Abstract
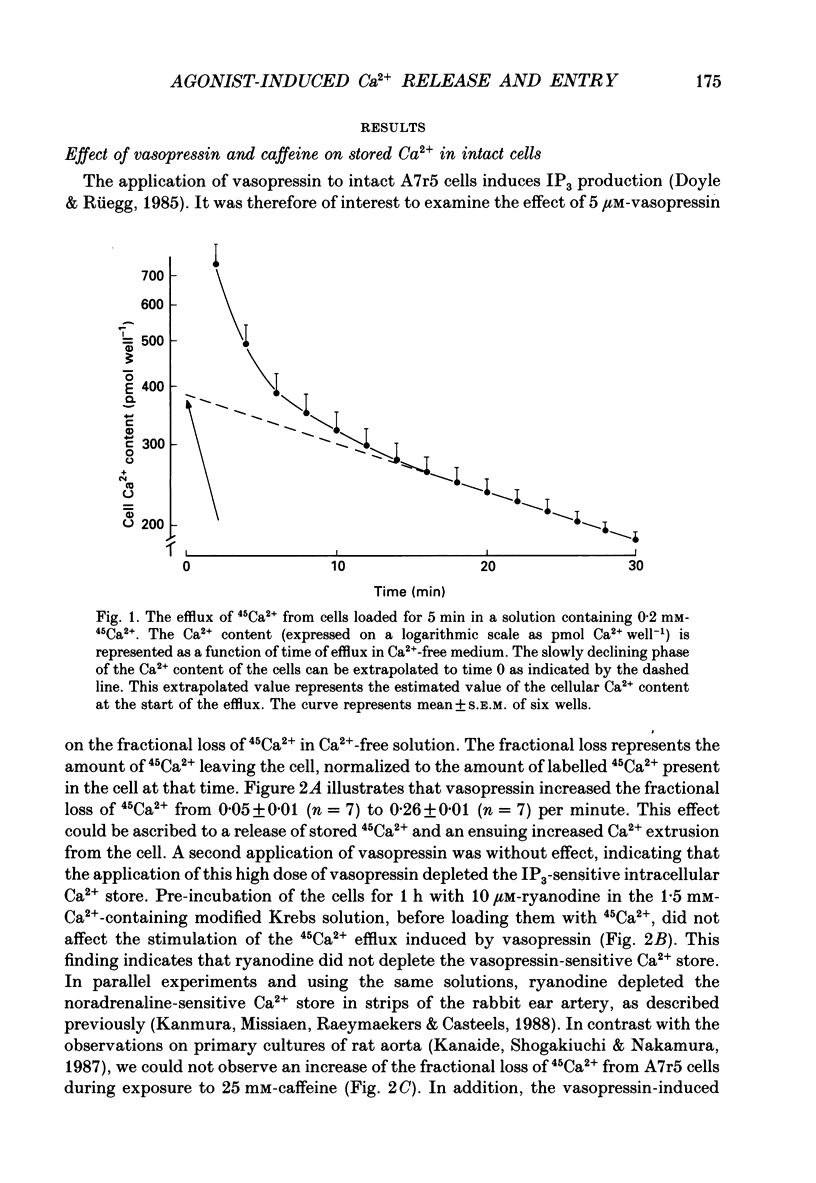
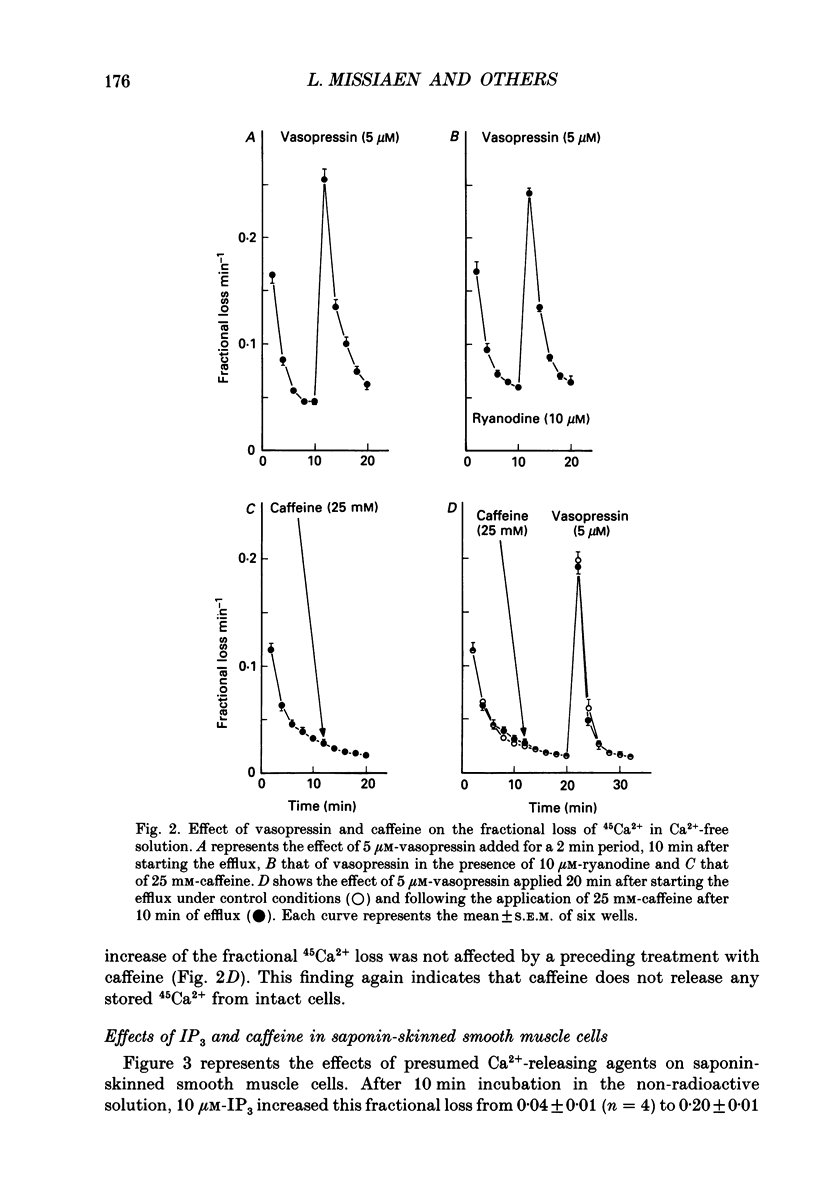
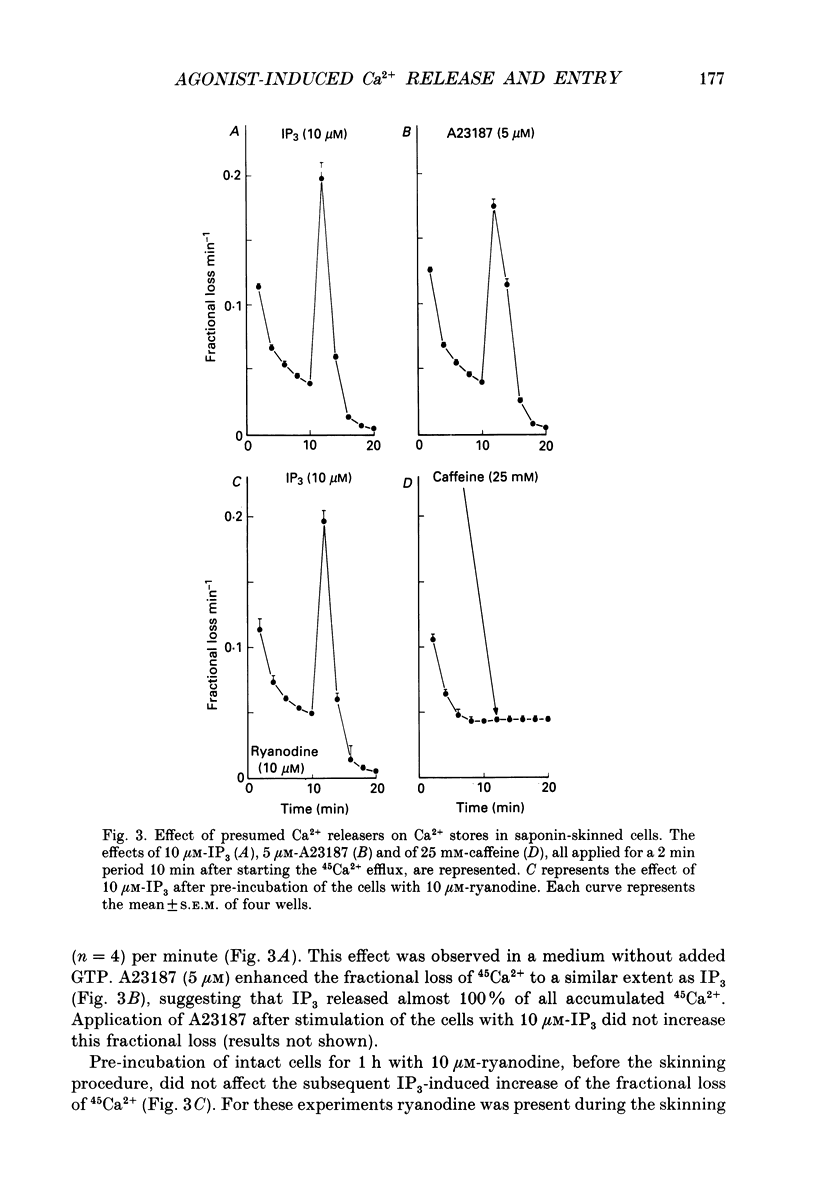
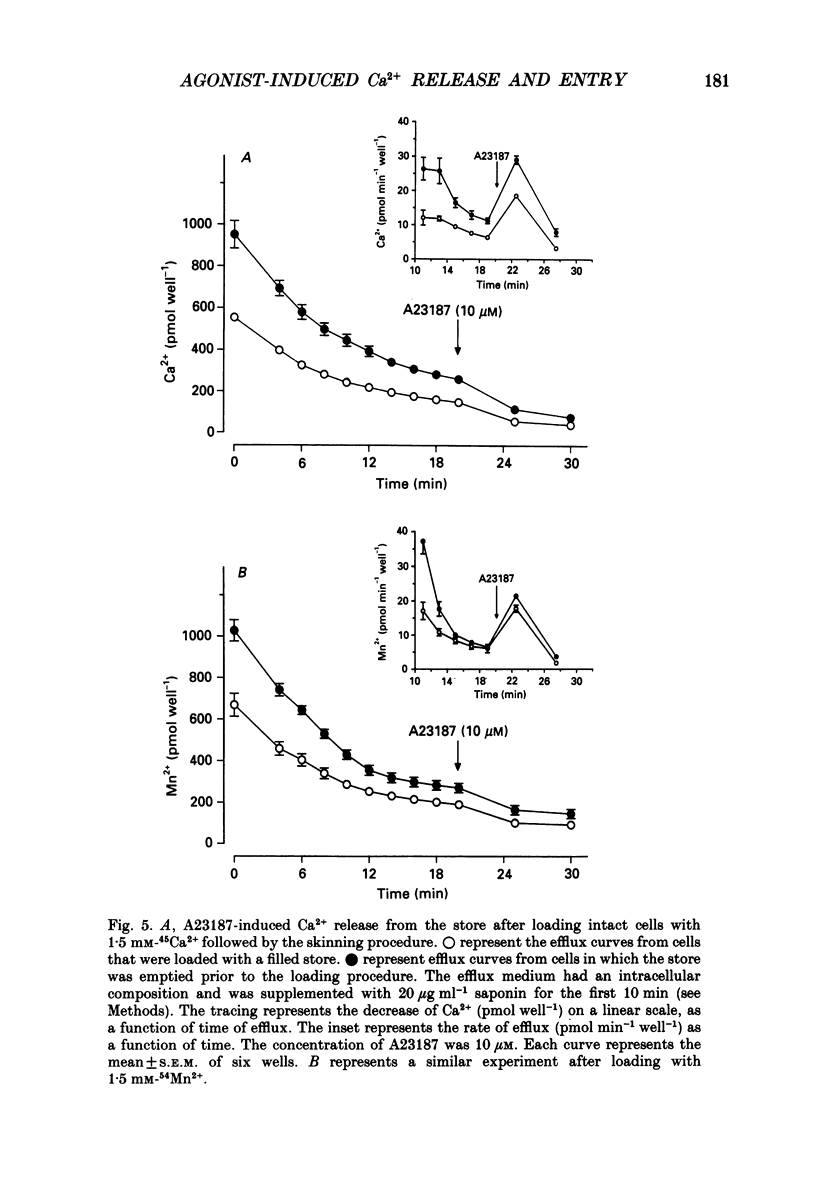
1. The properties of intracellular Ca2+ stores of intact- and of saponin-skinned A7r5 (an established cell line from embryonic rat aorta) smooth muscle cells were studied by measuring 45Ca2+ and 54Mn2+ fluxes. 2. Application of 5 microM-vasopressin to intact cells increased the fractional loss of 45Ca2+ in Ca2(+)-free solution by a factor of 5.2. This effect was not influenced by a pre-incubation with 10 microM-ryanodine. Caffeine (25 mM) did not stimulate the fractional loss of 45Ca2+ from intact cells. 3. In skinned cells 10 microM-IP3 (inositol 1,4,5-trisphosphate) and 5 microM-A23187 (a calcium ionophore) released the same amount of 45Ca2+. This release did not require GTP and was not affected by a pre-incubation with 10 microM-ryanodine. Caffeine (25 mM) did not release stored Ca2+. 4. NaF (1 mM) plus 10 microM-AlCl3 inhibited by 72% the 45Ca2+ uptake by the IP3-sensitive store of skinned cells at 0.15 microM-Ca2+. Cyclic AMP-dependent protein kinase did not stimulate this ATP-dependent 45Ca2+ uptake, nor could the presence of phospholamban be demonstrated immunologically. 5. The 45Ca2+ uptake by cells which had been depleted of Ca2+ with 5 microM-vasopressin was 69% higher than the uptake obtained without such proceeding depletion. This enhanced 45Ca2+ uptake did not occur through voltage-operated Ca2+ channels, because blockade of these channels with verapamil, or depolarization of the plasma membrane by increasing [K+] from 5.9 to 59 mM in the presence of verapamil, did not modify this uptake. 6. A similar increase of the 54Mn2+ uptake occurred in intact cells with a depleted Ca2+ store. If, however, the cells were first skinned and subsequently exposed to 54Mn2+, the ATP-dependent 54Mn2+ uptake amounted to less than 6% of the ATP-dependent 45Ca2+ uptake. 7. If intact cells were first exposed to a 45Ca2(+)- or 54Mn2(+)-containing solution, and subsequently skinned in a non-radioactive intracellular solution, the addition of 10 microM-A23187 to these cells released stored Ca2+ or Mn2+. The amount of released Ca2+ was only slightly larger than the amount of released Mn2+. If the intracellular store was depleted before loading, the amount of Ca2+ or Mn2+ released by the ionophore increased by 68 and 28%, respectively. 8. It is concluded that A7r5 smooth muscle cells do not express a Ca2(+)-induced Ca2+ release mechanism, but do contain an IP3-induced Ca2+ release mechanism which can release approximately all intracellularly accumulated 45Ca2+.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



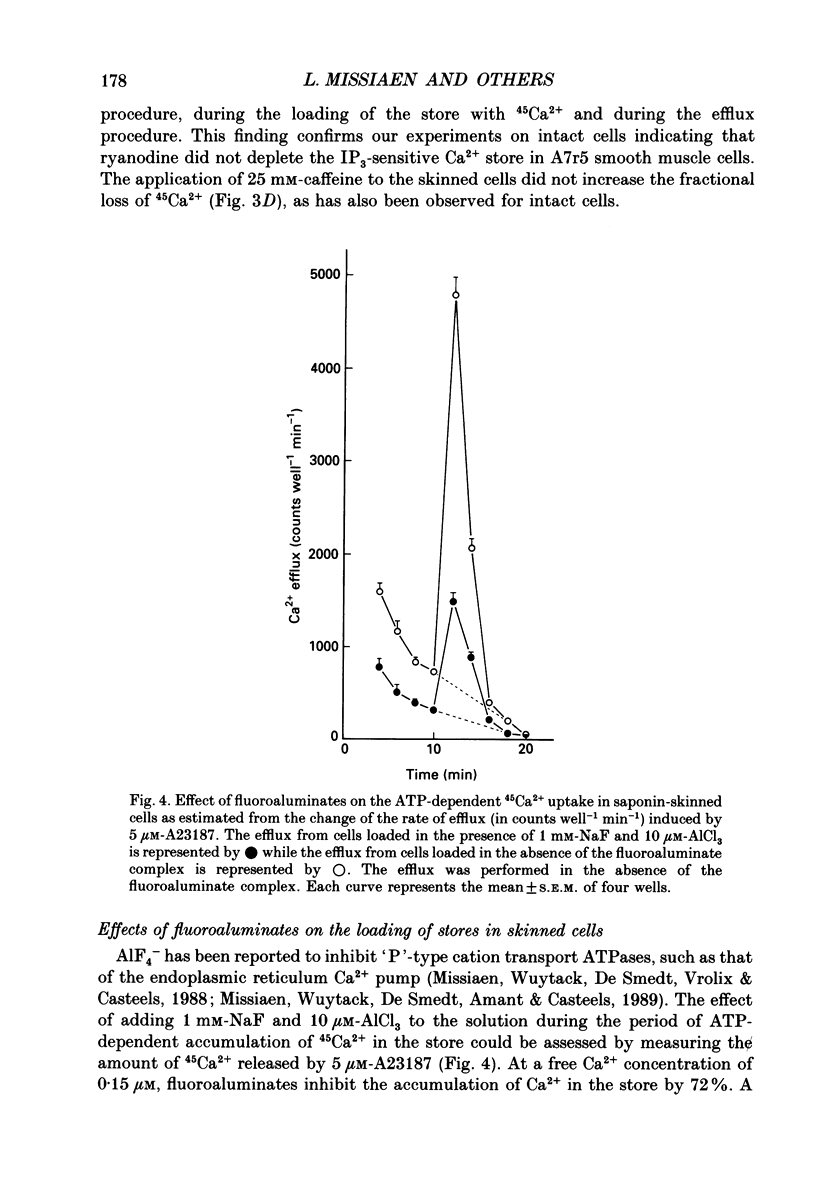







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. The Croonian lecture, 1988. Inositol lipids and calcium signalling. Proc R Soc Lond B Biol Sci. 1988 Sep 22;234(1277):359–378. doi: 10.1098/rspb.1988.0054. [DOI] [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981 Aug;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle V. M., Rüegg U. T. Vasopressin induced production of inositol trisphosphate and calcium efflux in a smooth muscle cell line. Biochem Biophys Res Commun. 1985 Aug 30;131(1):469–476. doi: 10.1016/0006-291x(85)91826-1. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont J. A., Vrolix M., Raeymaekers L., Wuytack F., Casteels R. Ca2+-transport ATPases of vascular smooth muscle. Circ Res. 1988 Feb;62(2):266–278. doi: 10.1161/01.res.62.2.266. [DOI] [PubMed] [Google Scholar]
- Ehrlich B. E., Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988 Dec 8;336(6199):583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Fill M., Coronado R. Ryanodine receptor channel of sarcoplasmic reticulum. Trends Neurosci. 1988 Oct;11(10):453–457. doi: 10.1016/0166-2236(88)90198-1. [DOI] [PubMed] [Google Scholar]
- Galizzi J. P., Qar J., Fosset M., Van Renterghem C., Lazdunski M. Regulation of calcium channels in aortic muscle cells by protein kinase C activators (diacylglycerol and phorbol esters) and by peptides (vasopressin and bombesin) that stimulate phosphoinositide breakdown. J Biol Chem. 1987 May 25;262(15):6947–6950. [PubMed] [Google Scholar]
- Gomes da Costa A., Madeira V. M. Magnesium and manganese ions modulate Ca2+ uptake and its energetic coupling in sarcoplasmic reticulum. Arch Biochem Biophys. 1986 Aug 15;249(1):199–206. doi: 10.1016/0003-9861(86)90575-8. [DOI] [PubMed] [Google Scholar]
- Hwang K. S., van Breemen C. Ryanodine modulation of 45Ca efflux and tension in rabbit aortic smooth muscle. Pflugers Arch. 1987 Apr;408(4):343–350. doi: 10.1007/BF00581127. [DOI] [PubMed] [Google Scholar]
- Inoue T., Kawashima H. 1,25-Dihydroxyvitamin D3 stimulates 45Ca2+-uptake by cultured vascular smooth muscle cells derived from rat aorta. Biochem Biophys Res Commun. 1988 May 16;152(3):1388–1394. doi: 10.1016/s0006-291x(88)80439-x. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Excitation--contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1981 Dec;321:513–535. doi: 10.1113/jphysiol.1981.sp014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Characteristics of inositol trisphosphate-mediated Ca2+ release from permeabilized hepatocytes. J Biol Chem. 1986 Nov 5;261(31):14658–14664. [PubMed] [Google Scholar]
- Kanaide H., Shogakiuchi Y., Nakamura M. The norepinephrine-sensitive Ca2+-storage site differs from the caffeine-sensitive site in vascular smooth muscle of the rat aorta. FEBS Lett. 1987 Apr 6;214(1):130–134. doi: 10.1016/0014-5793(87)80027-3. [DOI] [PubMed] [Google Scholar]
- Kanmura Y., Missiaen L., Raeymaekers L., Casteels R. Ryanodine reduces the amount of calcium in intracellular stores of smooth-muscle cells of the rabbit ear artery. Pflugers Arch. 1988 Dec;413(2):153–159. doi: 10.1007/BF00582525. [DOI] [PubMed] [Google Scholar]
- Kawashima H. 1,25-Dihydroxyvitamin D3 stimulates Ca-ATPase in a vascular smooth muscle cell line. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1138–1143. doi: 10.1016/0006-291x(88)90747-4. [DOI] [PubMed] [Google Scholar]
- Liu Q. Y., Lai F. A., Rousseau E., Jones R. V., Meissner G. Multiple conductance states of the purified calcium release channel complex from skeletal sarcoplasmic reticulum. Biophys J. 1989 Mar;55(3):415–424. doi: 10.1016/S0006-3495(89)82835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Jacob R., Hallam T. J. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem. 1989 Jan 25;264(3):1522–1527. [PubMed] [Google Scholar]
- Missiaen L., Wuytack F., De Smedt H., Amant F., Casteels R. AIF4-induced inhibition of the ATPase activity, the Ca2+-transport activity and the phosphoprotein-intermediate formation of plasma-membrane and endo(sarco)plasmic-reticulum Ca2+-transport ATPases in different tissues. Evidence for a tissue-dependent functional difference. Biochem J. 1989 Jul 15;261(2):655–660. doi: 10.1042/bj2610655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L., Wuytack F., De Smedt H., Vrolix M., Casteels R. AlF4- reversibly inhibits 'P'-type cation-transport ATPases, possibly by interacting with the phosphate-binding site of the ATPase. Biochem J. 1988 Aug 1;253(3):827–833. doi: 10.1042/bj2530827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyses L., Reinlib L., Carafoli E. Phosphorylation of the Ca2+-pumping ATPase of heart sarcolemma and erythrocyte plasma membrane by the cAMP-dependent protein kinase. J Biol Chem. 1985 Aug 25;260(18):10283–10287. [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Raeymaekers L., Jones L. R. Evidence for the presence of phospholamban in the endoplasmic reticulum of smooth muscle. Biochim Biophys Acta. 1986 Jun 19;882(2):258–265. doi: 10.1016/0304-4165(86)90163-7. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Hallam T. J. Calcium signalling in non-excitable cells: notes on oscillations and store refilling. Cell Calcium. 1989 Jul;10(5):385–395. doi: 10.1016/0143-4160(89)90064-x. [DOI] [PubMed] [Google Scholar]
- Rüegg U. T., Doyle V. M., Zuber J. F., Hof R. P. A smooth muscle cell line suitable for the study of voltage sensitive calcium channels. Biochem Biophys Res Commun. 1985 Jul 16;130(1):447–453. doi: 10.1016/0006-291x(85)90437-1. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Merritt J. E., Hallam T. J., Rink T. J. Receptor-mediated calcium entry in fura-2-loaded human platelets stimulated with ADP and thrombin. Dual-wavelengths studies with Mn2+. Biochem J. 1989 Mar 15;258(3):923–926. doi: 10.1042/bj2580923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind M. S., McCoy C. E., Chobanian A., Schwartz J. H. Regulation of intracellular calcium by cell pH in vascular smooth muscle cells. Am J Physiol. 1989 Feb;256(2 Pt 1):C234–C240. doi: 10.1152/ajpcell.1989.256.2.C234. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968 Jan;159(1):129–145. [PubMed] [Google Scholar]
- Sperti G., Colucci W. S. Phorbol ester-stimulated bidirectional transmembrane calcium flux in A7r5 vascular smooth muscle cells. Mol Pharmacol. 1987 Jul;32(1):37–42. [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Dawson A. P., Scharff O., Foder B., Cullen P. J., Drøbak B. K., Bjerrum P. J., Christensen S. B., Hanley M. R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989 Apr;27(1-2):17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Romey G., Lazdunski M. Vasopressin modulates the spontaneous electrical activity in aortic cells (line A7r5) by acting on three different types of ionic channels. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9365–9369. doi: 10.1073/pnas.85.23.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne P., Breittmayer J. P., Duval D., Frelin C., Lazdunski M. The Na+/Ca2+ antiporter in aortic smooth muscle cells. Characterization and demonstration of an activation by phorbol esters. J Biol Chem. 1988 Jun 15;263(17):8078–8083. [PubMed] [Google Scholar]
- Vigne P., Breittmayer J. P., Frelin C., Lazdunski M. Dual control of the intracellular pH in aortic smooth muscle cells by a cAMP-sensitive HCO3-/Cl- antiporter and a protein kinase C-sensitive Na+/H+ antiporter. J Biol Chem. 1988 Dec 5;263(34):18023–18029. [PubMed] [Google Scholar]
- Vigne P., Breittmayer J. P., Lazdunski M., Frelin C. The regulation of the cytoplasmic free Ca2+ concentration in aortic smooth muscle cells (A7r5 line) after stimulation by vasopressin and bombesin. Eur J Biochem. 1988 Sep 1;176(1):47–52. doi: 10.1111/j.1432-1033.1988.tb14249.x. [DOI] [PubMed] [Google Scholar]
- Wuytack F., De Schutter G., Casteels R. Purification of (Ca2+ + Mg2+)-ATPase from smooth muscle by calmodulin affinity chromatography. FEBS Lett. 1981 Jul 6;129(2):297–300. doi: 10.1016/0014-5793(81)80187-1. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., Deranleau D. A., Baggiolini M. Calcium fluxes and calcium buffering in human neutrophils. J Biol Chem. 1986 Aug 5;261(22):10163–10168. [PubMed] [Google Scholar]


