Abstract
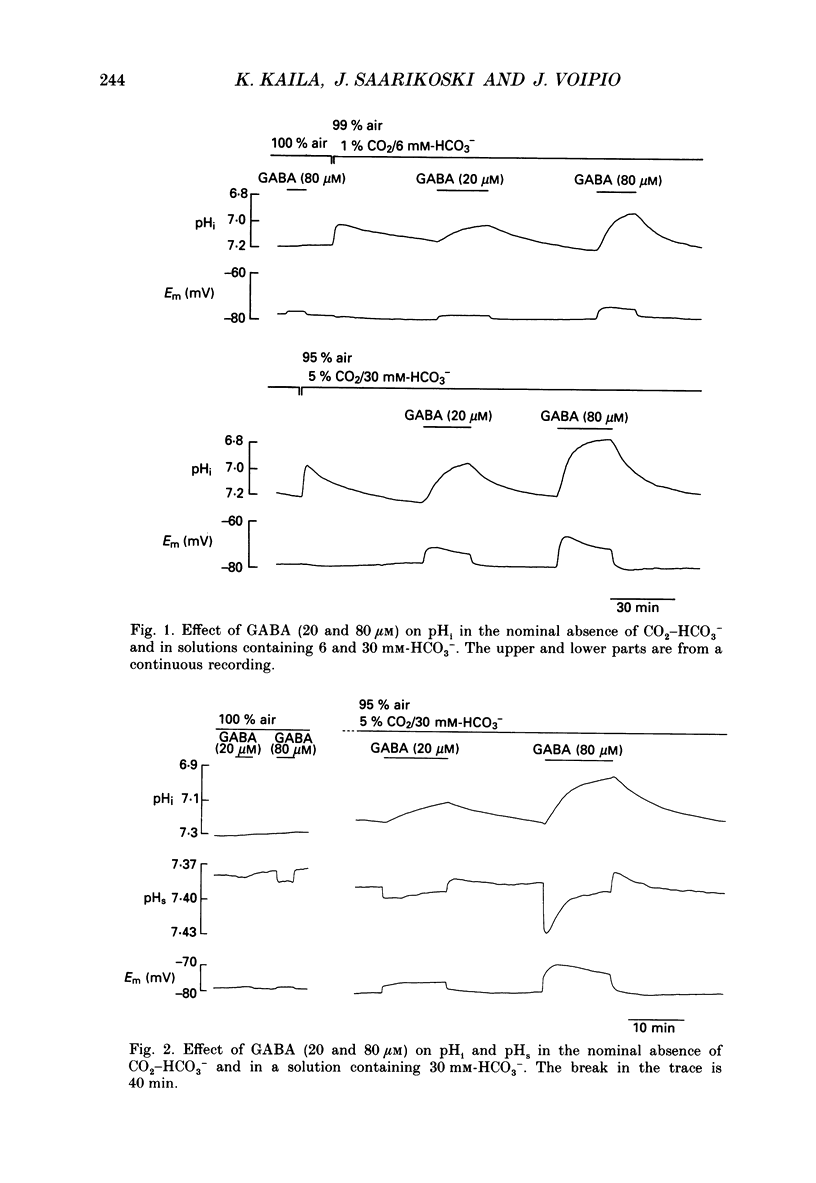
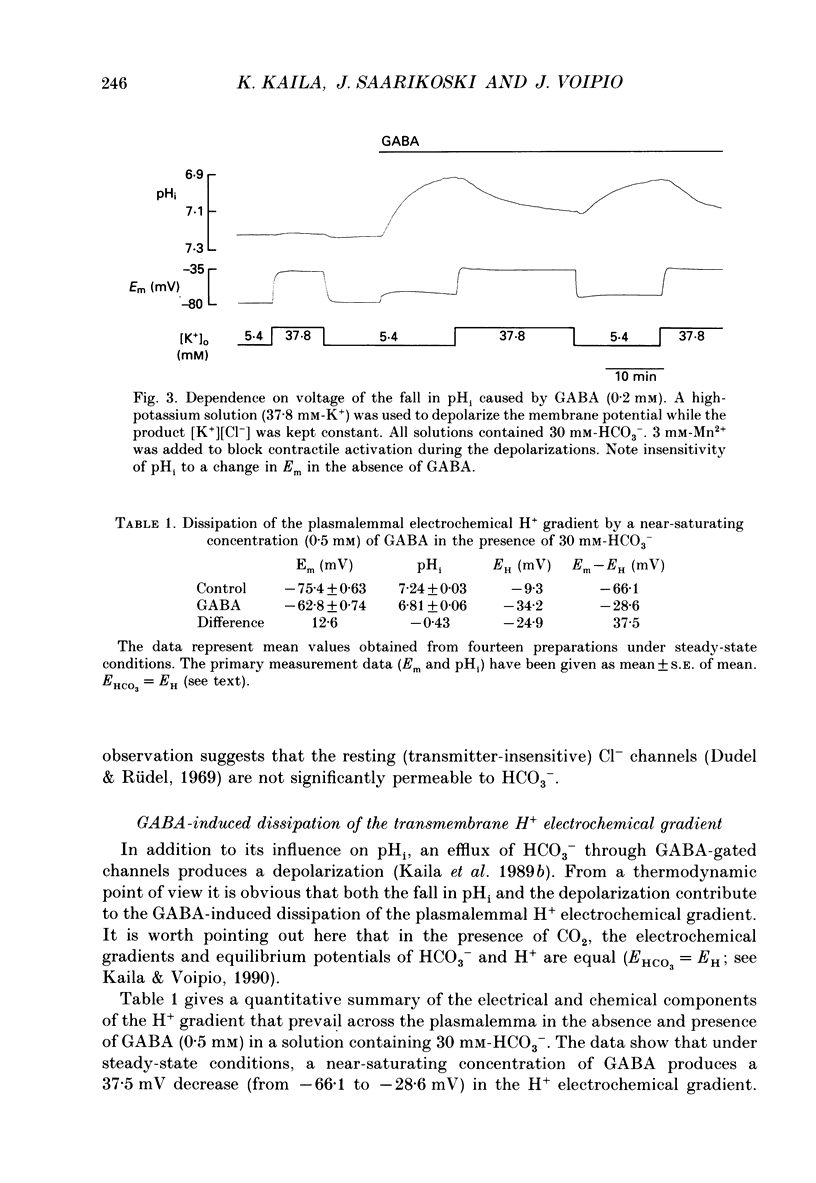
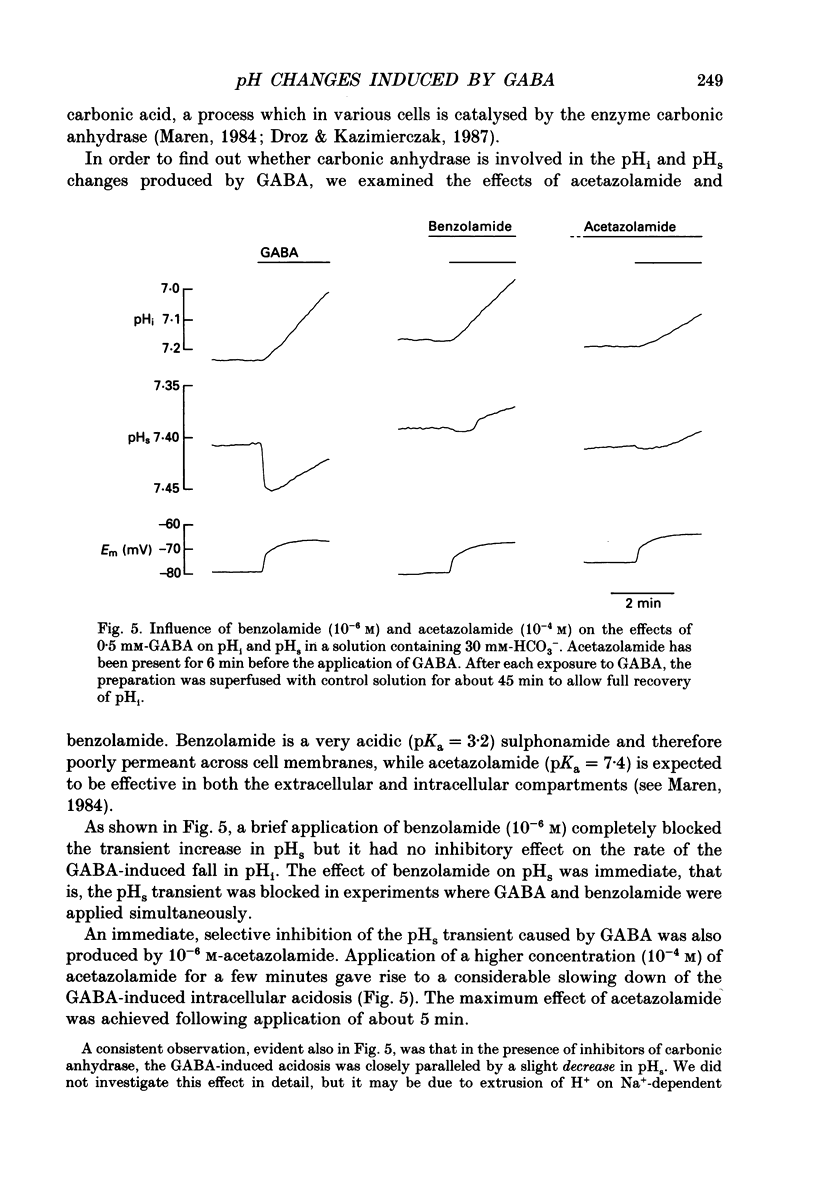
1. The mode of action of gamma-aminobutyric acid (GABA) on intracellular pH (pHi) and surface pH (pHs) was studied in crayfish muscle fibres using H(+)-selective microelectrodes. The extracellular HCO3- concentration was varied (0-30 mM) at constant pH (7.4). 2. GABA (5 x 10(-6)-10(-3) M) produced a reversible fall in pHi which showed a dependence on the concentrations of both GABA and HCO3-. The fall in pHi was associated with a transient increase in pHs and it was inhibited by a K(+)-induced depolarization. 3. In the presence of 30 mM-HCO3-, a near-saturating concentration of GABA (0.5 mM) produced a mean fall in pHi of 0.43 units. This change in pHi accounted for about two-thirds of the GABA-induced decrease (from -66 to -29 mV) in the sarcolemmal H+ driving force, while the rest was due to the simultaneous depolarization. 4. The apparent net efflux of HCO3- (JHCO3e) produced by a given concentration of GABA was estimated on the basis of the instantaneous rate of change of pHi. In the presence of 30 mM-HCO3-, JHCO3e following exposure to 0.5 mM-GABA had a mean value of 8.0 mmol l-1 min-1. Under steady-state conditions (at plateau acidosis), the intracellular acid load produced by 0.5 mM-GABA was about 25% of that seen at the onset of the application. 5. The GABA-induced HCO3- permeability, calculated on the basis of the flux data, showed a concentration dependence similar to that of the GABA-activated conductance described in previous work. 6. The GABA-induced increase in pHs was immediately blocked by both a membrane-permeant inhibitor of carbonic anhydrase (acetazolamide, 10(-6) M) and by a poorly permeant inhibitor (benzolamide, 10(-6) M). 7. Application of acetazolamide (10(-4) M) for 5 min or more produced a decrease of up to 60% in the maximum rate of fall of pHi at GABA concentrations higher than 20 microM. 8. The recovery of the GABA-induced acidosis was associated with a fall in pHs. The recovery was completely blocked in solutions devoid of Na+ or of Cl-, as well as by DIDS (4,4'-diisothiocyanostilbene-2,2'-disulphonic acid, 10(-5) M). This indicates that the maintenance of a non-equilibrium H+ gradient at plateau acidosis and the recovery of pHi are attributable to Na(+)-dependent Cl(-)-HCO3- exchange. 9. We conclude that the effects of GABA on pHi and pHs are due to electrodiffusion of HCO3- across postsynaptic anion channels.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




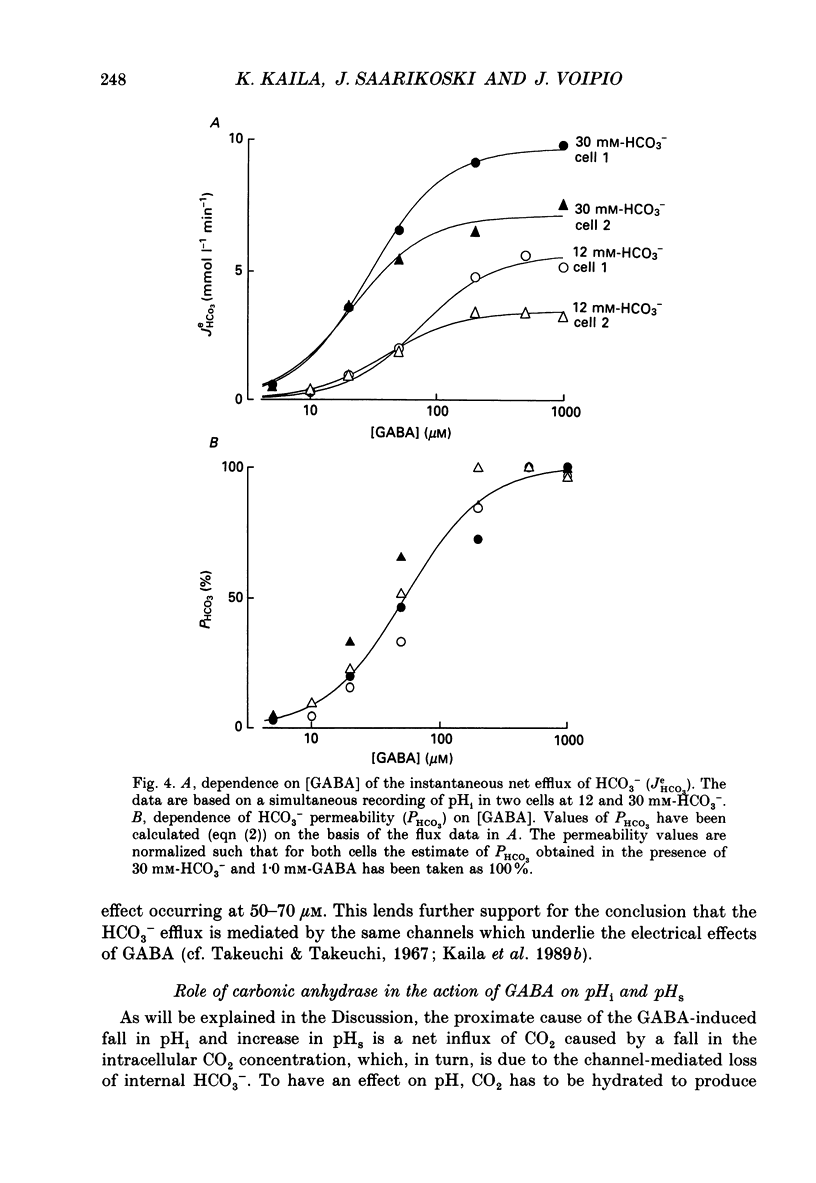









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie R. F., Putnam R. W., Roos A. The intracellular pH of frog skeletal muscle: its regulation in isotonic solutions. J Physiol. 1983 Dec;345:175–187. doi: 10.1113/jphysiol.1983.sp014973. [DOI] [PMC free article] [PubMed] [Google Scholar]
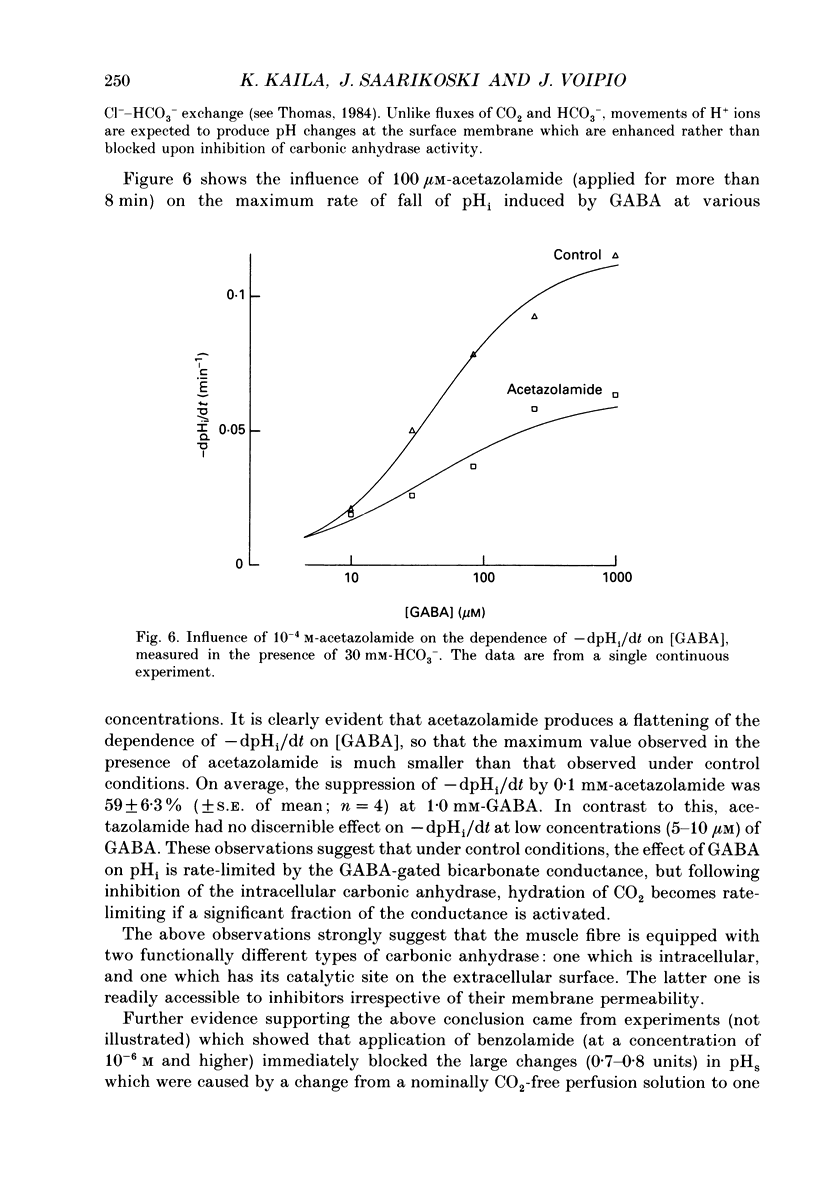
- Aldskogius H., Arvidsson J., Hansson P. Carbonic anhydrase enzyme histochemistry of cranial nerve primary sensory afferent neurons in the rat. Histochemistry. 1988;88(2):151–154. doi: 10.1007/BF00493297. [DOI] [PubMed] [Google Scholar]
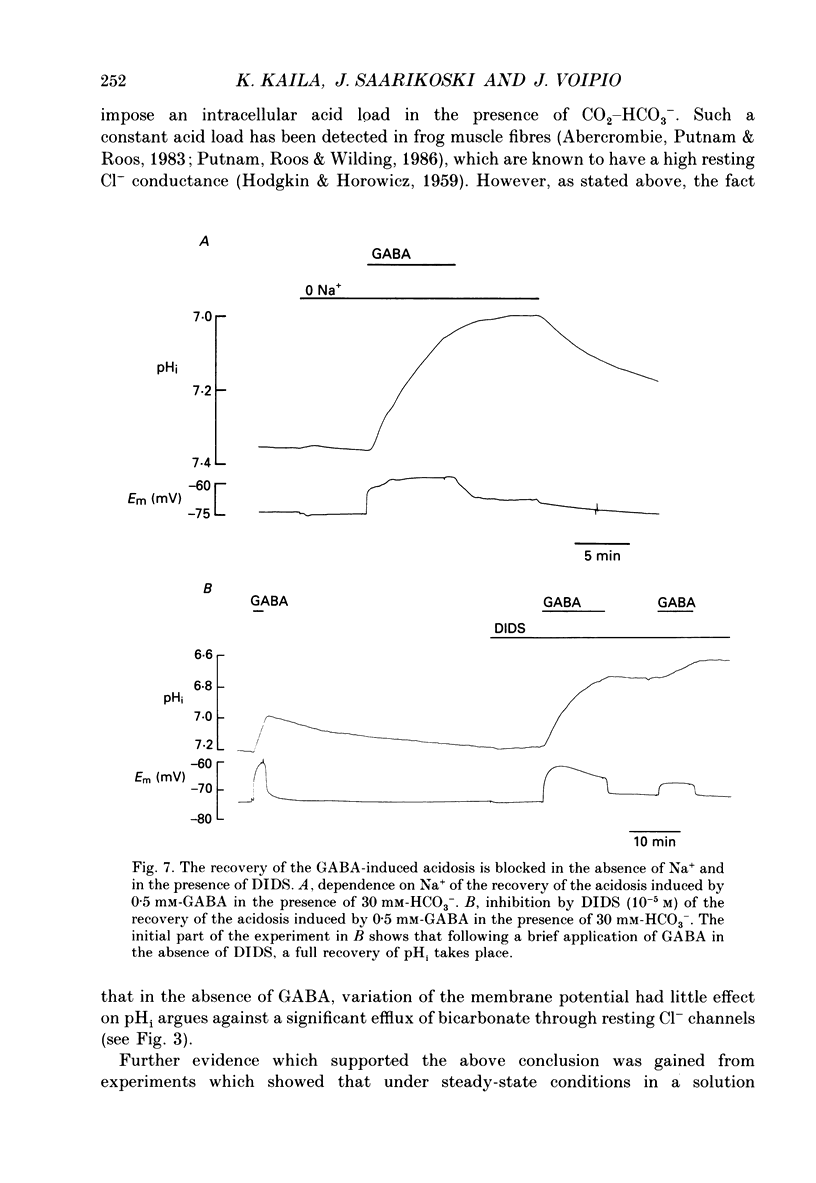
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983 Jan;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C., Kaila K., Vaughan-Jones R. D. Mechanism of rate-dependent pH changes in the sheep cardiac Purkinje fibre. J Physiol. 1988 Dec;406:483–501. doi: 10.1113/jphysiol.1988.sp017392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. D., Elliott A. C., Lau K. R. Indirect evidence for the presence of non-specific anion channels in rabbit mandibular salivary gland acinar cells. J Physiol. 1989 Jul;414:415–431. doi: 10.1113/jphysiol.1989.sp017696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M., Chan C. Y. Stimulus-induced extracellular pH transients in the in vitro turtle cerebellum. Neuroscience. 1988 Dec;27(3):941–948. doi: 10.1016/0306-4522(88)90197-2. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Schlue W. R. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol. 1989 Apr;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droz B., Kazimierczak J. Carbonic anhydrase in primary sensory neurons of dorsal root ganglia. Comp Biochem Physiol B. 1987;88(3):713–717. doi: 10.1016/0305-0491(87)90233-1. [DOI] [PubMed] [Google Scholar]
- Dudel J., Rüdel R. Voltage controlled contractions and current voltage relations of crayfish muscle fibers in chloride-free solutions. Pflugers Arch. 1969;308(4):291–314. doi: 10.1007/BF00587182. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Nakamura J., Shinnick-Gallagher P. The effects of temperature, pH and Cl-pump inhibitors on GABA responses recorded from cat dorsal root ganglia. Brain Res. 1983 May 16;267(2):249–259. doi: 10.1016/0006-8993(83)90877-6. [DOI] [PubMed] [Google Scholar]
- Galler S., Moser H. The ionic mechanism of intracellular pH regulation in crayfish muscle fibres. J Physiol. 1986 May;374:137–151. doi: 10.1113/jphysiol.1986.sp016071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers C., Gros G., Gärtner A. Extracellular carbonic anhydrase of skeletal muscle associated with the sarcolemma. J Appl Physiol (1985) 1985 Aug;59(2):548–558. doi: 10.1152/jappl.1985.59.2.548. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan J. W., Alles W. P., Lewis S. A. Single anion-selective channels in basolateral membrane of a mammalian tight epithelium. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7791–7795. doi: 10.1073/pnas.82.22.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata N., Oomura Y., Akaike N., Edwards C. The anion selectivity of the gamma-aminobutyric acid controlled chloride channel in the perfused spinal ganglion cell of frog. Neurosci Res. 1986 Jul;3(5):371–383. doi: 10.1016/0168-0102(86)90029-5. [DOI] [PubMed] [Google Scholar]
- Kaila K., Mattsson K., Voipio J. Fall in intracellular pH and increase in resting tension induced by a mitochondrial uncoupling agent in crayfish muscle. J Physiol. 1989 Jan;408:271–293. doi: 10.1113/jphysiol.1989.sp017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K., Pasternack M., Saarikoski J., Voipio J. Influence of GABA-gated bicarbonate conductance on potential, current and intracellular chloride in crayfish muscle fibres. J Physiol. 1989 Sep;416:161–181. doi: 10.1113/jphysiol.1989.sp017755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K., Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987 Nov 12;330(6144):163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Kelly J. S., Krnjević K., Morris M. E., Yim G. K. Anionic permeability of cortical neurones. Exp Brain Res. 1969;7(1):11–31. doi: 10.1007/BF00236105. [DOI] [PubMed] [Google Scholar]
- Kraig R. P., Ferreira-Filho C. R., Nicholson C. Alkaline and acid transients in cerebellar microenvironment. J Neurophysiol. 1983 Mar;49(3):831–850. doi: 10.1152/jn.1983.49.3.831. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Osipchuk Y. V., Shelest T. N., Smirnoff S. V. Rapid extracellular pH transients related to synaptic transmission in rat hippocampal slices. Brain Res. 1987 Dec 15;436(2):352–356. doi: 10.1016/0006-8993(87)91678-7. [DOI] [PubMed] [Google Scholar]
- Lau K. R., Elliott A. C., Brown P. D. Acetylcholine-induced intracellular acidosis in rabbit salivary gland acinar cells. Am J Physiol. 1989 Feb;256(2 Pt 1):C288–C295. doi: 10.1152/ajpcell.1989.256.2.C288. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Maren T. H. The general physiology of reactions catalyzed by carbonic anhydrase and their inhibition by sulfonamides. Ann N Y Acad Sci. 1984;429:568–579. doi: 10.1111/j.1749-6632.1984.tb12389.x. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Mattsson K., Pasternack M., Voipio J., Kaila K. Postsynaptic fall in intracellular pH and increase in surface pH caused by efflux of formate and acetate anions through GABA-gated channels in crayfish muscle fibres. Neuroscience. 1990;34(2):359–368. doi: 10.1016/0306-4522(90)90145-t. [DOI] [PubMed] [Google Scholar]
- Melvin J. E., Moran A., Turner R. J. The role of HCO3- and Na+/H+ exchange in the response of rat parotid acinar cells to muscarinic stimulation. J Biol Chem. 1988 Dec 25;263(36):19564–19569. [PubMed] [Google Scholar]
- Moody W., Jr Effects of intracellular H+ on the electrical properties of excitable cells. Annu Rev Neurosci. 1984;7:257–278. doi: 10.1146/annurev.ne.07.030184.001353. [DOI] [PubMed] [Google Scholar]
- Putnam R. W., Roos A., Wilding T. J. Properties of the intracellular pH-regulating systems of frog skeletal muscle. J Physiol. 1986 Dec;381:205–219. doi: 10.1113/jphysiol.1986.sp016323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sapirstein V. S., Strocchi P., Gilbert J. M. Properties and function of brain carbonic anhydrase. Ann N Y Acad Sci. 1984;429:481–493. doi: 10.1111/j.1749-6632.1984.tb12375.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. A study of the action of picrotoxin on the inhibitory neuromuscular junction of the crayfish. J Physiol. 1969 Nov;205(2):377–391. doi: 10.1113/jphysiol.1969.sp008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Anion permeability of the inhibitory post-synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1967 Aug;191(3):575–590. doi: 10.1113/jphysiol.1967.sp008269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol. 1984 Sep;354:3P–22P. doi: 10.1113/jphysiol.1984.sp015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A. Intracellular pH in depolarized cardiac Purkinje strands. Pflugers Arch. 1985 Sep;405(2):118–126. doi: 10.1007/BF00584532. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. An investigation of chloride-bicarbonate exchange in the sheep cardiac Purkinje fibre. J Physiol. 1986 Oct;379:377–406. doi: 10.1113/jphysiol.1986.sp016259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. M., Miller C. A voltage-gated anion channel from the electric organ of Torpedo californica. J Biol Chem. 1979 Oct 25;254(20):10161–10166. [PubMed] [Google Scholar]
- Wistrand P. J. Properties of membrane-bound carbonic anhydrase. Ann N Y Acad Sci. 1984;429:195–206. doi: 10.1111/j.1749-6632.1984.tb12333.x. [DOI] [PubMed] [Google Scholar]
- Wong V., Barrett C. P., Donati E. J., Guth L. Distribution of carbonic anhydrase activity in neurons of the rat. J Comp Neurol. 1987 Mar 1;257(1):122–129. doi: 10.1002/cne.902570109. [DOI] [PubMed] [Google Scholar]
- de Hemptinne A., Marrannes R., Vanheel B. Influence of organic acids on intracellular pH. Am J Physiol. 1983 Sep;245(3):C178–C183. doi: 10.1152/ajpcell.1983.245.3.C178. [DOI] [PubMed] [Google Scholar]
- de Hemptinne A., Marrannes R., Vanheel B. Surface pH and the control of intracellular pH in cardiac and skeletal muscle. Can J Physiol Pharmacol. 1987 May;65(5):970–977. doi: 10.1139/y87-154. [DOI] [PubMed] [Google Scholar]


