Abstract
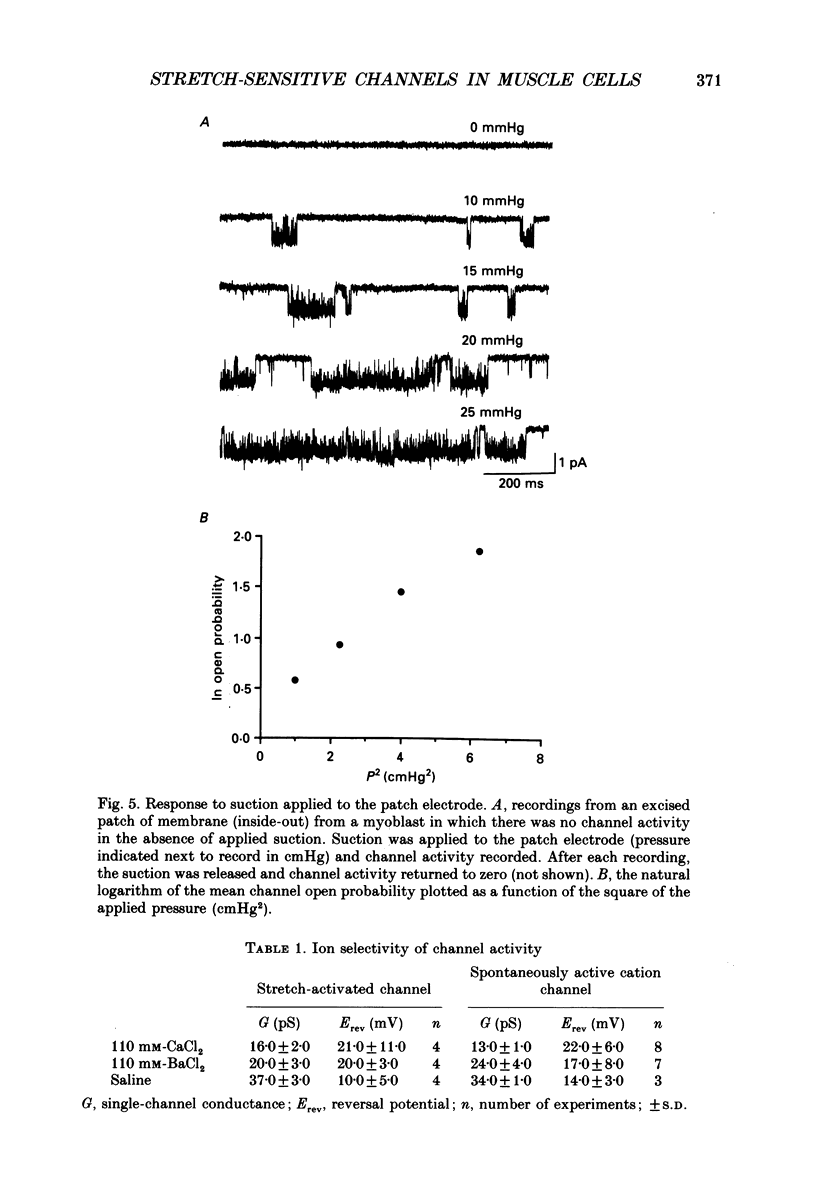
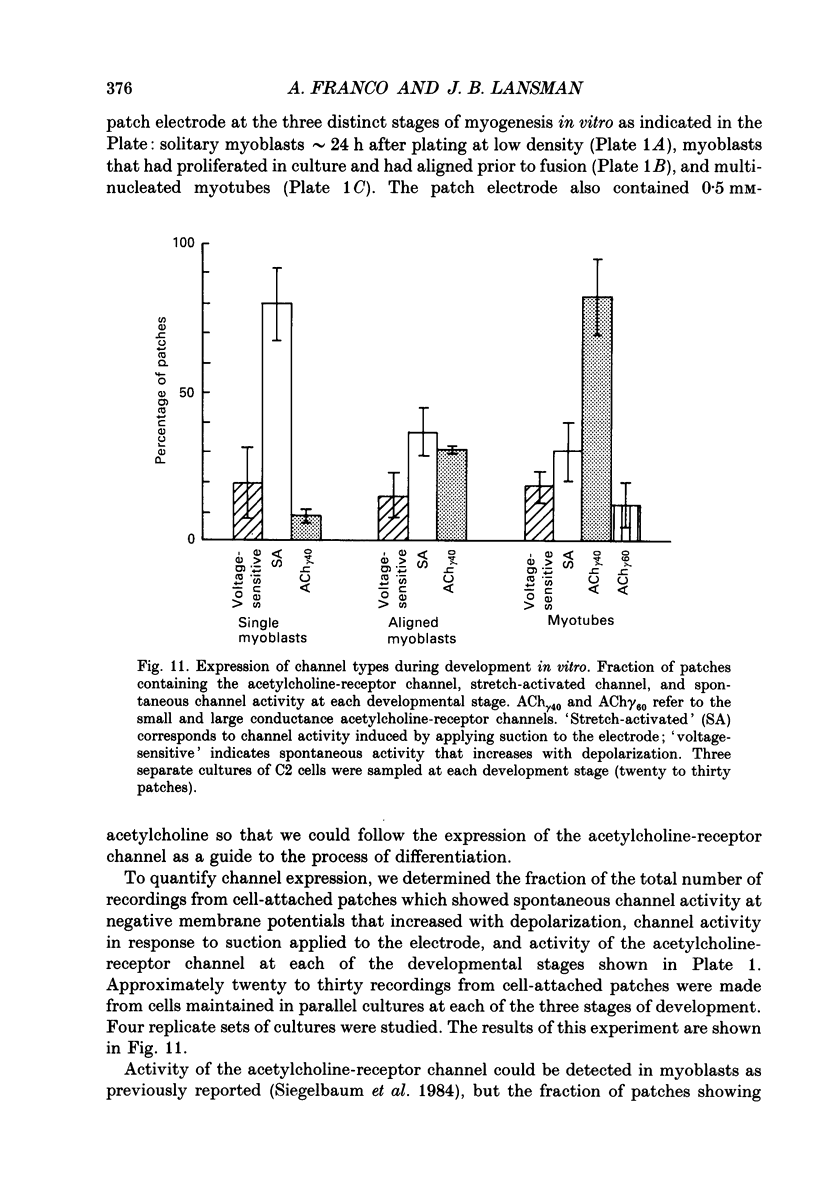
1. Recordings of single-channel activity were made from cell-attached patches on mouse C2 muscle cells at morphologically identifiable stages of myogenesis in vitro. We have identified Ca2(+)-permeable, cation-selective channels that are gated by applying suction to the patch electrode and by changes in membrane potential and have analysed single-channel properties as well as channel expression during myogenesis. 2. Single-channel activity could be detected when the membrane was held at steady negative potentials. With monovalent cations in the electrode, the single-channel current-voltage (i-V) relations were linear. The channel is permeable to Li+, Na+, K+, Rb+ and Cs+, but is not strongly selective among the monovalent cations as judged by measurements of single-channel conductance and reversal potential. 3. With 110 mM of either CaCl2 or BaCl2 as the only inward change carrier, slope conductances were approximately 13 and 24 pS and currents reversed at approximately +22 and +17 mV, respectively. The relative permeability of Ca2+ to K+ calculated from the constant-field equation was PCa/PK = approximately 2. 4. Channel openings occurred as bursts of brief openings and closings separated by much longer closed periods. Closed-time histograms were best fitted with three exponential components, while histograms of burst duration were best fitted with two exponential components, reflecting the short and long bursts in the single-channel records. 5. Applying suction to the patch electrode while recording at steady negative membrane potentials produced channel openings to discrete current levels. Mean channel open probability depended linearly on the square of the applied pressure and was greater at positive membrane potentials. The permeability of the channel to monovalent and divalent cations was indistinguishable from the spontaneous activity recorded at steady negative potentials. 6. Channel activity recorded from cell-attached patches in the absence of applied pressure depended on membrane potential increasing approximately e-fold per 38 mV with depolarization. Analysis of the kinetics of the response to membrane potential showed that the depolarization reduced the duration of the slowest component of the closed-time distribution. 7. The lanthanide cation gadolinium (Gd) reduced the amplitude of the unitary currents in a concentration-dependent manner. The amplitudes of both inward and outward currents were reduced to the same extent suggesting block is voltage-independent. Gd produced half-maximal inhibition of the unitary current at approximately 6 microM.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodensteiner J. B., Engel A. G. Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: a study of 567,000 muscle fibers in 114 biopsies. Neurology. 1978 May;28(5):439–446. doi: 10.1212/wnl.28.5.439. [DOI] [PubMed] [Google Scholar]
- Bregestovski P. D., Miledi R., Parker I. Calcium conductance of acetylcholine-induced endplate channels. Nature. 1979 Jun 14;279(5714):638–639. doi: 10.1038/279638a0. [DOI] [PubMed] [Google Scholar]
- Brehm P., Kullberg R., Moody-Corbett F. Properties of non-junctional acetylcholine receptor channels on innervated muscle of Xenopus laevis. J Physiol. 1984 May;350:631–648. doi: 10.1113/jphysiol.1984.sp015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey J. M., Brown A. M., Schneider M. D. Mitogens and oncogenes can block the induction of specific voltage-gated ion channels. Science. 1987 May 1;236(4801):570–573. doi: 10.1126/science.2437651. [DOI] [PubMed] [Google Scholar]
- David J. D., Higginbotham C. A. Fusion of chick embryo skeletal myoblasts: interactions of prostaglandin E1, adenosine 3':5' monophosphate, and calcium influx. Dev Biol. 1981 Mar;82(2):308–316. doi: 10.1016/0012-1606(81)90454-1. [DOI] [PubMed] [Google Scholar]
- David J. D., See W. M., Higginbotham C. A. Fusion of chick embryo skeletal myoblasts: role of calcium influx preceding membrane union. Dev Biol. 1981 Mar;82(2):297–307. doi: 10.1016/0012-1606(81)90453-x. [DOI] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Mechanotransducer ion channels in chick skeletal muscle: the effects of extracellular pH. J Physiol. 1985 Jun;363:119–134. doi: 10.1113/jphysiol.1985.sp015699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin M. C., Zhou X. L., Martinac B., Kung C. A mechanosensitive ion channel in the yeast plasma membrane. Science. 1988 Nov 4;242(4879):762–765. doi: 10.1126/science.2460920. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Inestrosa N. C., Miller J. B., Silberstein L., Ziskind-Conhaim L., Hall Z. W. Developmental regulation of 16S acetylcholinesterase and acetylcholine receptors in a mouse muscle cell line. Exp Cell Res. 1983 Sep;147(2):393–405. doi: 10.1016/0014-4827(83)90221-5. [DOI] [PubMed] [Google Scholar]
- Konieczny S. F., McKay J., Coleman J. R. Isolation and characterization of terminally differentiated chicken and rat skeletal muscle myoblasts. Dev Biol. 1982 May;91(1):11–26. doi: 10.1016/0012-1606(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. High selectivity of calcium channels in single dialysed heart cells of the guinea-pig. J Physiol. 1984 Sep;354:253–272. doi: 10.1113/jphysiol.1984.sp015374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkhart T. A., Clegg C. H., Hauschika S. D. Myogenic differentiation in permanent clonal mouse myoblast cell lines: regulation by macromolecular growth factors in the culture medium. Dev Biol. 1981 Aug;86(1):19–30. doi: 10.1016/0012-1606(81)90311-0. [DOI] [PubMed] [Google Scholar]
- Martinac B., Buechner M., Delcour A. H., Adler J., Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Sternberg E., Hu J. S., Spizz G., Wilcox C. Regulation of myogenic differentiation by type beta transforming growth factor. J Cell Biol. 1986 Nov;103(5):1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F. Mechanical transduction in biological systems. Crit Rev Biomed Eng. 1988;16(2):141–169. [PubMed] [Google Scholar]
- Siegelbaum S. A., Trautmann A., Koenig J. Single acetylcholine-activated channel currents in developing muscle cells. Dev Biol. 1984 Aug;104(2):366–379. doi: 10.1016/0012-1606(84)90092-7. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J. The fusion of myoblasts. Biochem J. 1985 May 15;228(1):1–12. doi: 10.1042/bj2280001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989 Feb 24;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]



