Abstract
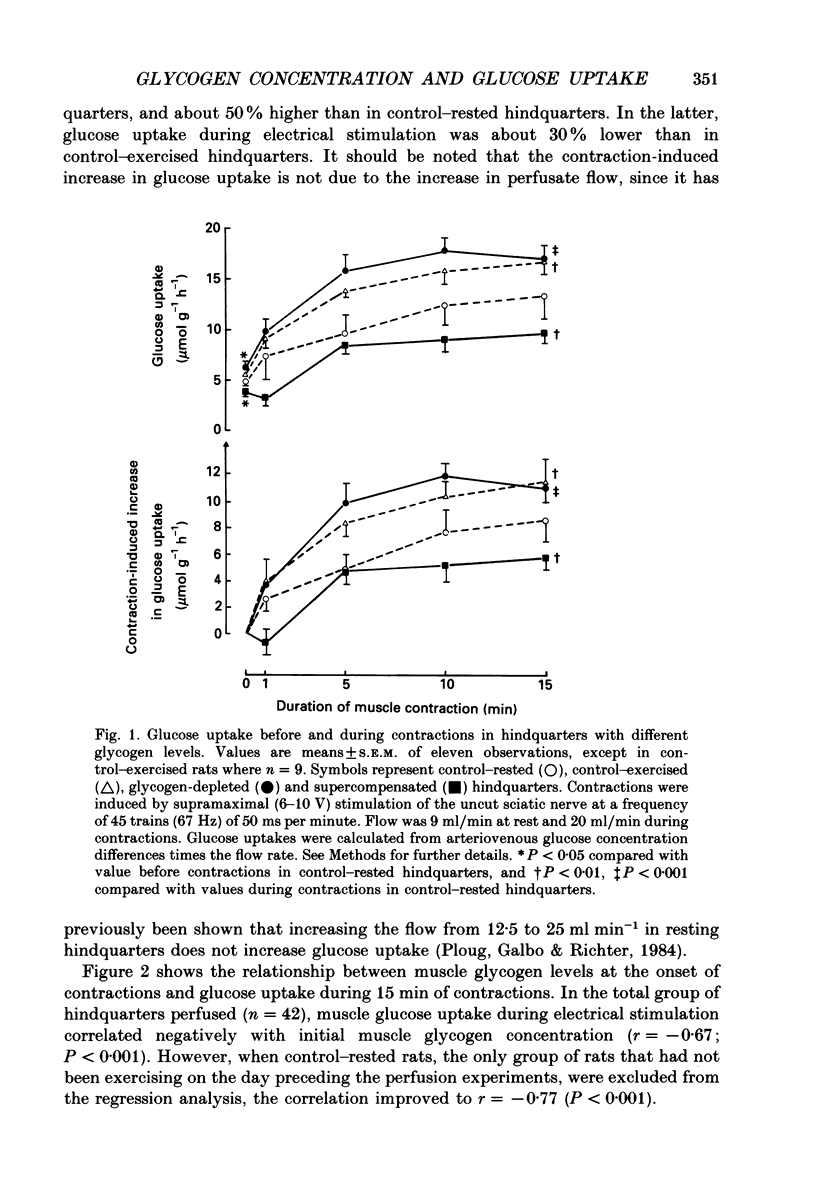
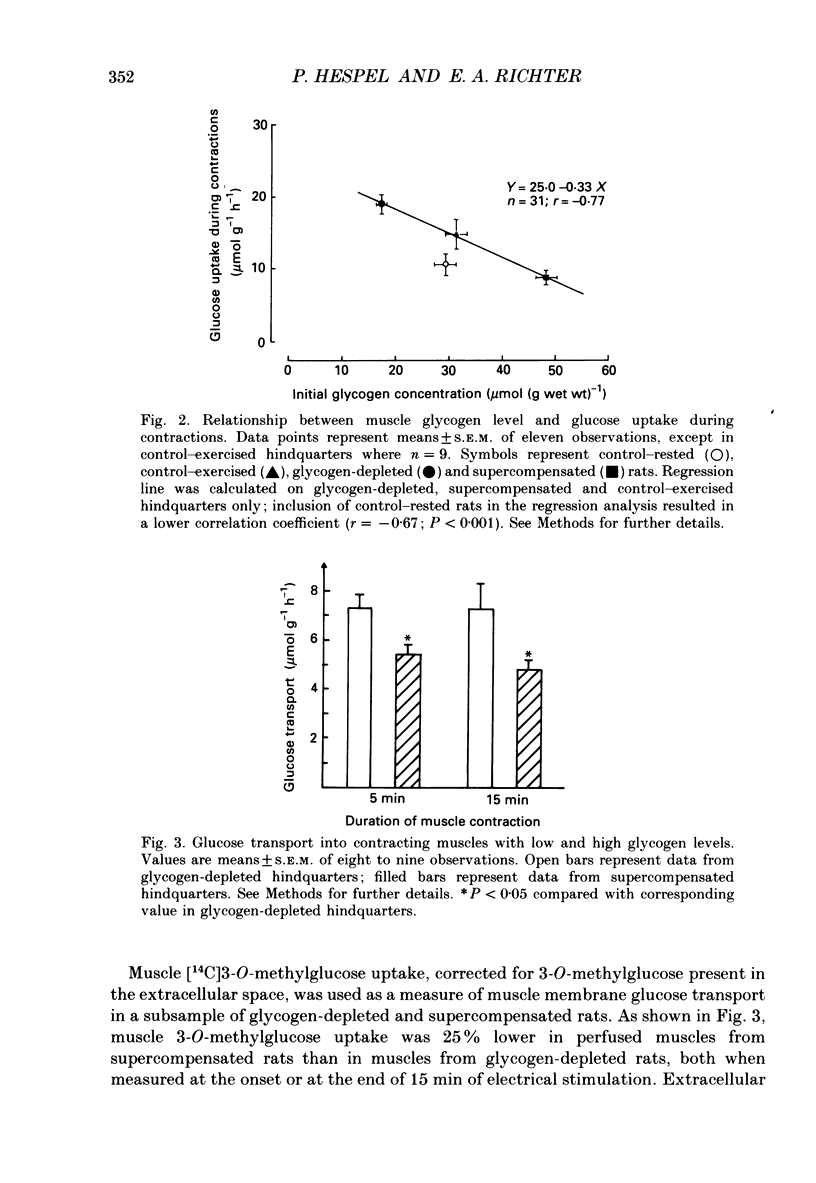
1. Glucose uptake and transport, muscle glycogen, free glucose and glucose-6-phosphate concentrations were studied in perfused resting and contracting rat skeletal muscle with different pre-contraction glycogen concentrations. Rats were pre-conditioned by a combination of swimming exercise and diet, resulting in either low (glycogen-depleted rats), normal (control rats) or high (supercompensated rats) muscle glycogen concentrations at the time their hindlimbs were perfused. 2. Compared with control rats, pre-contraction muscle glycogen concentration was approximately 40% lower in glycogen-depleted rats, whereas it was 40% higher in supercompensated rats. Muscle glycogen break-down correlated positively (r = 0.76; P less than 0.001) with pre-contraction muscle glycogen concentration. 3. Glucose uptake during contractions was approximately 50% higher in glycogen-depleted hindquarters than in control hindquarters; in supercompensated hindquarters it was 30% lower. When rats with similar muscle glycogen concentrations were compared, glucose uptake in hindquarters from rats that had exercised on the preceding day was approximately 20% higher than in hindquarters from rats that had not exercised on the preceding day. 4. Muscle membrane glucose transport, as measured by the rate of accumulation of 14C-3-O-methylglucose in the contracting muscles, was 25% lower in supercompensated than in glycogen-depleted muscles at the onset as well as at the end of the 15 min contraction period. 5. Intracellular concentrations of free glucose and glucose-6-phosphate were higher at rest and during the entire 15-min stimulation period in supercompensated muscles than in glycogen-depleted muscles, and glucose uptake during contractions correlated negatively with free glucose (r = -0.52; P less than 0.01) as well as with glucose-6-phosphate (r = -0.49; P less than 0.01) concentrations. 6. It is concluded that: (a) The rate of glucose uptake in contracting skeletal muscle is dependent on the pre-contraction muscle glycogen concentration. Regulating mechanisms include limitations of membrane glucose transport as well as of glucose metabolism. (b) Contractions on the preceding day have a stimulating effect on glucose uptake during contractions of the same muscles on the next day.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergström J., Hermansen L., Hultman E., Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967 Oct-Nov;71(2):140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- Cartee G. D., Young D. A., Sleeper M. D., Zierath J., Wallberg-Henriksson H., Holloszy J. O. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol. 1989 Apr;256(4 Pt 1):E494–E499. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- Douen A. G., Ramlal T., Klip A., Young D. A., Cartee G. D., Holloszy J. O. Exercise-induced increase in glucose transporters in plasma membranes of rat skeletal muscle. Endocrinology. 1989 Jan;124(1):449–454. doi: 10.1210/endo-124-1-449. [DOI] [PubMed] [Google Scholar]
- Foley J. E., Huecksteadt T. P. Glucose 6-phosphate effects on deoxyglucose, glucose and methylglucose transport in rat adipocytes. Evidence for intracellular regulation of sugar transport by glucose metabolites. Biochim Biophys Acta. 1984 Nov 13;805(3):313–316. doi: 10.1016/0167-4889(84)90088-0. [DOI] [PubMed] [Google Scholar]
- Galbo H., Holst J. J., Christensen N. J. The effect of different diets and of insulin on the hormonal response to prolonged exercise. Acta Physiol Scand. 1979 Sep;107(1):19–32. doi: 10.1111/j.1748-1716.1979.tb06438.x. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Piehl K., Saubert C. W., 4th, Armstrong R. B., Saltin B. Diet, exercise, and glycogen changes in human muscle fibers. J Appl Physiol. 1972 Oct;33(4):421–425. doi: 10.1152/jappl.1972.33.4.421. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Ruderman N. B., Aoki T. T. Glucose and amino acid metabolism in perfused skeletal muscle. Effect of dichloroacetate. Diabetes. 1978 Nov;27(11):1065–1074. doi: 10.2337/diab.27.11.1065. [DOI] [PubMed] [Google Scholar]
- Jansson E. Diet and muscle metabolism in man with reference to fat and carbohydrate utilization and its regulation. Acta Physiol Scand Suppl. 1980;487:1–24. [PubMed] [Google Scholar]
- Karlsson J., Diamant B., Saltin B. Muscle metabolites during submaximal and maximal exercise in man. Scand J Clin Lab Invest. 1970 Dec;26(4):385–394. doi: 10.3109/00365517009046250. [DOI] [PubMed] [Google Scholar]
- Kubo K., Foley J. E. Rate-limiting steps for insulin-mediated glucose uptake into perfused rat hindlimb. Am J Physiol. 1986 Jan;250(1 Pt 1):E100–E102. doi: 10.1152/ajpendo.1986.250.1.E100. [DOI] [PubMed] [Google Scholar]
- Ploug T., Galbo H., Richter E. A. Increased muscle glucose uptake during contractions: no need for insulin. Am J Physiol. 1984 Dec;247(6 Pt 1):E726–E731. doi: 10.1152/ajpendo.1984.247.6.E726. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Galbo H. High glycogen levels enhance glycogen breakdown in isolated contracting skeletal muscle. J Appl Physiol (1985) 1986 Sep;61(3):827–831. doi: 10.1152/jappl.1986.61.3.827. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Ploug T., Galbo H. Increased muscle glucose uptake after exercise. No need for insulin during exercise. Diabetes. 1985 Oct;34(10):1041–1048. doi: 10.2337/diab.34.10.1041. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Ruderman N. B., Gavras H., Belur E. R., Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. Am J Physiol. 1982 Jan;242(1):E25–E32. doi: 10.1152/ajpendo.1982.242.1.E25. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K., Broberg S., Katz A. Glucose formation in human skeletal muscle. Influence of glycogen content. Biochem J. 1989 Mar 15;258(3):911–913. doi: 10.1042/bj2580911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht E., Barnard R. J., Grimditch G. K. Exercise and insulin stimulate skeletal muscle glucose transport through different mechanisms. Am J Physiol. 1989 Feb;256(2 Pt 1):E227–E230. doi: 10.1152/ajpendo.1989.256.2.E227. [DOI] [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Holloszy J. O. Contractile activity increases glucose uptake by muscle in severely diabetic rats. J Appl Physiol Respir Environ Exerc Physiol. 1984 Oct;57(4):1045–1049. doi: 10.1152/jappl.1984.57.4.1045. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Young A. A., Lamkin C., Foley J. E. Kinetics of glucose disposal in whole body and across the forearm in man. J Clin Invest. 1987 Jun;79(6):1713–1719. doi: 10.1172/JCI113011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. C., Garthwaite S. M., Bryan J. E., Cartier L. J., Holloszy J. O. Carbohydrate feeding speeds reversal of enhanced glucose uptake in muscle after exercise. Am J Physiol. 1983 Nov;245(5 Pt 1):R684–R688. doi: 10.1152/ajpregu.1983.245.5.R684. [DOI] [PubMed] [Google Scholar]


