Abstract
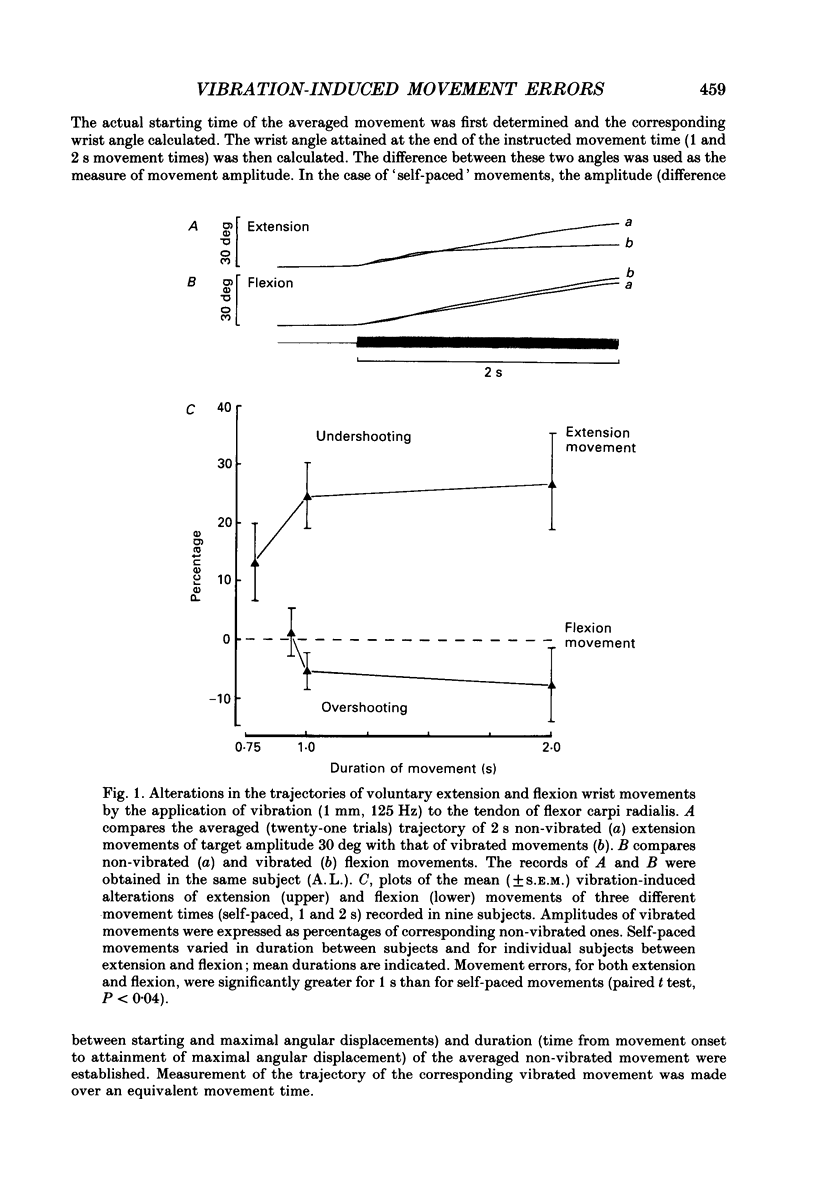
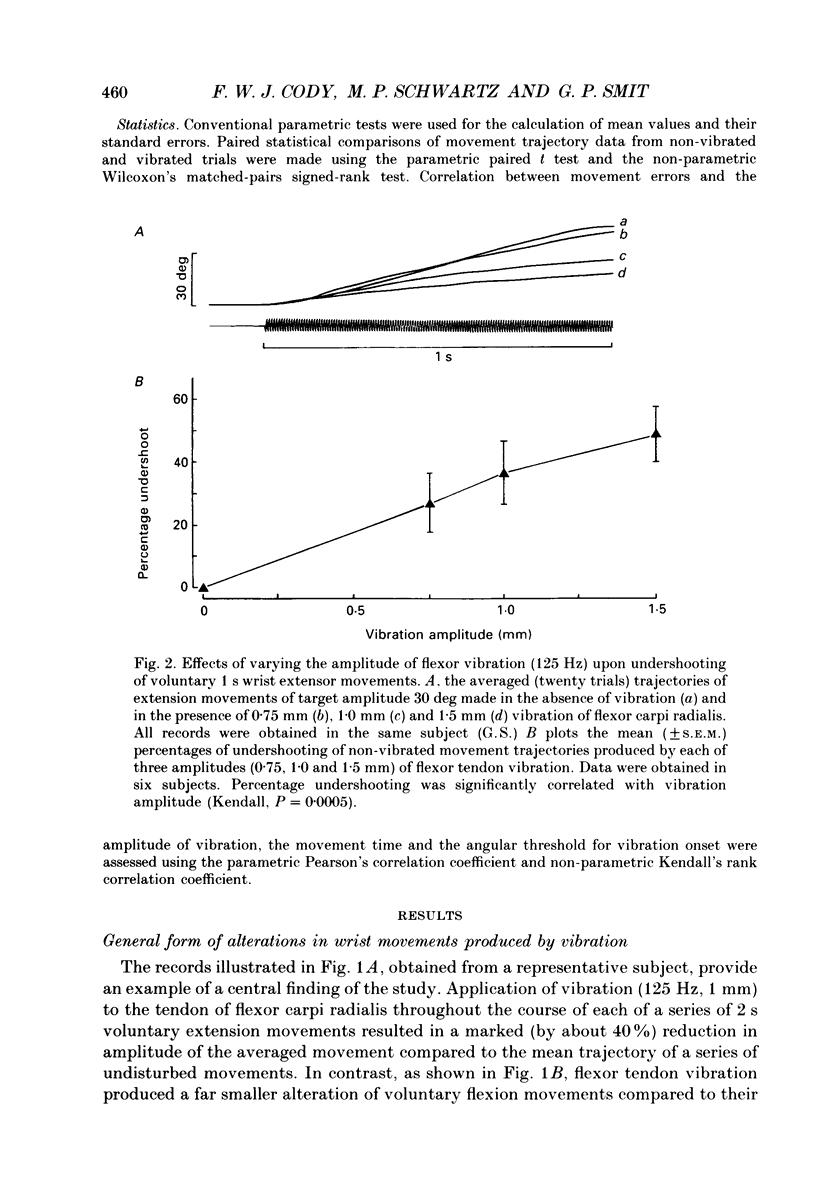
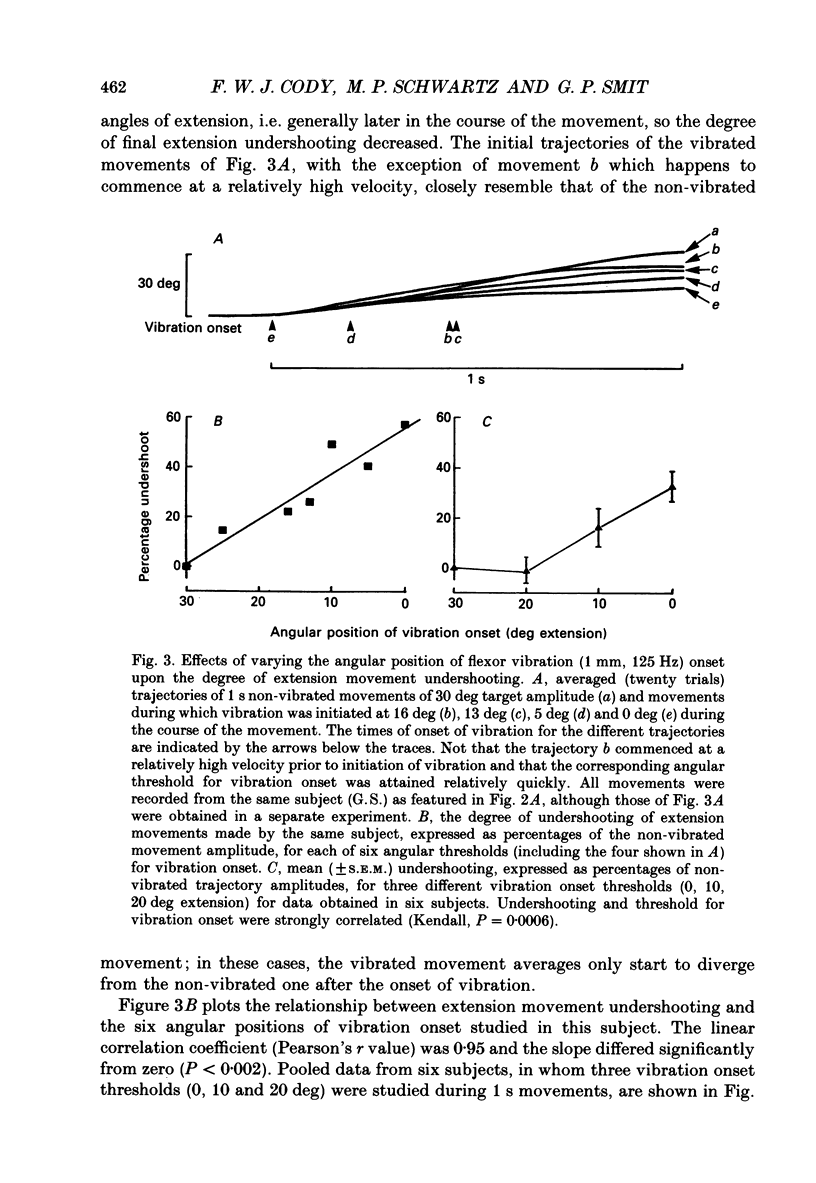
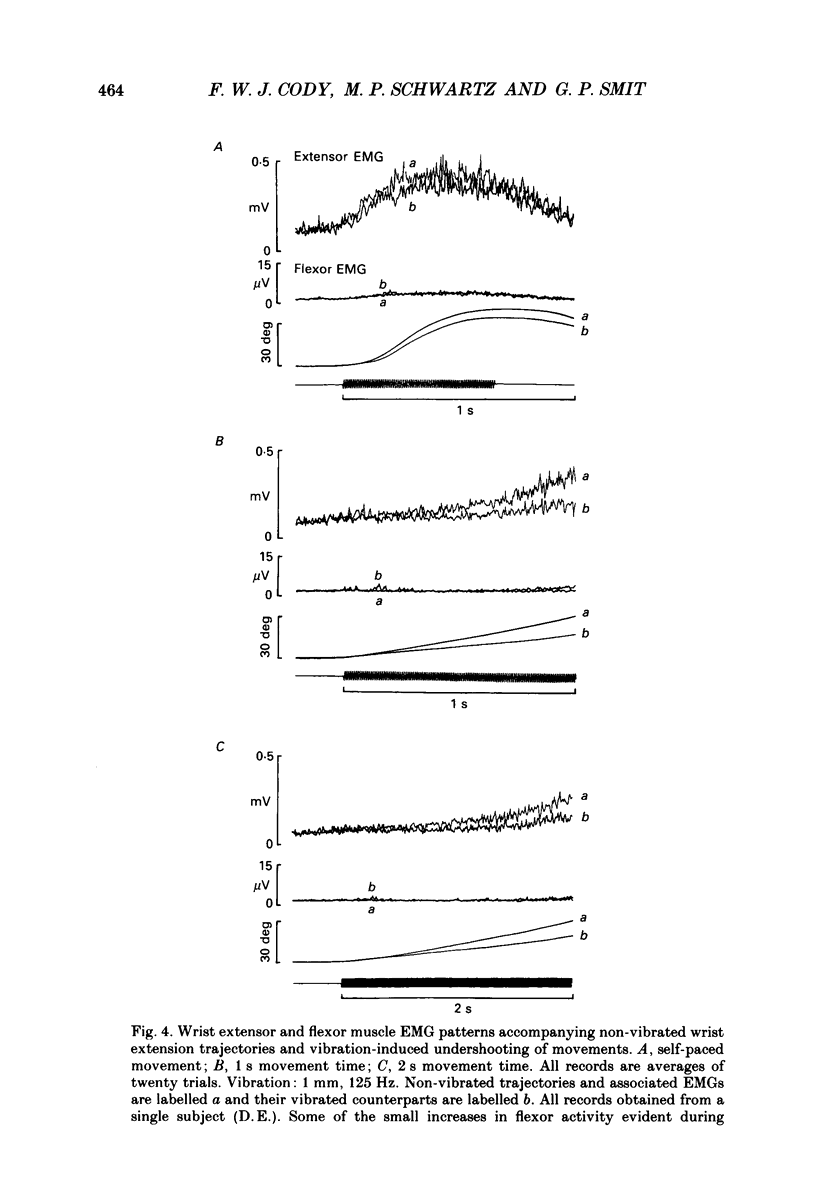
1. The alterations in voluntary wrist extension and flexion movement trajectories induced by application of vibration to the tendon of flexor carpi radialis throughout the course of the movement, together with the associated EMG patterns, have been studied in normal human subjects. Both extension and flexion movements were routinely of a target amplitude of 30 deg and made against a torque load of 0.32 N m. Flexor tendon vibration consistently produced undershooting of voluntary extension movements. In contrast, voluntary flexion movements were relatively unaffected. 2. The degree of vibration-induced undershooting of 1 s voluntary extension movements was graded according to the amplitude (0.75, 1.0 and 1.5 mm) of flexor tendon vibration. 3. As flexor vibration was initiated progressively later (at greater angular thresholds) during the course of 1 s voluntary extension movements, and the period of vibration was proportionately reduced, so the degree of vibration-induced undershooting showed a corresponding decline. 4. Varying the torque loads (0.32, 0.65 and 0.97 N m) against which 1 s extension movements were made, and thereby the strength of voluntary extensor contraction, produced no systematic changes in the degree of flexor vibration-induced undershooting. 5. Analysis of EMG patterns recorded from wrist flexor and extensor muscles indicated that vibration-induced undershooting of extension movements resulted largely from a reduction in activity in the prime-mover rather than increased antagonist activity. The earliest reductions in extensor EMG commenced some 40 ms after the onset of vibration, i.e. well before voluntary reaction time; these initial responses were considered to be 'automatic' in nature. 6. These results support the view that the central nervous system utilizes proprioceptive information in the continuous regulation of moderately slow voluntary wrist movements. Proprioceptive sensory input from the passively lengthening antagonist muscle, presumably arising mainly from muscle spindle I a afferents, appears to be particularly important and to act mainly in the reciprocal control of the prime-mover.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berardelli A., Day B. L., Marsden C. D., Rothwell J. C. Evidence favouring presynaptic inhibition between antagonist muscle afferents in the human forearm. J Physiol. 1987 Oct;391:71–83. doi: 10.1113/jphysiol.1987.sp016726. [DOI] [PMC free article] [PubMed] [Google Scholar]
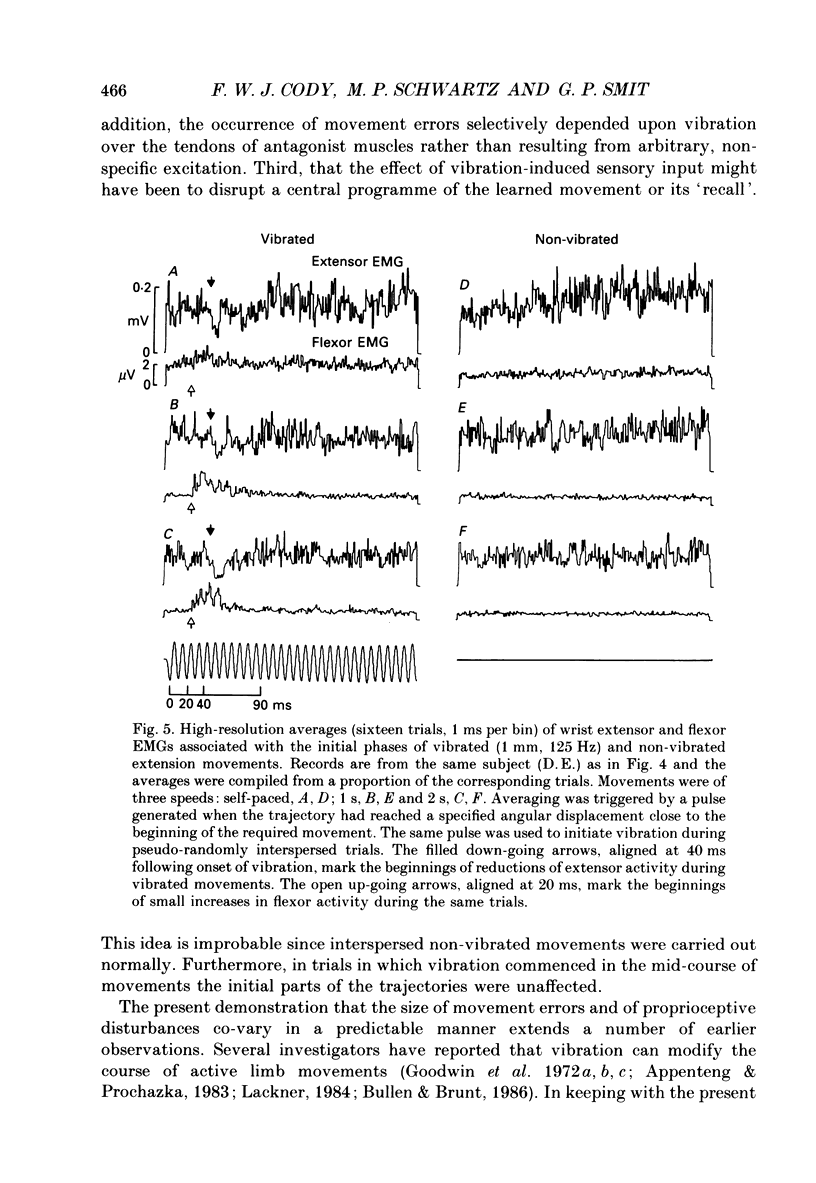
- Bullen A. R., Brunt D. Effects of tendon vibration on unimanual and bimanual movement accuracy. Exp Neurol. 1986 Aug;93(2):311–319. doi: 10.1016/0014-4886(86)90192-5. [DOI] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L., Wallin B. G. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976 Oct;261(3):695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L., Wallin B. G. The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol. 1976 Oct;261(3):673–693. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C., Cooke J. D. The effects of muscle vibration on the attainment of intended final position during voluntary human arm movements. Exp Brain Res. 1981;42(2):228–230. doi: 10.1007/BF00236912. [DOI] [PubMed] [Google Scholar]
- Capaday C., Cooke J. D. Vibration-induced changes in movement-related EMG activity in humans. Exp Brain Res. 1983;52(1):139–146. doi: 10.1007/BF00237158. [DOI] [PubMed] [Google Scholar]
- Cody F. W., Plant T. Vibration-evoked reciprocal inhibition between human wrist muscles. Exp Brain Res. 1989;78(3):613–623. doi: 10.1007/BF00230249. [DOI] [PubMed] [Google Scholar]
- Gilhodes J. C., Roll J. P., Tardy-Gervet M. F. Perceptual and motor effects of agonist-antagonist muscle vibration in man. Exp Brain Res. 1986;61(2):395–402. doi: 10.1007/BF00239528. [DOI] [PubMed] [Google Scholar]
- Goodwin G. M., McCloskey D. I., Matthews P. B. Proprioceptive illusions induced by muscle vibration: contribution by muscle spindles to perception? Science. 1972 Mar 24;175(4028):1382–1384. doi: 10.1126/science.175.4028.1382. [DOI] [PubMed] [Google Scholar]
- Goodwin G. M., McCloskey D. I., Matthews P. B. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain. 1972;95(4):705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Goodwin G. M., McCloskey D. I., Matthews P. B. The persistence of appreciable kinesthesia after paralysing joint afferents but preserving muscle afferents. Brain Res. 1972 Feb 25;37(2):326–329. doi: 10.1016/0006-8993(72)90679-8. [DOI] [PubMed] [Google Scholar]
- Hallett M., Shahani B. T., Young R. R. EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiatry. 1975 Dec;38(12):1154–1162. doi: 10.1136/jnnp.38.12.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner J. R. Some influences of tonic vibration reflexes on the position sense of the contralateral limb. Exp Neurol. 1984 Jul;85(1):107–113. doi: 10.1016/0014-4886(84)90165-1. [DOI] [PubMed] [Google Scholar]
- Matthews P. B. Observations on the time course of the electromyographic response reflexly elicited by muscle vibration in man. J Physiol. 1984 Aug;353:447–461. doi: 10.1113/jphysiol.1984.sp015346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton P. A. Human position sense and sense of effort. Symp Soc Exp Biol. 1964;18:387–400. [PubMed] [Google Scholar]
- Moberg E. The role of cutaneous afferents in position sense, kinaesthesia, and motor function of the hand. Brain. 1983 Mar;106(Pt 1):1–19. doi: 10.1093/brain/106.1.1. [DOI] [PubMed] [Google Scholar]
- Roll J. P., Vedel J. P. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res. 1982;47(2):177–190. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- Rothwell J. C., Traub M. M., Day B. L., Obeso J. A., Thomas P. K., Marsden C. D. Manual motor performance in a deafferented man. Brain. 1982 Sep;105(Pt 3):515–542. doi: 10.1093/brain/105.3.515. [DOI] [PubMed] [Google Scholar]
- Sittig A. C., Denier van der Gon J. J., Gielen C. C. Separate control of arm position and velocity demonstrated by vibration of muscle tendon in man. Exp Brain Res. 1985;60(3):445–453. doi: 10.1007/BF00236930. [DOI] [PubMed] [Google Scholar]
- Sittig A. C., Denier van der Gon J. J., Gielen C. C. The contribution of afferent information on position and velocity to the control of slow and fast human forearm movements. Exp Brain Res. 1987;67(1):33–40. doi: 10.1007/BF00269450. [DOI] [PubMed] [Google Scholar]


