Abstract
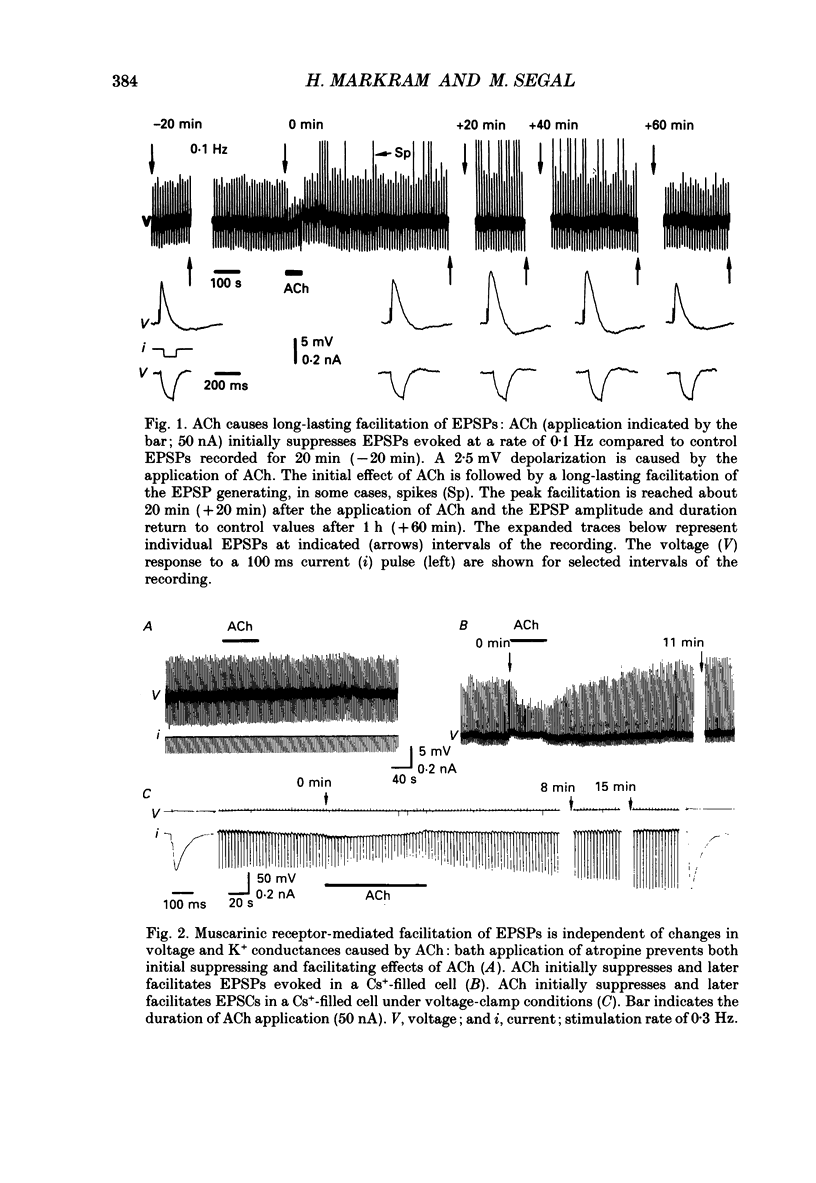
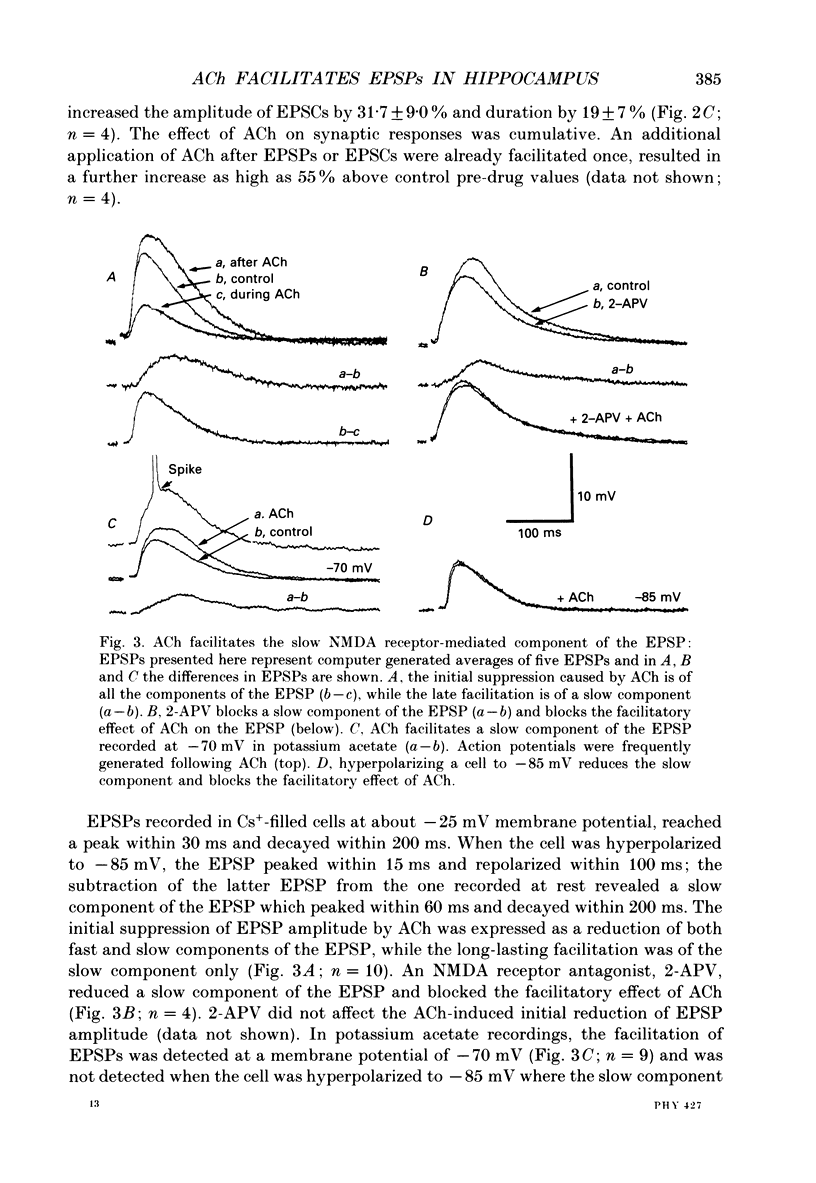
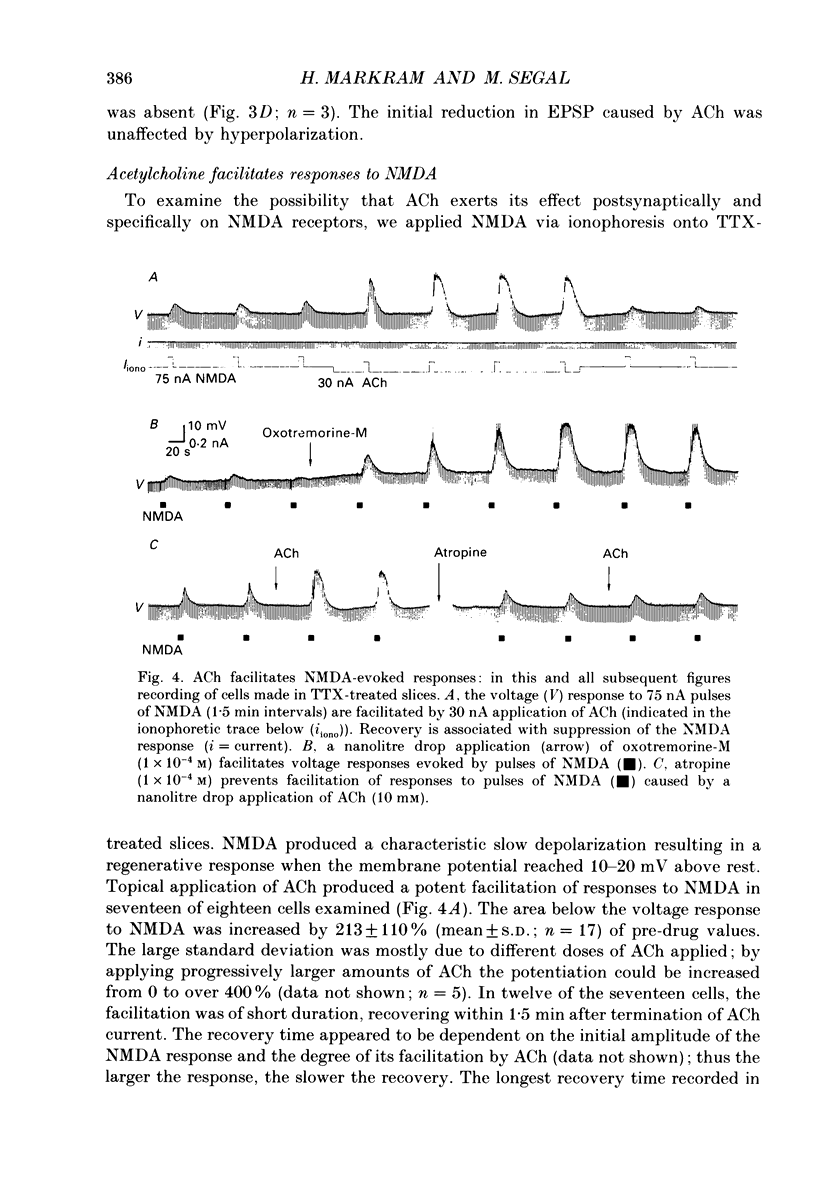
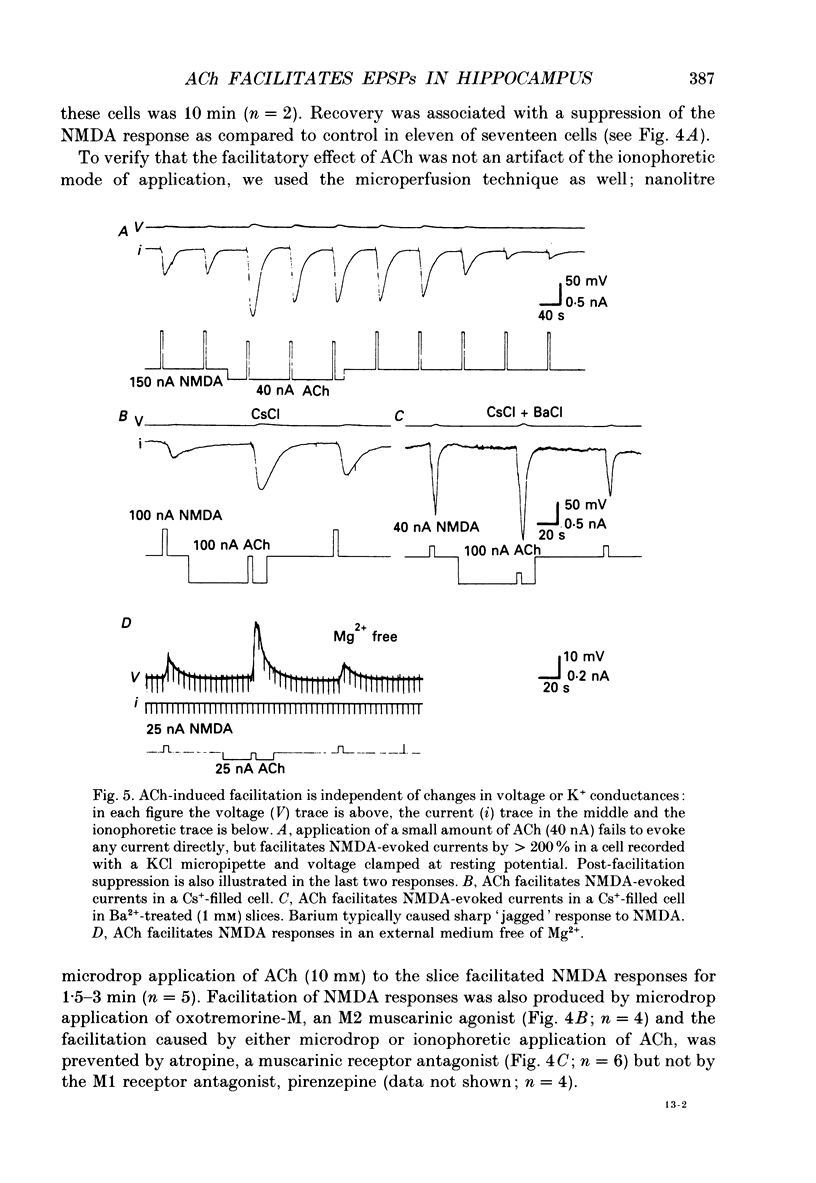
1. The effects of acetylcholine (ACh) on excitatory postsynaptic potentials (EPSPs) evoked by stimulating Schaffer-commissural afferents and on ionophoretically applied L-glutamate ligands, were investigated in CA1 neurones of hippocampal slices using current- and voltage-clamp techniques. 2. ACh produced a transient suppression followed by a long-lasting facilitation of EPSPs. The facilitation was also seen in Cs(+)-filled cells under voltage-clamp conditions. Both suppressing and facilitating effects were blocked by atropine. 3. All components of the EPSP were reduced in the initial phase of ACh action, while only the slow component was enhanced during the later phase. The facilitation was blocked by an N-methyl-D-aspartate (NMDA) receptor antagonist, d-2-amino-5-phosphonovalerate (2-APV) and by hyperpolarization. 4. ACh also facilitated responses to ionophoretically applied NMDA in voltage-clamped, Cs(+)-filled cells in Ba2(+)-treated slices. ACh facilitated responses to L-glutamate which was blocked by 2-APV. ACh failed to affect responses to kainate or quisqualate. 5. We conclude that ACh, acting on muscarinic receptors, exerts a primary effect in the hippocampus to specifically amplify NMDA receptor-mediated synaptic responses and thereby facilitate EPSPs.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURESOVA O., BURES J., BOHDANECKY Z., WEISS T. EFFECT OF ATROPINE ON LEARNING, EXTINCTION, RETENTION AND RETRIEVAL IN RATS. Psychopharmacologia. 1964 Mar 11;5:255–263. doi: 10.1007/BF02341258. [DOI] [PubMed] [Google Scholar]
- Bartus R. T., Dean R. L., 3rd, Beer B., Lippa A. S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982 Jul 30;217(4558):408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Acetylcholine induced modulation of hippocampal pyramidal neurons. Brain Res. 1981 Apr 27;211(1):227–234. doi: 10.1016/0006-8993(81)90089-5. [DOI] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):333–344. doi: 10.1016/0006-8993(82)90067-1. [DOI] [PubMed] [Google Scholar]
- Bland B. H., Colom L. V., Konopacki J., Roth S. H. Intracellular records of carbachol-induced theta rhythm in hippocampal slices. Brain Res. 1988 May 3;447(2):364–368. doi: 10.1016/0006-8993(88)91141-9. [DOI] [PubMed] [Google Scholar]
- Bland B. H. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26(1):1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science. 1983 Sep 23;221(4617):1299–1301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croucher M. J., Collins J. F., Meldrum B. S. Anticonvulsant action of excitatory amino acid antagonists. Science. 1982 May 21;216(4548):899–901. doi: 10.1126/science.7079744. [DOI] [PubMed] [Google Scholar]
- Davis W. M., Hatoum N. S. Synergism of the toxicity of physostigmine and neostigmine by lithium or by a reserpine-like agent (Ro4-1284). Toxicology. 1980;17(1):1–7. doi: 10.1016/0300-483x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Deutsch J. A. The cholinergic synapse and the site of memory. Science. 1971 Nov 19;174(4011):788–794. doi: 10.1126/science.174.4011.788. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Hynes M. A., King G. L. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol. 1986 Nov;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J., Dingledine R., Kelly J. S. The excitatory action of acetylcholine on hippocampal neurones of the guinea pig and rat maintained in vitro. Brain Res. 1981 Feb 23;207(1):109–127. doi: 10.1016/0006-8993(81)90682-x. [DOI] [PubMed] [Google Scholar]
- Drachman D. A., Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974 Feb;30(2):113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- Freund T. F., Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988 Nov 10;336(6195):170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. Muscarine affects calcium-currents in rat hippocampal pyramidal cells in vitro. Neurosci Lett. 1987 May 19;76(3):301–306. doi: 10.1016/0304-3940(87)90419-8. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Dreifuss J. J. Multiple actions of acetylcholine on hippocampal pyramidal cells in organotypic explant cultures. Neuroscience. 1982 May;7(5):1243–1256. doi: 10.1016/0306-4522(82)91131-9. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Hirotsu I., Hori N., Katsuda N., Ishihara T. Effect of anticholinergic drug on long-term potentiation in rat hippocampal slices. Brain Res. 1989 Mar 13;482(1):194–197. doi: 10.1016/0006-8993(89)90561-1. [DOI] [PubMed] [Google Scholar]
- Hwa G. G., Avoli M. NMDA receptor antagonists CPP and MK-801 partially suppress the epileptiform discharges induced by the convulsant drug bicuculline in the rat neocortex. Neurosci Lett. 1989 Mar 27;98(2):189–193. doi: 10.1016/0304-3940(89)90508-9. [DOI] [PubMed] [Google Scholar]
- Ito T., Miura Y., Kadokawa T. Effects of physostigmine and scopolamine on long-term potentiation of hippocampal population spikes in rats. Can J Physiol Pharmacol. 1988 Aug;66(8):1010–1016. doi: 10.1139/y88-165. [DOI] [PubMed] [Google Scholar]
- Jope R. S., Morrisett R. A., Snead O. C. Characterization of lithium potentiation of pilocarpine-induced status epilepticus in rats. Exp Neurol. 1986 Mar;91(3):471–480. doi: 10.1016/0014-4886(86)90045-2. [DOI] [PubMed] [Google Scholar]
- Jope R. S., Simonato M., Lally K. Acetylcholine content in rat brain is elevated by status epilepticus induced by lithium and pilocarpine. J Neurochem. 1987 Sep;49(3):944–951. doi: 10.1111/j.1471-4159.1987.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Kesner R. P. Reevaluation of the contribution of the basal forebrain cholinergic system to memory. Neurobiol Aging. 1988 Sep-Dec;9(5-6):609–616. doi: 10.1016/s0197-4580(88)80122-2. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Reiffenstein R. J., Ropert N. Disinhibitory action of acetylcholine in the rat's hippocampus: extracellular observations. Neuroscience. 1981;6(12):2465–2474. doi: 10.1016/0306-4522(81)90092-0. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Ropert N. Electrophysiological and pharmacological characteristics of facilitation of hippocampal population spikes by stimulation of the medial septum. Neuroscience. 1982;7(9):2165–2183. doi: 10.1016/0306-4522(82)90128-2. [DOI] [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C., Silver A. Confirmation from choline acetylase analyses of a massive cholinergic innervation to the rat hippocampus. J Physiol. 1967 Jul;191(1):215–224. doi: 10.1113/jphysiol.1967.sp008246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Lancaster B., Nicoll R. A. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987 Mar;7(3):733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi M., Raiteri M. Interaction acetylcholine-glutamate in rat hippocampus: involvement of two subtypes of M-2 muscarinic receptors. J Pharmacol Exp Ther. 1989 Mar;248(3):1255–1260. [PubMed] [Google Scholar]
- Muller D., Joly M., Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988 Dec 23;242(4886):1694–1697. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Nakajima S., Leonard R. J., Yamaguchi K. Acetylcholine raises excitability by inhibiting the fast transient potassium current in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1986 May;83(9):3022–3026. doi: 10.1073/pnas.83.9.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ordy J. M., Thomas G. J., Volpe B. T., Dunlap W. P., Colombo P. M. An animal model of human-type memory loss based on aging, lesion, forebrain ischemia, and drug studies with the rat. Neurobiol Aging. 1988 Sep-Dec;9(5-6):667–683. doi: 10.1016/s0197-4580(88)80131-3. [DOI] [PubMed] [Google Scholar]
- Pitler T. A., McCarren M., Alger B. E. Calcium-dependent pirenzepine-sensitive muscarinic response in the rat hippocampal slice. Neurosci Lett. 1988 Aug 31;91(2):177–182. doi: 10.1016/0304-3940(88)90764-1. [DOI] [PubMed] [Google Scholar]
- Robinson G. B. Enhanced long-term potentiation induced in rat dentate gyrus by coactivation of septal and entorhinal inputs: temporal constraints. Brain Res. 1986 Jul 30;379(1):56–62. doi: 10.1016/0006-8993(86)90254-4. [DOI] [PubMed] [Google Scholar]
- Robinson G. B., Racine R. J. Interactions between septal and entorhinal inputs to the rat dentate gyrus: facilitation effects. Brain Res. 1986 Jul 30;379(1):63–67. doi: 10.1016/0006-8993(86)90255-6. [DOI] [PubMed] [Google Scholar]
- Segal M. Multiple action of acetylcholine at a muscarinic receptor studied in the rat hippocampal slice. Brain Res. 1982 Aug 19;246(1):77–87. doi: 10.1016/0006-8993(82)90144-5. [DOI] [PubMed] [Google Scholar]
- Segal M. Synaptic activation of a cholinergic receptor in rat hippocampus. Brain Res. 1988 Jun 14;452(1-2):79–86. doi: 10.1016/0006-8993(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Stringer J. L., Greenfield L. J., Hackett J. T., Guyenet P. G. Blockade of long-term potentiation by phencyclidine and sigma opiates in the hippocampus in vivo and in vitro. Brain Res. 1983 Nov 28;280(1):127–138. doi: 10.1016/0006-8993(83)91180-0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Sakurai M., Hayashi S. Effect of scopolamine and HP 029, a cholinesterase inhibitor, on long-term potentiation in hippocampal slices of the guinea pig. Neurosci Lett. 1989 Mar 27;98(2):179–183. doi: 10.1016/0304-3940(89)90506-5. [DOI] [PubMed] [Google Scholar]
- Teyler T. J., DiScenna P. The role of hippocampus in memory: a hypothesis. Neurosci Biobehav Rev. 1985 Fall;9(3):377–389. doi: 10.1016/0149-7634(85)90016-8. [DOI] [PubMed] [Google Scholar]
- Teyler T. J., Discenna P. Long-term potentiation as a candidate mnemonic device. Brain Res. 1984 Mar;319(1):15–28. doi: 10.1016/0165-0173(84)90027-4. [DOI] [PubMed] [Google Scholar]
- Tse F. W., MacVicar B. A. Phosphoinositides and GTP binding proteins involved in muscarinic generation of hippocampal rhythmic slow activity. Neurosci Lett. 1989 Jul 17;102(1):58–63. doi: 10.1016/0304-3940(89)90307-8. [DOI] [PubMed] [Google Scholar]
- Walden J., Speckmann E. J., Bingmann D. Augmentation of glutamate responses by GABA in the rat's motorcortex in vivo. Neurosci Lett. 1989 Jun 19;101(2):209–213. doi: 10.1016/0304-3940(89)90532-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto C., Kawai N. Presynaptic action of acetylcholine in thin sections from the guinea pig dentate gyrus in vitro. Exp Neurol. 1967 Oct;19(2):176–187. doi: 10.1016/0014-4886(67)90016-7. [DOI] [PubMed] [Google Scholar]


