Abstract
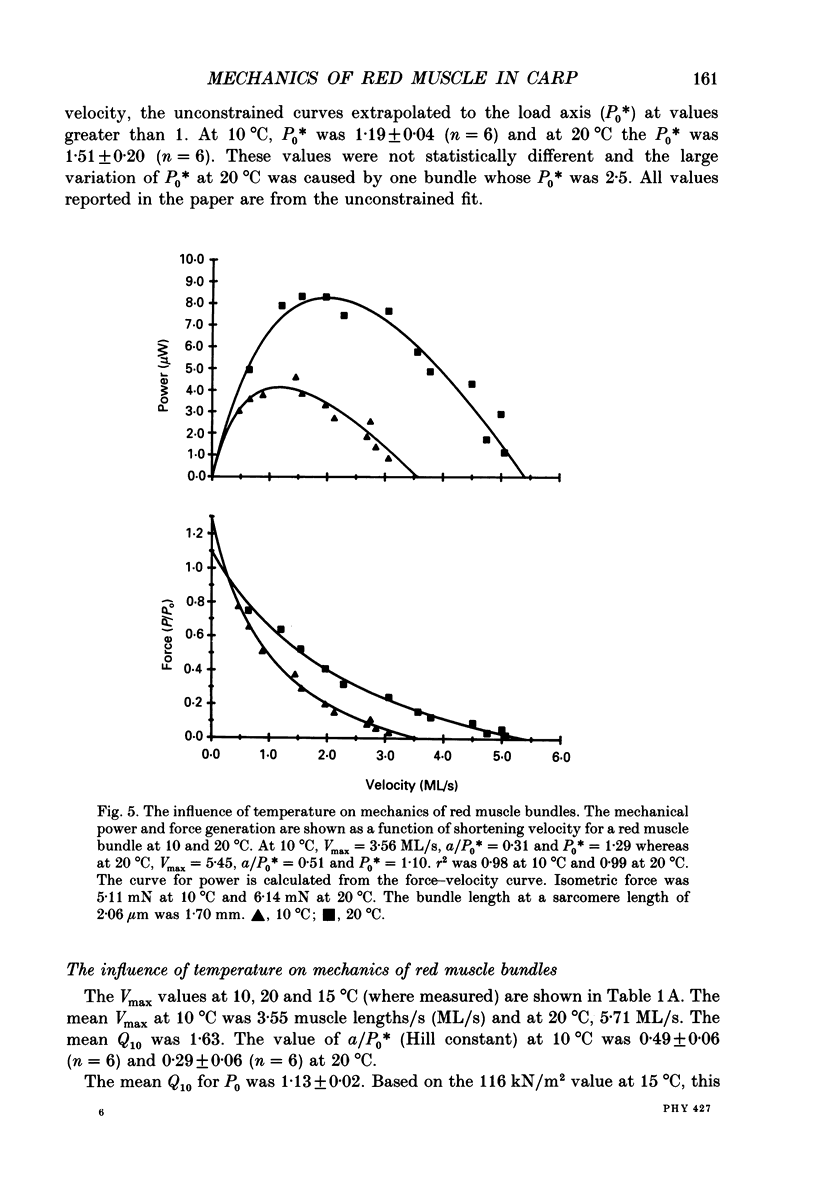
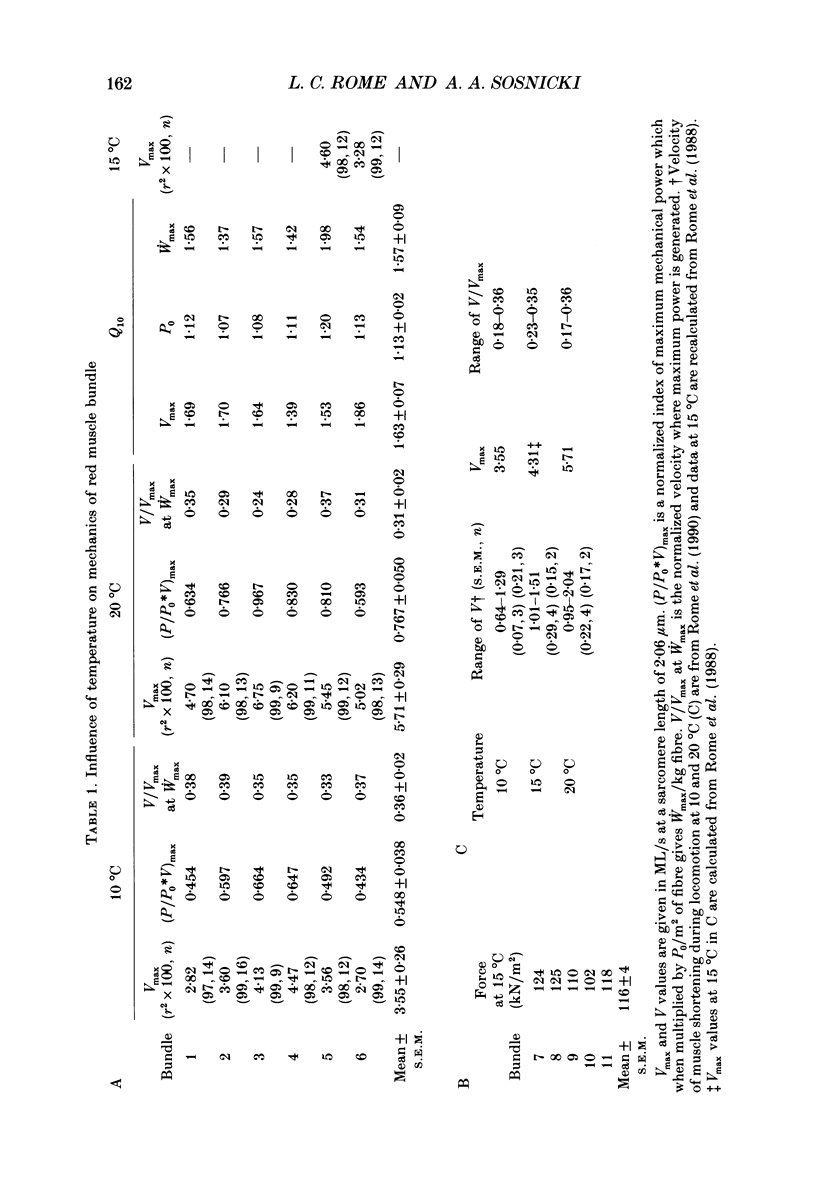
1. We measured the influence of temperature on maximum velocity of shortening (Vmax) of red muscle in carp in order to better understand the influence of temperature on locomotory performance. 2. A stable red muscle bundle preparation containing about 100 muscle fibres was developed. The bundles could not be activated directly by electrical stimulation, but rather contained sufficient nervous tissue so that acetylcholine released from the nerve terminals caused activation of the muscle. A high level of activation was achieved (116 kN/m2) by adding a combination of a 1 mM-caffeine and 10(-5) g/ml eserine to physiological Ringer solution and electrically stimulating the preparation. 3. Force-velocity characteristics were determined at 10 and 20 degrees C by the force clamp method. The data were well fitted by a hyperbola not constrained to pass through P0 = 1 (where P0 is the isometric force). The mean Vmax at 10 degrees C was 3.55 +/- 0.26 muscle lengths/s (ML/s) (n = 6) and at 20 degrees C, 5.71 +/- 0.29 ML/s (n = 6). The mean Q10 for Vmax was 1.63 +/- 0.07 (n = 6). The a/P0* (Hill constant) and Po* (where P0* is the extrapolated load at zero velocity) were 0.49 +/- 0.06 (n = 6) and 1.19 +/- 0.04 (n = 6) respectively at 10 degrees C and 0.29 +/- 0.06 (n = 6) and 1.51 +/- 0.20 (n = 6) respectively at 20 degrees C. 4. The mean Q10 for maximum isometric tension was 1.13 +/- 0.02 (n = 6). The maximal power generation was 59.7 +/- 2.3 W/kg (n = 6) at 10 degrees C and 94.3 +/- 3.2 W/kg (n = 6) at 20 degrees C representing a Q10 of 1.58. The Q10 is less than the product of Q10s for P0 and Vmax because of the greater curvature of the force-velocity curve at 20 degrees C. 5. The 1.63-fold higher Vmax at 20 degrees C than at 10 degrees C enables fish to swim with a 1.6-fold faster muscle shortening velocity, V, at the higher temperature. Thus at both 10 and 20 degrees C, red muscle is used only over the same narrow range of V/Vmax (0.18-0.36), where isolated muscle experiments suggest that power and efficiency are maximal. Thus V/Vmax appears to be an effective design constraint which limits the range of velocities over which muscle is used in vivo at different temperatures.
Full text
PDF


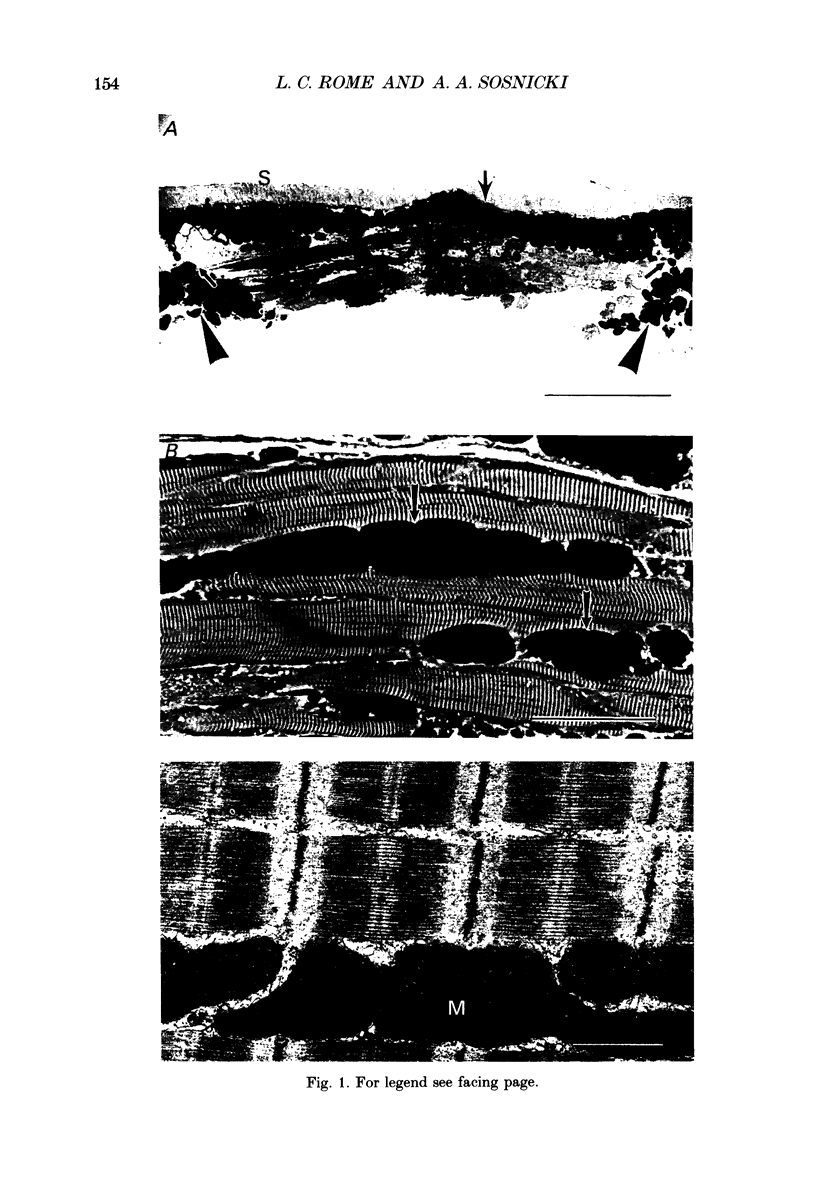

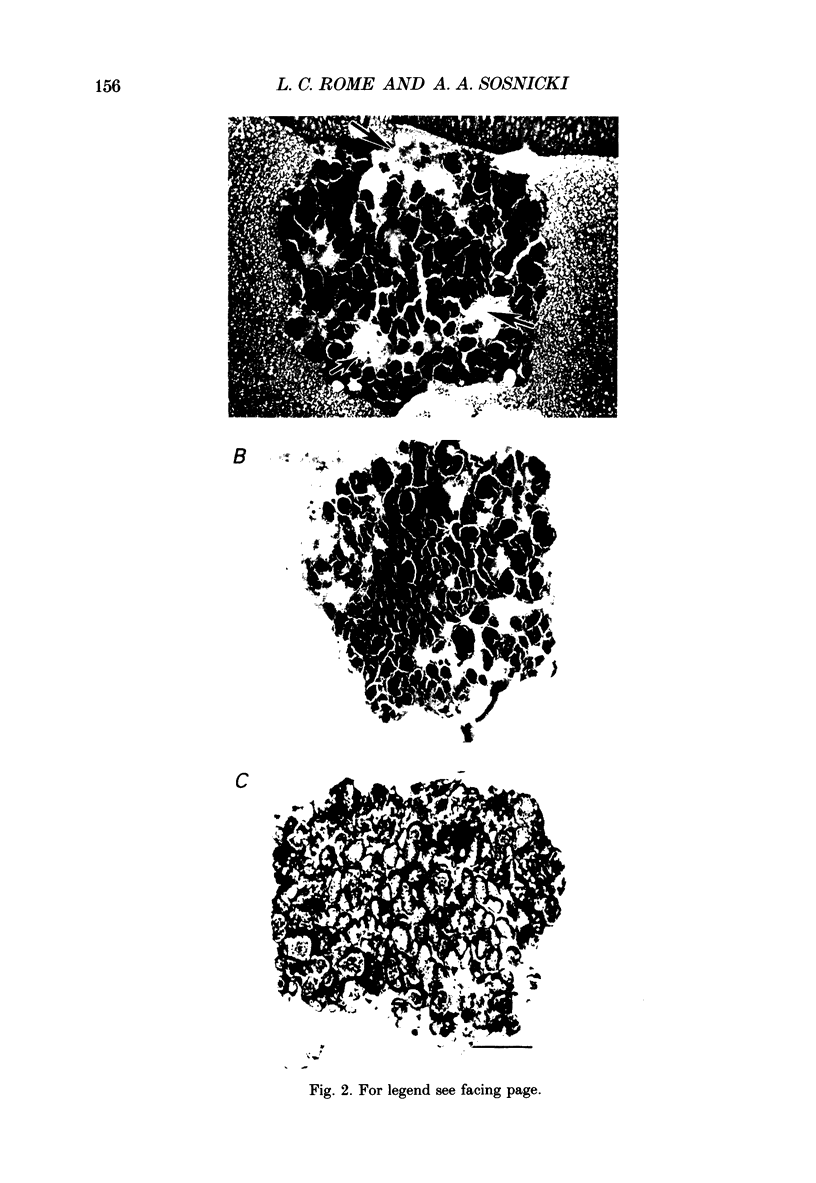











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akster H. A., Granzier H. L., ter Keurs H. E. A comparison of quantitative ultrastructural and contractile characteristics of muscle fibre types of the perch, Perca fluviatilis L. J Comp Physiol B. 1985;155(6):685–691. doi: 10.1007/BF00694582. [DOI] [PubMed] [Google Scholar]
- Altringham J. D., Johnston I. A. The mechanical properties of polyneuronally innervated, myotomal muscle fibres isolated from a teleost fish (Myoxocephalus scorpius). Pflugers Arch. 1988 Oct;412(5):524–529. doi: 10.1007/BF00582542. [DOI] [PubMed] [Google Scholar]
- Bennett A. F. Thermal dependence of muscle function. Am J Physiol. 1984 Aug;247(2 Pt 2):R217–R229. doi: 10.1152/ajpregu.1984.247.2.R217. [DOI] [PubMed] [Google Scholar]
- Claflin D. R., Faulkner J. A. Shortening velocity extrapolated to zero load and unloaded shortening velocity of whole rat skeletal muscle. J Physiol. 1985 Feb;359:357–363. doi: 10.1113/jphysiol.1985.sp015589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Johnston I. A., Sidell B. D., Driedzic W. R. Force-velocity characteristics and metabolism of carp muscle fibres following temperature acclimation. J Exp Biol. 1985 Nov;119:239–249. doi: 10.1242/jeb.119.1.239. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol. 1986 Jan;370:181–199. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Davies R. E. The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Appendix. Free energy and enthalpy of atp hydrolysis in the sarcoplasm. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):315–353. doi: 10.1098/rspb.1969.0096. [DOI] [PubMed] [Google Scholar]
- PAGE S. G., HUXLEY H. E. FILAMENT LENGTHS IN STRIATED MUSCLE. J Cell Biol. 1963 Nov;19:369–390. doi: 10.1083/jcb.19.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga K. W. The force-velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J Physiol. 1984 Jun;351:517–529. doi: 10.1113/jphysiol.1984.sp015260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome L. C., Funke R. P., Alexander R. M., Lutz G., Aldridge H., Scott F., Freadman M. Why animals have different muscle fibre types. Nature. 1988 Oct 27;335(6193):824–827. doi: 10.1038/335824a0. [DOI] [PubMed] [Google Scholar]
- Rome L. C., Kushmerick M. J. Energetics of isometric contractions as a function of muscle temperature. Am J Physiol. 1983 Jan;244(1):C100–C109. doi: 10.1152/ajpcell.1983.244.1.C100. [DOI] [PubMed] [Google Scholar]
- Rome L. C., Loughna P. T., Goldspink G. Muscle fiber activity in carp as a function of swimming speed and muscle temperature. Am J Physiol. 1984 Aug;247(2 Pt 2):R272–R279. doi: 10.1152/ajpregu.1984.247.2.R272. [DOI] [PubMed] [Google Scholar]
- Rome L. C., Loughna P. T., Goldspink G. Temperature acclimation: improved sustained swimming performance in carp at low temperatures. Science. 1985 Apr 12;228(4696):194–196. doi: 10.1126/science.228.4696.194. [DOI] [PubMed] [Google Scholar]
- Sosnicki A. A., Lutz G. J., Rome L. C., Goble D. O. Histochemical and molecular determination of fiber types in chemically skinned single equine skeletal muscle fibers. J Histochem Cytochem. 1989 Nov;37(11):1731–1738. doi: 10.1177/37.11.2530270. [DOI] [PubMed] [Google Scholar]
- Woledge R. C. The energetics of tortoise muscle. J Physiol. 1968 Aug;197(3):685–707. doi: 10.1113/jphysiol.1968.sp008582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuurveld J. G., Veerkamp J. H., Wirtz P. Isolated myofibers from rat skeletal muscle in suspension: cellular morphology and calcium homeostasis. Muscle Nerve. 1985 Nov-Dec;8(9):750–759. doi: 10.1002/mus.880080903. [DOI] [PubMed] [Google Scholar]




