTD) of the evoked PSP was observed in fourteen cells and long-term potentiation (LTP) in ten cells. There was no significant change in the steady-state membrane properties and in the latency of the response. 9. In 64% of the cells that showed LTD and 70% of those that showed LTP of synaptic efficacy, the latency of the enhanced or depressed component of the PSP was the same as the control.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
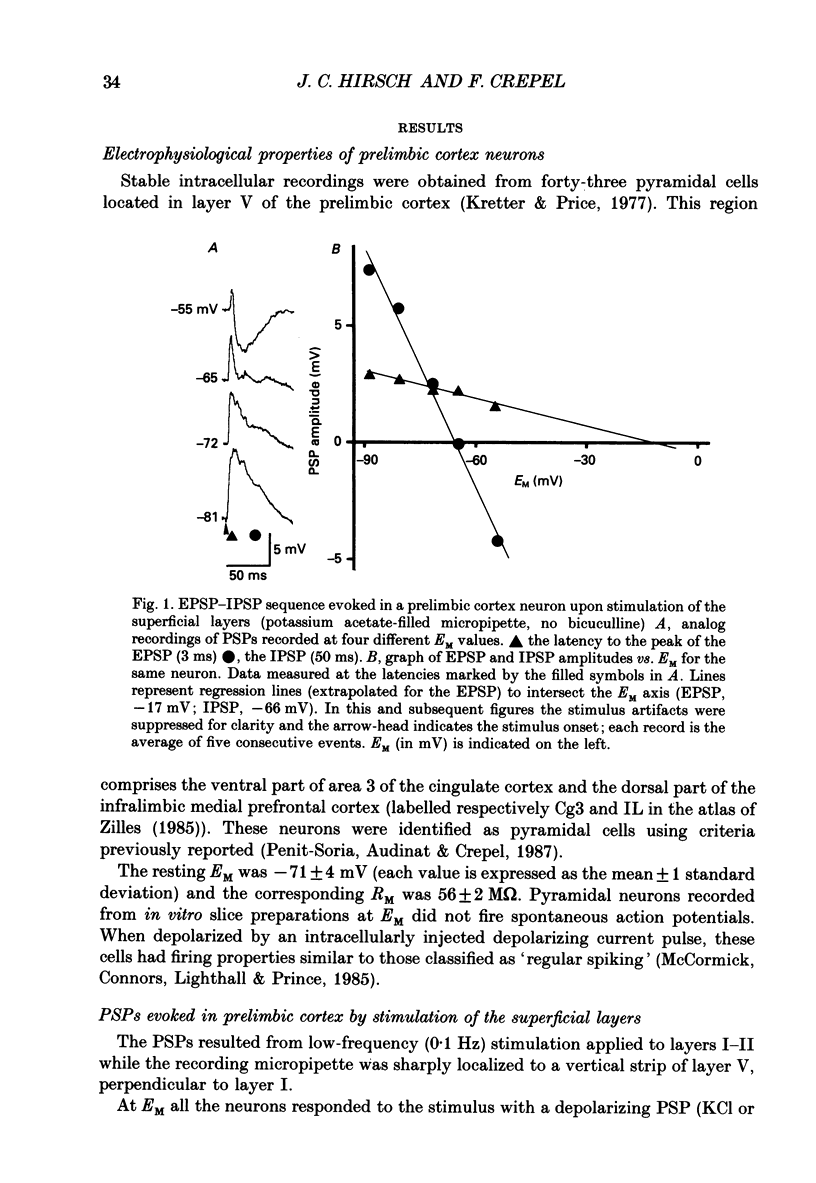
PDF



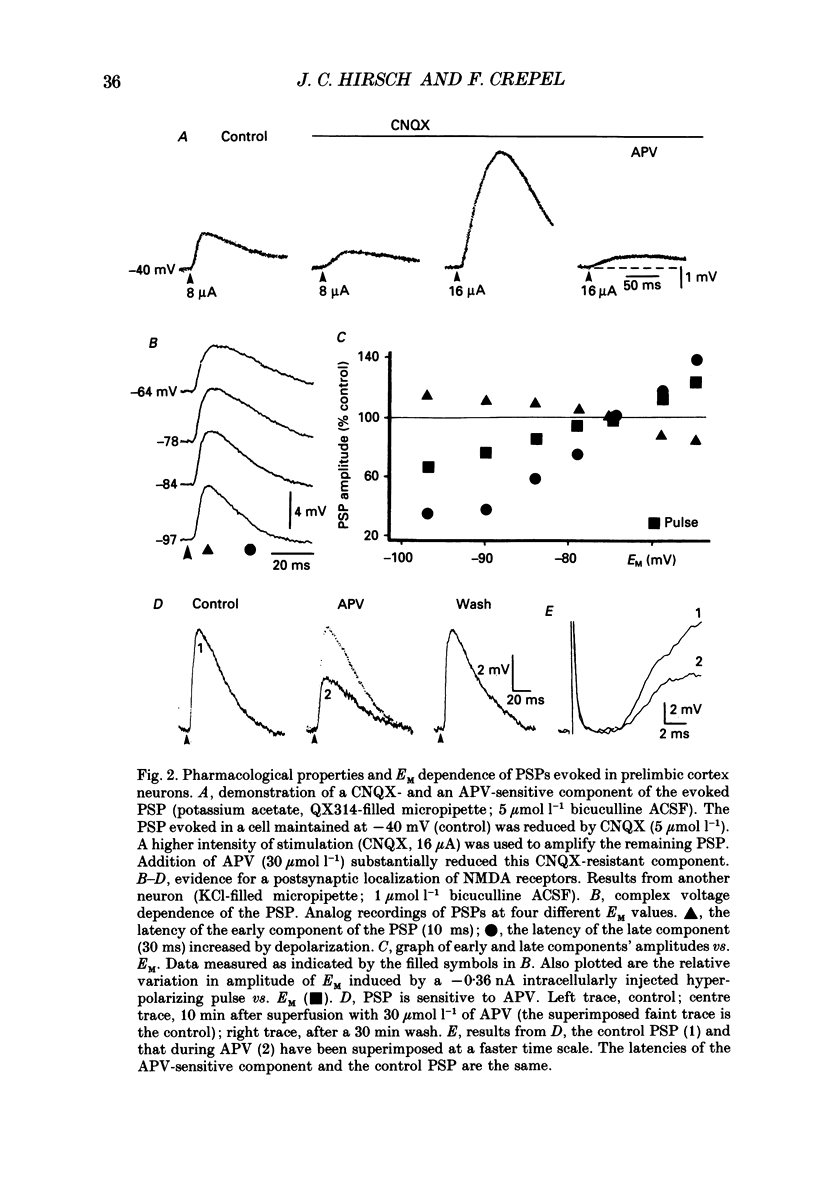







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
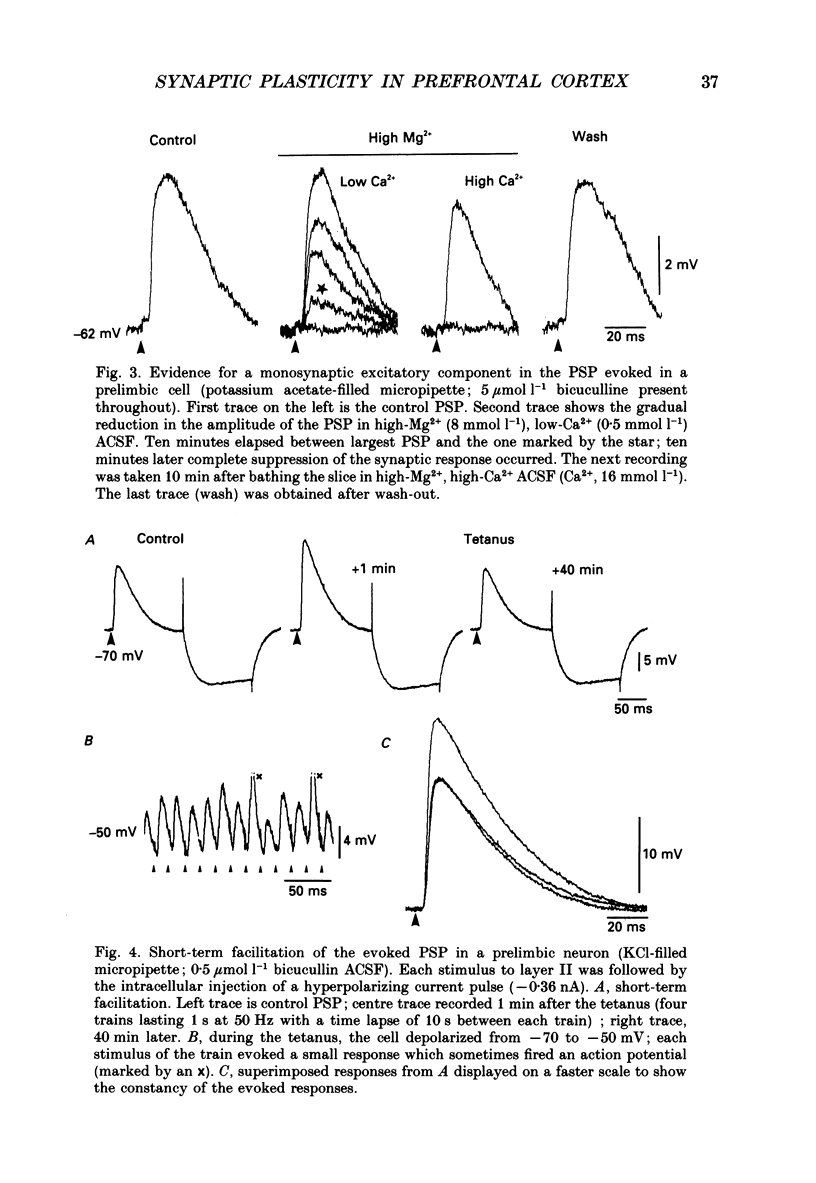
- Andreasen M., Lambert J. D., Jensen M. S. Effects of new non-N-methyl-D-aspartate antagonists on synaptic transmission in the in vitro rat hippocampus. J Physiol. 1989 Jul;414:317–336. doi: 10.1113/jphysiol.1989.sp017690. [DOI] [PMC free article] [PubMed] [Google Scholar]
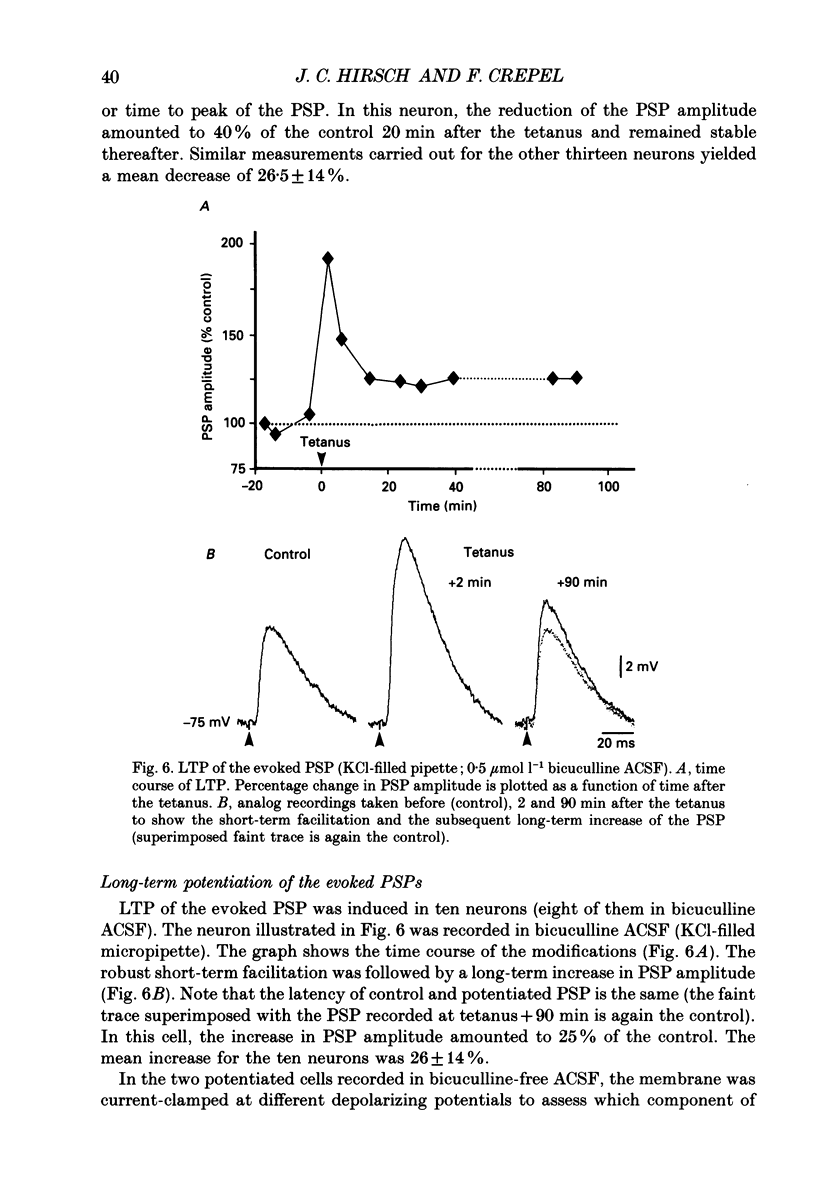
- Artola A., Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987 Dec 17;330(6149):649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
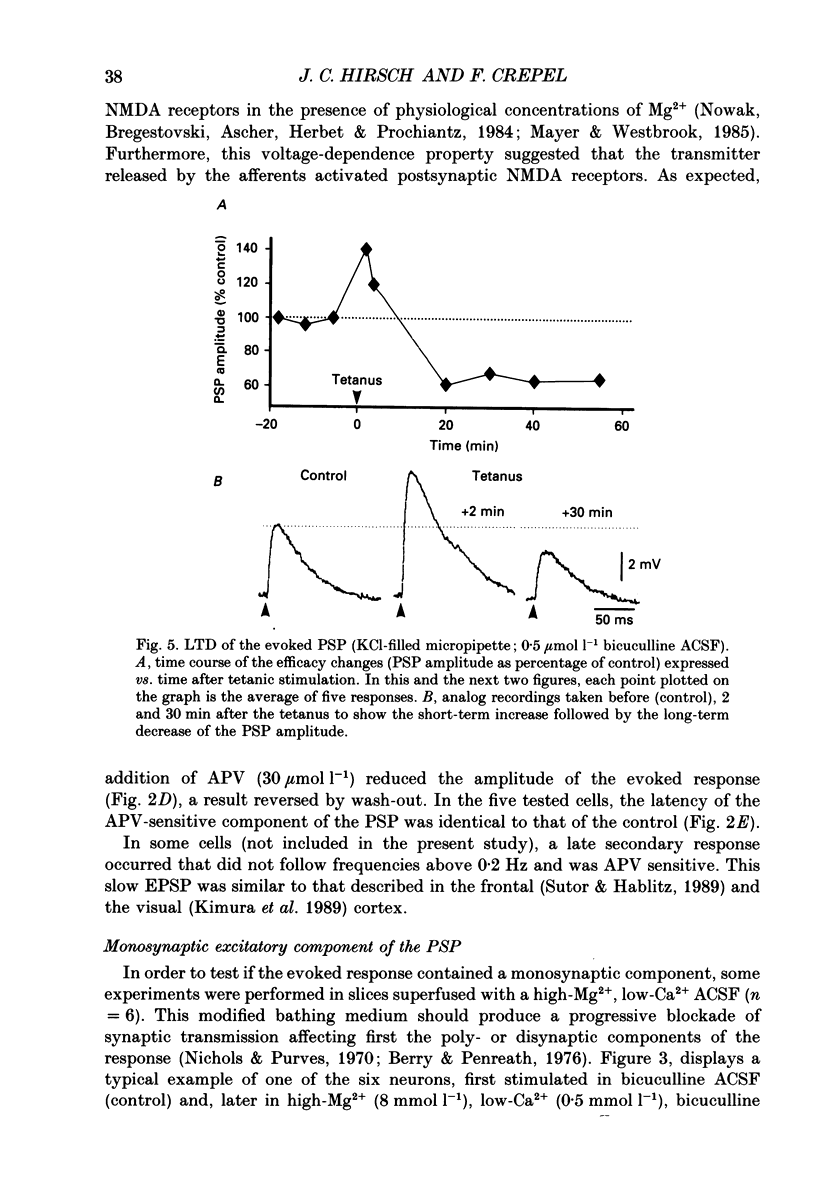
- Audinat E., Hermel J. M., Crépel F. Neurotensin-induced excitation of neurons of the rat's frontal cortex studied intracellularly in vitro. Exp Brain Res. 1989;78(2):358–368. doi: 10.1007/BF00228907. [DOI] [PubMed] [Google Scholar]
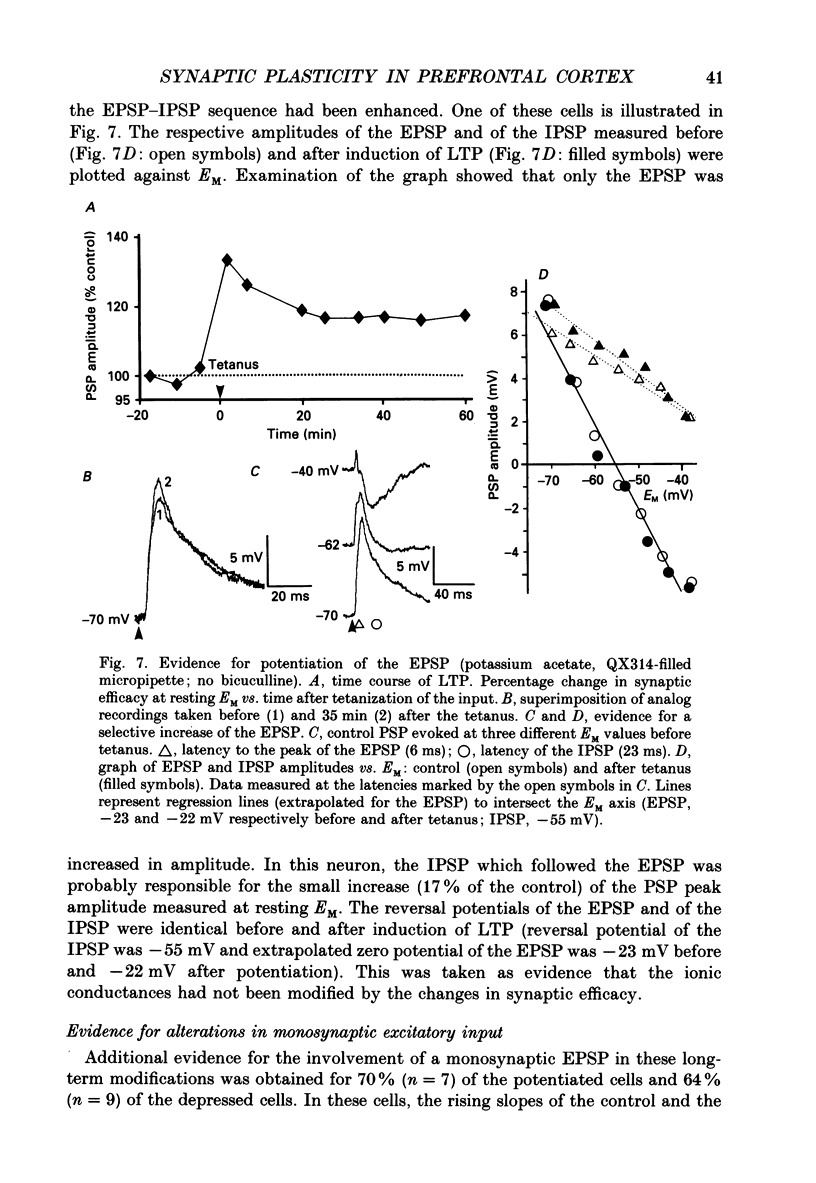
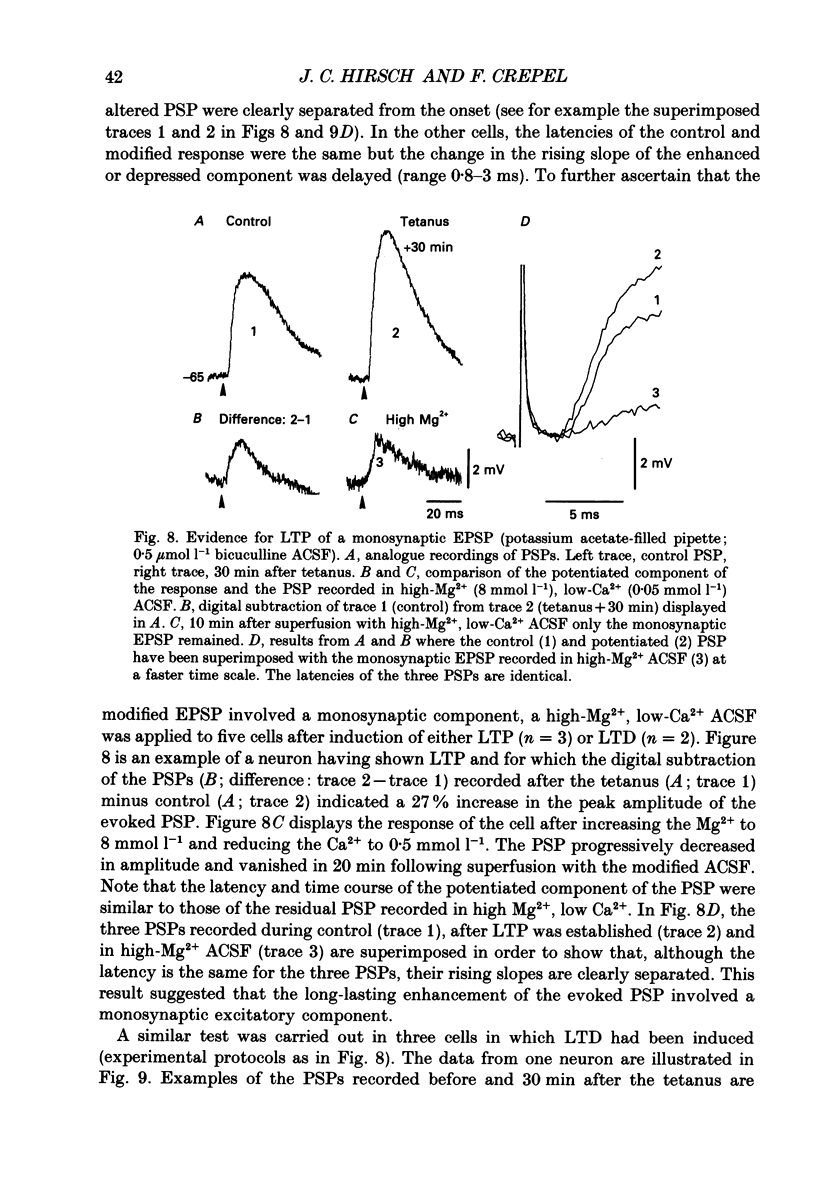
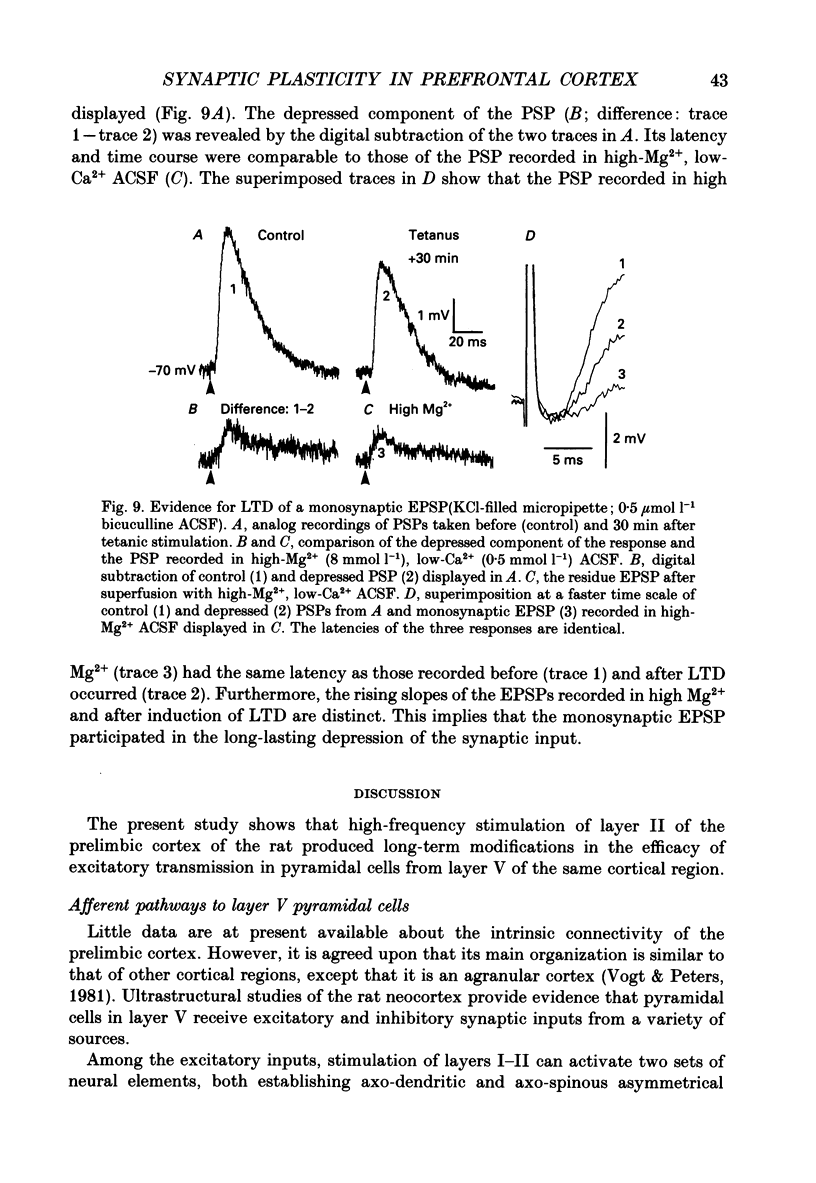
- Avoli M. Inhibitory potentials in neurons of the deep layers of the in vitro neocortical slice. Brain Res. 1986 Apr 2;370(1):165–170. doi: 10.1016/0006-8993(86)91118-2. [DOI] [PubMed] [Google Scholar]
- Baranyi A., Szente M. B. Long-lasting potentiation of synaptic transmission requires postsynaptic modifications in the neocortex. Brain Res. 1987 Oct 13;423(1-2):378–384. doi: 10.1016/0006-8993(87)90867-5. [DOI] [PubMed] [Google Scholar]
- Barrionuevo G., Kelso S. R., Johnston D., Brown T. H. Conductance mechanism responsible for long-term potentiation in monosynaptic and isolated excitatory synaptic inputs to hippocampus. J Neurophysiol. 1986 Mar;55(3):540–550. doi: 10.1152/jn.1986.55.3.540. [DOI] [PubMed] [Google Scholar]
- Berry M. S., Pentreath V. W. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976 Mar 19;105(1):1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- Berry R. L., Teyler T. J., Han T. Z. Induction of LTP in rat primary visual cortex: tetanus parameters. Brain Res. 1989 Mar 6;481(2):221–227. doi: 10.1016/0006-8993(89)90797-x. [DOI] [PubMed] [Google Scholar]
- Bindman L. J., Murphy K. P., Pockett S. Postsynaptic control of the induction of long-term changes in efficacy of transmission at neocortical synapses in slices of rat brain. J Neurophysiol. 1988 Sep;60(3):1053–1065. doi: 10.1152/jn.1988.60.3.1053. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Burns B. D., Uttley A. M. Factors affecting the conductivity of pathways in the cerebral cortex. J Physiol. 1968 Mar;195(2):339–367. doi: 10.1113/jphysiol.1968.sp008462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradler J. E., Barrioneuvo G. Long-term potentiation in hippocampal CA3 neurons: tetanized input regulates heterosynaptic efficacy. Synapse. 1989;4(2):132–142. doi: 10.1002/syn.890040207. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Prince D. A. Effects of local anesthetic QX-314 on the membrane properties of hippocampal pyramidal neurons. J Pharmacol Exp Ther. 1982 Mar;220(3):476–481. [PubMed] [Google Scholar]
- Crepel F., Krupa M. Activation of protein kinase C induces a long-term depression of glutamate sensitivity of cerebellar Purkinje cells. An in vitro study. Brain Res. 1988 Aug 23;458(2):397–401. doi: 10.1016/0006-8993(88)90486-6. [DOI] [PubMed] [Google Scholar]
- Davies S. N., Collingridge G. L. Role of excitatory amino acid receptors in synaptic transmission in area CA1 of rat hippocampus. Proc R Soc Lond B Biol Sci. 1989 May 22;236(1285):373–384. doi: 10.1098/rspb.1989.0028. [DOI] [PubMed] [Google Scholar]
- Frégnac Y., Shulz D., Thorpe S., Bienenstock E. A cellular analogue of visual cortical plasticity. Nature. 1988 May 26;333(6171):367–370. doi: 10.1038/333367a0. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Baughman R. W. NMDA- and non-NMDA-receptor components of excitatory synaptic potentials recorded from cells in layer V of rat visual cortex. J Neurosci. 1988 Sep;8(9):3522–3534. doi: 10.1523/JNEUROSCI.08-09-03522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura F., Nishigori A., Shirokawa T., Tsumoto T. Long-term potentiation and N-methyl-D-aspartate receptors in the visual cortex of young rats. J Physiol. 1989 Jul;414:125–144. doi: 10.1113/jphysiol.1989.sp017680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Res. 1984 Nov;320(1):65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Komatsu Y., Fujii K., Maeda J., Sakaguchi H., Toyama K. Long-term potentiation of synaptic transmission in kitten visual cortex. J Neurophysiol. 1988 Jan;59(1):124–141. doi: 10.1152/jn.1988.59.1.124. [DOI] [PubMed] [Google Scholar]
- Krettek J. E., Price J. L. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977 Jan 15;171(2):157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Lynch G. S., Dunwiddie T., Gribkoff V. Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature. 1977 Apr 21;266(5604):737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Miller J. P. Postsynaptic hyperpolarization during conditioning reversibly blocks induction of long-term potentiation. Nature. 1986 Apr 10;320(6062):529–530. doi: 10.1038/320529a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Connors B. W., Lighthall J. W., Prince D. A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985 Oct;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Nicholls J. G., Purves D. Monosynaptic chemical and electrical connexions between sensory and motor cells in the central nervous system of the leech. J Physiol. 1970 Aug;209(3):647–667. doi: 10.1113/jphysiol.1970.sp009184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Olton D. S. Frontal cortex, timing and memory. Neuropsychologia. 1989;27(1):121–130. doi: 10.1016/0028-3932(89)90094-8. [DOI] [PubMed] [Google Scholar]
- Penit-Soria J., Audinat E., Crepel F. Excitation of rat prefrontal cortical neurons by dopamine: an in vitro electrophysiological study. Brain Res. 1987 Nov 10;425(2):263–274. doi: 10.1016/0006-8993(87)90509-9. [DOI] [PubMed] [Google Scholar]
- Stanton P. K., Sejnowski T. J. Associative long-term depression in the hippocampus induced by hebbian covariance. Nature. 1989 May 18;339(6221):215–218. doi: 10.1038/339215a0. [DOI] [PubMed] [Google Scholar]
- Sutor B., Hablitz J. J. Long-term potentiation in frontal cortex: role of NMDA-modulated polysynaptic excitatory pathways. Neurosci Lett. 1989 Feb 13;97(1-2):111–117. doi: 10.1016/0304-3940(89)90148-1. [DOI] [PubMed] [Google Scholar]
- Szentágothai J. The Ferrier Lecture, 1977. The neuron network of the cerebral cortex: a functional interpretation. Proc R Soc Lond B Biol Sci. 1978 May 16;201(1144):219–248. doi: 10.1098/rspb.1978.0043. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. A magnesium-sensitive post-synaptic potential in rat cerebral cortex resembles neuronal responses to N-methylaspartate. J Physiol. 1986 Jan;370:531–549. doi: 10.1113/jphysiol.1986.sp015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B. A., Peters A. Form and distribution of neurons in rat cingulate cortex: areas 32, 24, and 29. J Comp Neurol. 1981 Feb 1;195(4):603–625. doi: 10.1002/cne.901950406. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]


